Published online Feb 14, 2012. doi: 10.3748/wjg.v18.i6.563
Revised: March 14, 2011
Accepted: March 21, 2011
Published online: February 14, 2012
AIM: To explore the potential risk factors related to gastrointestinal cancer in northern China.
METHODS: A total of 3314 cases of gastrointestinal cancer (esophageal, gastric, pancreatic and biliary) and 2223 controls (including healthy individuals, glioma and thyroid cancer) were analyzed by case-control study. Multivariable logistic regression analysis was applied to evaluate the association between different cancers and hepatitis B surface antigen, sex, age, blood type, diabetes, or family history of cancer.
RESULTS: Type 2 diabetes was significantly associated with gastric, biliary and pancreatic cancer with an OR of 2.0-3.0. Blood type B was significantly associated with esophageal cancer [odd ratio (OR) = 1.53, 95% confidence interval (CI) = 1.10-2.14] and biliary cancer (OR = 1.49, 95% CI = 1.09-2.05). The prevalence of type 2 diabetes was significantly higher in gastric, biliary and pancreatic cancers compared with other groups, with ORs ranging between 2.0 and 3.0. Family history of cancer was strongly associated with gastrointestinal compared with other cancers.
CONCLUSION: Blood type B individuals are susceptible to esophageal and biliary cancer. Type 2 diabetes is significantly associated with gastric, biliary and especially pancreatic cancer.
- Citation: Gong Y, Yang YS, Zhang XM, Su M, Wang J, Han JD, Guo MZ. ABO blood type, diabetes and risk of gastrointestinal cancer in northern China. World J Gastroenterol 2012; 18(6): 563-569
- URL: https://www.wjgnet.com/1007-9327/full/v18/i6/563.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i6.563
The incidence and mortality of gastrointestinal cancers (e.g., esophagus, stomach, pancreas, and biliary tract) are higher in China compared with Western countries, but the reason remains unclear.
In contrast to most Western countries, esophageal squamous cell cancer is common in many areas of China[1]. Gastric cancer ranks as the third most common cancer in China for both men and women, with 5-year survival rate < 20%[2]. Pancreatic cancer is the sixth leading cause of death among malignant diseases in China, with an overall cumulative 5-year survival rate of only 1%-3%[3].
A recent seroepidemiological study conducted in mainland China showed that hepatitis B surface antigen (HBsAg) prevalence was 7.2% in the general population and 1.1% in children under 5 years of age[4]. Hepatitis B virus (HBV) is considered to be hepatotropic and is significantly associated with end-stage chronic liver diseases, including hepatocellular carcinoma (HCC) and cholangiocarcinoma[5]. HBV may travel through the bloodstream and deposit in other organs[6]. The possible relationship between HBV and pancreatic or bile duct cancer has been reported[7,8], but the inverse relationship has been reported by others[9].
The most common type of diabetes mellitus, type 2, seems to be associated with cancers of the biliary tract and pancreas. It is significant that the greatest risk of cancer in diabetic patients is to the organs in which concentrations of endogenous insulin reach particularly high levels (i.e., liver and pancreas)[10]. Meta-analyses have indicated that diabetes mellitus is associated with a 1.7-fold increased risk of pancreatic cancer and a 2.5-fold increased risk of HCC[11].
Studies conducted several decades ago have suggested a link between inherited human blood group antigens and the risk of various malignancies[12]. Human blood antigens are glycoproteins expressed on the surface of red blood cells and a few other cell types, including cells from the gastrointestinal tract. The sugar residues of these glycoproteins are attached to a protein backbone, the H antigen, by a glycosyltransferase that is encoded by the ABO gene[13]. Alterations of surface glycoconjugates may lead to modifications and could be related to tumor development and spread.
Recently, Wolpin et al[14] have found that ABO blood type is significantly associated with the risk of pancreatic cancer. Hassan et al[7] have reported a higher risk of pancreatic cancer in HBV carriers.
Although it seems reasonable that ABO blood type, HBsAg, and type 2 diabetes may have a close relationship with gastrointestinal tract tumors in Western countries, to the best of our knowledge, no previous studies have been conducted to investigate the possible association between these factors and the risk of gastrointestinal cancers in China. Therefore, we embarked on the present large case-control study to evaluate whether ABO blood type, HBsAg, sex, age, type 2 diabetes, and family history of cancer are associated with gastrointestinal cancer in northern China.
This study is a retrospective hospital-based, case-control investigation conducted in Beijing, China, at the Chinese PLA General Hospital. A total of 3314 cases and 2223 controls were recruited from January 2004 to November 2008. Cases were patients with newly diagnosed gastrointestinal tract tumors, who were evaluated and treated at the Chinese PLA General Hospital. The population mainly came from northern China and the majority was Han people. The inclusion criteria for cases were as follows: pathologically confirmed diagnosis of gastrointestinal tract cancer; laboratory data available for ABO blood type, HBsAg, and diabetes screening; and detailed record of disease course and history. The exclusion criteria were the presence of other types of digestive disease (such as neuroendocrine tumors, adenomas, cysts or unknown primary tumors) and the absence of laboratory data on blood types, HBsAg and plasma glucose.
Controls were healthy cohorts (undergoing physical examination in Chinese PLA Hospital, mainly from northern China) or patients with other cancers (such as glioma and thyroid cancer). The inclusion criteria for controls were the same as those for cases, except for the cancer diagnosis. The cases and controls were selected at the same period, with integrated laboratory data on blood types, HBsAg and plasma glucose to reduce the bias inherent in retrospective studies. The research proposal was approved by the hospital institutional review board and ethics committee.
The fasting blood samples were collected from patients and controls. Plasma samples were separated and tested for the presence of HBsAg using a third-generation ELISA or chemiluminescence assay, venous glucose by enzyme methods, and blood type by immune assay. The laboratory researcher running these assays was blinded to the disease status (cases or controls) of the subjects’ blood samples. Patients whose fasting glucose was > 7.8 mmol/L at least twice were diagnosed as having diabetes. The blood type was ABO. Family history of cancer was defined as any of the first-degree relatives (parents, brother or sister, children) with a tumor history.
Cases were comprised of a total of 855 esophageal, 824 gastric, 809 pancreatic and 826 biliary cancer patients. Controls included 674 glioma and 798 thyroid cancer patients, as well as 751 healthy controls. Stata software version 10 (http://www.stata.com/stata10/) was used for data management and statistical analysis. We compared the proportions of potential risk factors among cases and controls. Student’s t test was used to compare mean age between cases and controls. The χ2 test was used to compare proportions. We performed multivariable unconditional logistic regression analyses using all variables significant at P < 0.05 in the single factor analyses. For each factor, we calculated the adjusted odds ratio and 95% CI using maximum likelihood estimation.
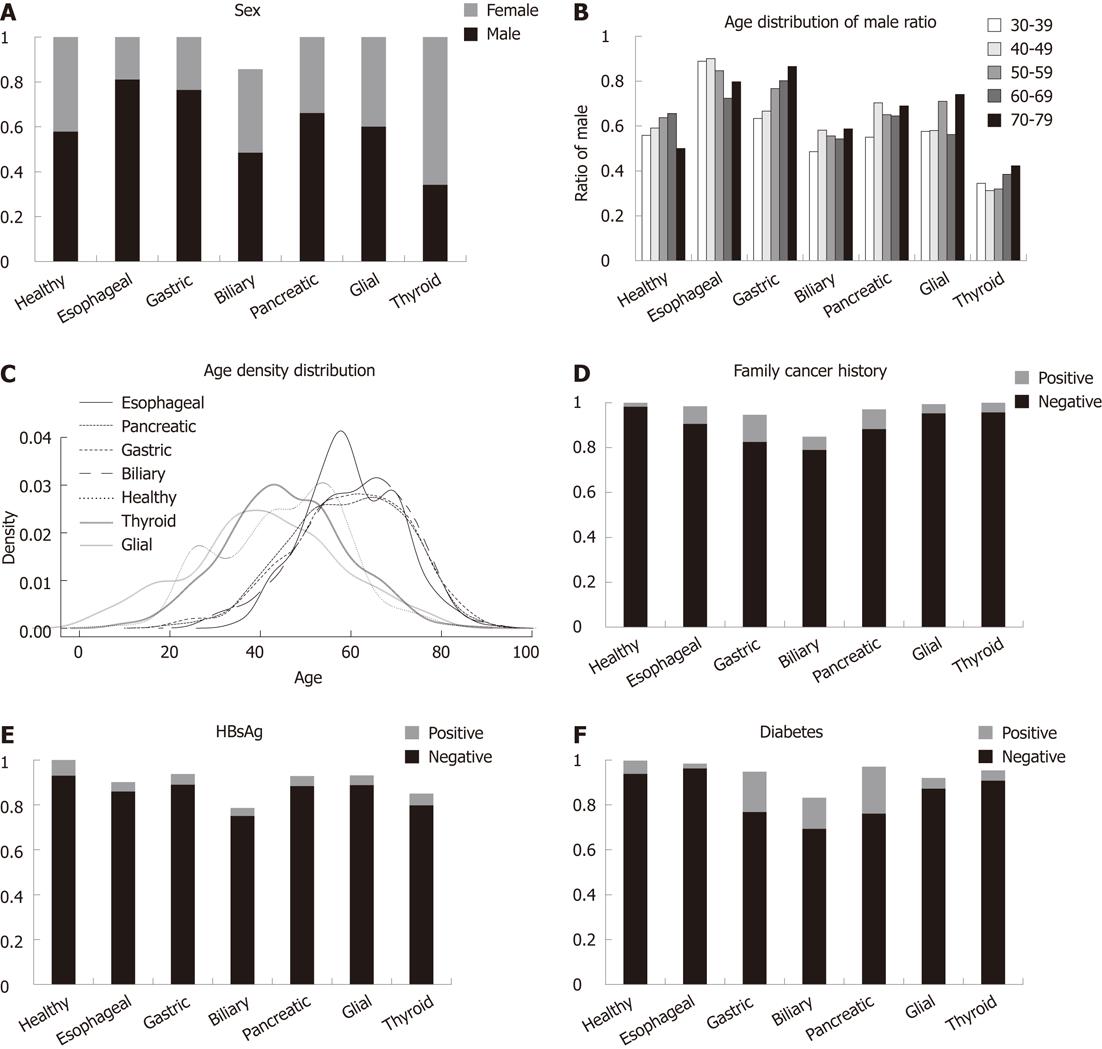
To determine whether sex, age, family history of cancer, HBsAg and diabetes were associated with gastrointestinal cancer, we evaluated the distribution of these clinical traits among the healthy controls, and patients with digestive system and other system cancers (Figure 1A), and calculated the OR to measure the association of each trait with gastrointestinal or other cancers, compared with healthy controls (Table 1), using multivariable logistic regression (Table 2).
| Population | Number |
| Healthy controls | 751 |
| Esophageal cancer | 855 |
| Gastric cancer | 824 |
| Biliary cancer | 826 |
| Pancreatic cancer | 809 |
| Glioma | 674 |
| Thyroid cancer | 798 |
| Cancer type | Sex | Age | Family history | HBsAg | Diabetes | |||||
| OR1 | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Esophageal | 3.22 | [2.42–4.28] | 1.12 | [1.10–1.13] | 6.20 | [3.03–12.68] | 0.66 | [0.39–1.11] | 0.29 | [0.15–0.54] |
| Gastric | 1.80 | [1.38–2.35] | 1.08 | [1.07–1.09] | 9.61 | [5.07–18.22] | 0.72 | [0.43–1.19] | 2.07 | [1.40–3.08] |
| Biliary | 0.86 | [0.67–1.10] | 1.10 | [1.09–1.11] | 5.27 | [2.58–10.75] | 0.94 | [0.56–1.58] | 2.13 | [1.43–3.17] |
| Pancreatic | 1.21 | [0.94–1.55] | 1.08 | [1.07–1.09] | 6.18 | [3.22–11.86] | 0.72 | [0.43–1.19] | 2.87 | [1.97–4.19] |
| Glioma | 1.17 | [0.93–1.47] | 0.98 | [0.97–0.99] | 2.83 | [1.42–5.64] | 0.68 | [0.42–1.09] | 1.06 | [0.65–1.72] |
| Thyroid | 0.37 | [0.30–0.47] | 1.00 | [1.00–1.01] | 2.46 | [1.24–4.88] | 0.95 | [0.61–1.47] | 0.89 | [0.54–1.45] |
Sex: The esophageal and gastric cancer groups were dominated by male patients, whereas female patients comprised the majority in the thyroid cancer group (Figure 1). Therefore, thyroid cancer showed a protective effect of male sex (OR = 0.37, 95% CI = 0.30-0.47, Table 2), whereas all other cancers were positively associated with male sex compared with healthy controls (Table 2).
The age peak of cancer occurrence was different among various cancers, therefore, we calculated sex ratios within different age groups to ensure the distribution was not skewed by any particular age group. Sex ratios were largely consistent among different age groups (Figure 1B). Thus, the association of sex with thyroid, esophageal and gastric cancers seems to be independent of age.
Age: The age distribution of patients with gastrointestinal cancers (esophageal, gastric, biliary and pancreatic) was similar, whereas that of healthy controls and patients with other cancers (glial and thyroid) had relatively younger age profiles (Figure 1C). These different age distributions suggested that older individuals were more susceptible to gastrointestinal cancer. Only glioma had a weak negative association with increasing age compared with healthy controls (OR = 0.98, 95% CI = 0.97-0.99). All the other cancers analyzed here showed weak positive association with age (Table 2).
Family history of cancer: The prevalence of a positive family history of cancer seemed to be much higher in the cases compared with controls (Figure 1D). Association analysis also revealed stronger associations of family cancer history with gastrointestinal cancer compared with other cancers (Table 2), although the two other cancers (glioma and thyroid) were also significantly associated with cancer family history when compared with healthy controls (OR = 2.83, 95% CI = 1.42-5.64; OR = 2.46, 95% CI = 1.24-4.88, respectively).
HBsAg: There were no associations found between HBsAg and any of the different patient groups (Figure 1E and Table 2).
Diabetes: Type 1 diabetes mellitus is relatively rare in most populations and cancer risk investigations have been scarce. Our study indicates that the prevalence of type 2 diabetes was significantly higher in gastric, biliary and pancreatic cancers compared with other groups (Figure 1F), with ORs ranging between 2.0 and 3.0 (Table 2). However, esophageal cancer was strongly inversely associated with type 2 diabetes compared with healthy controls (OR = 0.29, 95% CI = 0.15-0.54).
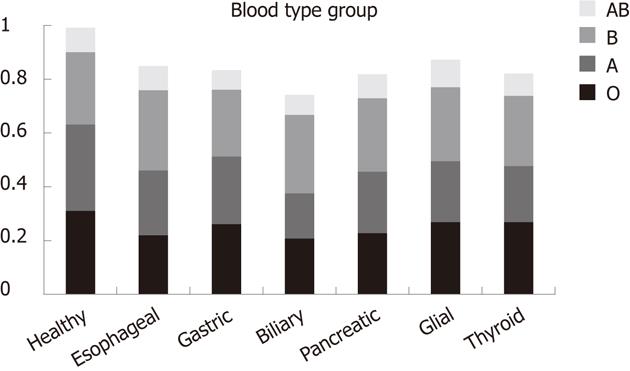
The risk of thyroid cancer was lower in blood type A than in type O (OR = 0.74, 95% CI = 0.56-0.99). The risk of esophageal cancer (OR = 1.53, 95% CI = 1.10-2.14) and biliary cancer (OR = 1.49, 95% CI = 1.09-2.05) (Table 3) was significantly associated with blood type B. These were confirmed by comparison of each blood type with a combination of all the other blood types (Table 3, Figure 2). In addition, the risk of thyroid cancer in blood type B was significantly higher when compared with the combination of all the other blood types (OR = 1.30, 95% CI = 1.02-1.66). The risk of biliary cancer and glioma was significantly lower in blood type A when compared with combination of all the other blood types (OR = 0.65, 95% CI = 0.49-0.86; OR = 0.77, 95% CI = 0.60-0.98).
| Cancer type | Control | Type A | Type B | Type AB | Type O | ||||
| OR1 | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Esophageal | Type O | 0.99 | [0.70–1.40] | 1.53 | [1.10–2.14] | 1.25 | [0.76–2.08] | – | – |
| All others | 0.80 | [0.60–1.05] | 1.49 | [1.12–1.97] | 1.11 | [0.71–1.74] | 0.80 | [0.60–1.07] | |
| Gastric | Type O | 0.99 | [0.71–1.37] | 1.03 | [0.74–1.46] | 1.28 | [0.78–2.10] | – | – |
| All others | 0.93 | [0.71–1.23] | 1.03 | [0.78–1.37] | 1.24 | [0.79–1.93] | 0.96 | [0.73–1.26] | |
| Biliary | Type O | 0.78 | [0.56–1.08] | 1.49 | [1.09–2.05] | 0.95 | [0.57–1.57] | – | – |
| All others | 0.65 | [0.49–0.86] | 1.66 | [1.27–2.17] | 0.91 | [0.58–1.43] | 0.92 | [0.70–1.21] | |
| Pancreatic | Type O | 1.05 | [0.76–1.44] | 1.26 | [0.91–1.75] | 1.44 | [0.89–2.31] | – | – |
| All others | 0.88 | [0.67–1.15] | 1.20 | [0.92–1.57] | 1.28 | [0.84–1.95] | 0.86 | [0.65–1.12] | |
| Glioma | Type O | 0.83 | [0.62–1.12] | 1.15 | [0.86–1.54] | 1.22 | [0.81–1.84] | – | – |
| All others | 0.77 | [0.60–0.98] | 1.21 | [0.95–1.56] | 1.21 | [0.84–1.76] | 0.99 | [0.78–1.26] | |
| Thyroid | Type O | 0.74 | [0.56–0.99] | 1.16 | [0.87–1.55] | 1.01 | [0.66–1.54] | – | – |
| All others | 0.70 | [0.54–0.89] | 1.30 | [1.02–1.66] | 1.07 | [0.73–1.57] | 1.07 | [0.84–1.35] | |
No significant associations of cancer risk were found with blood type AB. This may have been caused by the small number of patients with this blood type in this study.
This was a retrospective study on the risk factors (including ABO blood type, HBsAg, diabetes, sex, age and family history of cancer) associated with digestive system cancer in northern China. To reduce the bias, we tried to enlarge our samples, and the cases and controls were selected using the same criteria and in the same period. The association of type 2 diabetes with gastric, biliary and pancreatic cancers was significant, and blood type B was significantly associated with esophageal and biliary cancers.
The ABO blood groups are defined by carbohydrate moieties displayed on the surface of red blood cells and attached to a protein backbone, known as the H antigen. Three variant alleles (A, B and O) of a single gene on chromosomes 9q34, the A, B or O gene, determine a person’s blood type by encoding three glycosyltransferases with different substrate specificities. In addition to their expression on the surface of red blood cells, the A, B and O antigens are highly expressed on the surface of epithelial cells of the gastrointestinal, bronchopulmonary, and urogenital tracts.
One hospital-based case-control study has shown some evidence of a positive association between blood type A and risk of pancreatic cancer[15] . Another study has demonstrated an increased prevalence of pancreatic cancer among patients with blood group B and a decreased prevalence in patients with blood group O[16]. Wolpin et al[14] have found that ABO blood type is significantly associated with the risk of pancreatic cancer. Compared with blood group O, patients with groups A, AB and B were more likely to develop pancreatic cancer (adjusted hazard ratios for incidence of pancreatic cancer were 1.32, 95% CI = 1.02-1.72; 1.51, 95% CI = 1.02-2.23; and 1.72, 95% CI = 1.25-2.38, respectively). However, in our study, no significant associations between A, B and O blood types and pancreatic cancer were found. Environmental and dietary factors may play an important role in pancreatic cancer. However, a lower risk of biliary cancer associated with blood type A was found in our study, whereas patients with blood type B were more susceptible to esophageal and biliary cancers.
The functional significance of ABO blood group distribution might be associated with biological characteristics such as differentiation, mean size of the tumor, venous invasion, and TNM stages of esophageal squamous cell cancer[17]. A previous study from China has shown that blood group B is associated with the incidence of upper esophageal squamous cell cancer in men[18]. Our results are consistent with these findings.
Previous studies have reported an association between hepatitis B and pancreatic and biliary cancers. Hassan et al[7] have reported a higher risk of pancreatic cancer in HBV carriers. Meanwhile, a case-control study in China has reported that HBV infection and hepatolithiasis are risk factors in the development of cholangiocarcinoma[8]. In Shanghai, chronic HBV infection was associated with a 2.4-fold increased risk of extrahepatic bile duct cancer[19]. However, no association was found between HBsAg and pancreatic or biliary cancer in our study. However, there were some limitations to this study for evaluation of HBV infection. We only acquired HBsAg data, and not data from occult HBV infection. Occult HBV infection has been described in patients who are negative for HBsAg, and who have been previously exposed to HBV and have recovered from acute or chronic infection[20,21]. Currently, it is generally accepted that occult HBV infection among patients without any serological evidence for infection is a risk factor for HCC development[22-24]. For biliary cancer, we did not separate our cases by extrahepatic and intrahepatic origin although most cases were extrahepatic in origin.
Diabetes has been found in previous studies to be associated with a number of cancers. Recent studies among Japanese men and women have shown that a past/present history of diabetes is associated with cancer risk for all sites in both sexes (OR = 1.44, 95% CI = 1.28-1.62; OR = 1.39, 95% CI = 1.19-1.62, respectively). A significantly increased risk was found for cancers of the pharynx, esophagus, colorectum, liver, pancreas, and lung among men, and the stomach, liver, lung and uterine cervix among women[25,26]. Diabetes mellitus and hyperglycemia have also been found to increase the risk of gastric cancer associated with Helicobacter pylori infection[27]. The relationship between diabetes mellitus and esophageal cancer has also been investigated in a Danish study that has shown a 30% increase in risk for esophageal cancer among men with diabetes. No increased risk was seen in women[28].
Diabetes often precedes pancreatic cancer and is thus regarded as a potential risk factor for malignancy. Conversely, pancreatic cancer may secrete diabetogenic factors. Given these findings, there is increasing interest in whether close monitoring of the glycemic profile may help with early detection of pancreatic cancer[29]. The success of a strategy using new-onset hyperglycemia and diabetes as a screening tool to identify people with a high likelihood of asymptomatic pancreatic cancer will depend largely on our ability to differentiate pancreatic-cancer-associated diabetes from the more common type 2 diabetes, by use of a serological biomarker[30].
The risk from diabetes varies according to tumor site, and in our study, diabetes had the strongest association with pancreatic cancer, a moderately increased risk for gastric and biliary cancers, and a decreased risk for esophageal cancer. Given the food intake difficulties of patients with advanced esophageal cancer, the inverse association of diabetes with esophageal cancer could be an effect of diet restriction. Substantial public investment in preventing diabetes mellitus is important to have a major impact on its adverse health effects including cancer.
In conclusion, we found that blood type A was associated with a lower risk of biliary cancer, glioma and thyroid cancer, while those with blood type B were more susceptible to esophageal, biliary and thyroid cancers. Patients with type 2 diabetes were at high risk of pancreatic cancer, moderate risk for gastric and biliary cancers, and decreased risk for esophageal cancer. We did not find any association between HBsAg and the four gastrointestinal cancers in the Chinese population that we studied here.
The incidence and mortality of gastrointestinal cancer (such as cancer of the esophagus, stomach, pancreas, and biliary tract) are higher in China compared with Western countries, but the reason remains unclear.
Although it seems reasonable that ABO blood type, hepatitis B surface antigen (HBsAg), and type 2 diabetes have a close relationship with gastrointestinal tract tumors in Western countries, to the best of our knowledge, no previous studies have been conducted to investigate the possible association between these factors and the risk of gastrointestinal cancer in China.
This was a retrospective study on the risk factors (including ABO blood type, HBsAg, diabetes, sex, age and family history of cancer) associated with digestive system cancer in northern China. The association of type 2 diabetes with gastric, biliary and pancreatic cancer was significant, and blood type B was significantly associated with esophageal and biliary cancers.
It is important for early cancer detection and prevention that type 2 diabetes is related to gastric, biliary, and particularly pancreatic cancers.
This study describes a very large case-control study of risk factors (age, sex, ABO blood type, diabetes, hepatitis B virus, and family cancer history) for gastrointestinal cancers in northern China. ABO blood group and type 2 diabetes are correlated with gastrointestinal cancer in northern China, and the family history findings are important. The study design is good and the methods are standard and reliable.
Peer reviewer: Xiao-Chun Xu, Associate Professor, Department of Clinical Cancer Prevention, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Unit 1360, Houston, TX 77030, United States
S- Editor Sun H L- Editor Kerr C E- Editor Xiong L
| 1. | Yang CS. Research on esophageal cancer in China: a review. Cancer Res. 1980;40:2633-2644. [PubMed] |
| 2. | Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol. 2006;12:17-20. [PubMed] |
| 3. | Guo X, Cui Z. Current diagnosis and treatment of pancreatic cancer in China. Pancreas. 2005;31:13-22. [PubMed] |
| 4. | Zhou YH, Wu C, Zhuang H. Vaccination against hepatitis B: the Chinese experience. Chin Med J (Engl). 2009;122:98-102. [PubMed] |
| 5. | Marcellin P. Hepatitis B and hepatitis C in 2009. Liver Int. 2009;29 Suppl 1:1-8. [PubMed] [DOI] [Full Text] |
| 6. | Dejean A, Lugassy C, Zafrani S, Tiollais P, Brechot C. Detection of hepatitis B virus DNA in pancreas, kidney and skin of two human carriers of the virus. J Gen Virol. 1984;65:651-655. [PubMed] |
| 7. | Hassan MM, Li D, El-Deeb AS, Wolff RA, Bondy ML, Davila M, Abbruzzese JL. Association between hepatitis B virus and pancreatic cancer. J Clin Oncol. 2008;26:4557-4562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Zhou YM, Yin ZF, Yang JM, Li B, Shao WY, Xu F, Wang YL, Li DQ. Risk factors for intrahepatic cholangiocarcinoma: a case-control study in China. World J Gastroenterol. 2008;14:632-635. [PubMed] |
| 9. | Fahal AH, el Razig SA, Suliman SH, Ibrahim SZ, Tigani AE. Gastrointestinal tract cancer in association with hepatitis and HIV infection. East Afr Med J. 1995;72:424-426. [PubMed] |
| 10. | Mlinar B, Marc J, Janez A, Pfeifer M. Molecular mechanisms of insulin resistance and associated diseases. Clin Chim Acta. 2007;375:20-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 175] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: A meta-analysis of prospective studies. Int J Cancer. 2007;120:1993-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 226] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 12. | Rai S, Saronwala KC, Singh R. ABO blood groups in cancer of the gastro-intestinal tract. Indian J Cancer. 1972;9:97-100. [PubMed] |
| 13. | Reid ME, Mohandas N. Red blood cell blood group antigens: structure and function. Semin Hematol. 2004;41:93-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Wolpin BM, Chan AT, Hartge P, Chanock SJ, Kraft P, Hunter DJ, Giovannucci EL, Fuchs CS. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2009;101:424-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 280] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 15. | Vioque J, Walker AM. [Pancreatic cancer and ABO blood types: a study of cases and controls]. Med Clin (Barc). 1991;96:761-764. [PubMed] |
| 16. | Annese V, Minervini M, Gabbrielli A, Gambassi G, Manna R. ABO blood groups and cancer of the pancreas. Int J Pancreatol. 1990;6:81-88. [PubMed] |
| 17. | Nozoe T, Ezaki T, Baba H, Kakeji Y, Maehara Y. Correlation of ABO blood group with clinicopathologic characteristics of patients with esophageal squamous cell carcinoma. Dis Esophagus. 2004;17:146-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Su M, Lu SM, Tian DP, Zhao H, Li XY, Li DR, Zheng ZC. Relationship between ABO blood groups and carcinoma of esophagus and cardia in Chaoshan inhabitants of China. World J Gastroenterol. 2001;7:657-661. [PubMed] |
| 19. | Hsing AW, Zhang M, Rashid A, McGlynn KA, Wang BS, Niwa S, Ortiz-Conde BA, Goedert JJ, Fraumeni JF, O'Brien TR. Hepatitis B and C virus infection and the risk of biliary tract cancer: a population-based study in China. Int J Cancer. 2008;122:1849-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Tamori A, Nishiguchi S, Kubo S, Narimatsu T, Habu D, Takeda T, Hirohashi K, Shiomi S. HBV DNA integration and HBV-transcript expression in non-B, non-C hepatocellular carcinoma in Japan. J Med Virol. 2003;71:492-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Kannangai R, Molmenti E, Arrazola L, Klein A, Choti M, Thomas DL, Torbenson M. Occult hepatitis B viral DNA in liver carcinomas from a region with a low prevalence of chronic hepatitis B infection. J Viral Hepat. 2004;11:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Paterlini P, Gerken G, Nakajima E, Terre S, D'Errico A, Grigioni W, Nalpas B, Franco D, Wands J, Kew M. Polymerase chain reaction to detect hepatitis B virus DNA and RNA sequences in primary liver cancers from patients negative for hepatitis B surface antigen. N Engl J Med. 1990;323:80-85. [PubMed] |
| 23. | Paterlini P, Driss F, Nalpas B, Pisi E, Franco D, Berthelot P, Bréchot C. Persistence of hepatitis B and hepatitis C viral genomes in primary liver cancers from HBsAg-negative patients: a study of a low-endemic area. Hepatology. 1993;17:20-29. [PubMed] |
| 24. | Sheu JC, Huang GT, Shih LN, Lee WC, Chou HC, Wang JT, Lee PH, Lai MY, Wang CY, Yang PM. Hepatitis C and B viruses in hepatitis B surface antigen-negative hepatocellular carcinoma. Gastroenterology. 1992;103:1322-1327. [PubMed] |
| 25. | Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med. 2006;166:1871-1877. [PubMed] |
| 26. | Kuriki K, Hirose K, Tajima K. Diabetes and cancer risk for all and specific sites among Japanese men and women. Eur J Cancer Prev. 2007;16:83-89. [PubMed] |
| 27. | Ikeda F, Doi Y, Yonemoto K, Ninomiya T, Kubo M, Shikata K, Hata J, Tanizaki Y, Matsumoto T, Iida M. Hyperglycemia increases risk of gastric cancer posed by Helicobacter pylori infection: a population-based cohort study. Gastroenterology. 2009;136:1234-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Wideroff L, Gridley G, Mellemkjaer L, Chow WH, Linet M, Keehn S, Borch-Johnsen K, Olsen JH. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst. 1997;89:1360-1365. [PubMed] |
| 29. | Meisterfeld R, Ehehalt F, Saeger HD, Solimena M. Pancreatic disorders and diabetes mellitus. Exp Clin Endocrinol Diabetes. 2008;116 Suppl 1:S7-S12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |