Published online Jan 28, 2010. doi: 10.3748/wjg.v16.i4.445
Revised: December 3, 2009
Accepted: December 10, 2009
Published online: January 28, 2010
AIM: To investigate the potential anti-Helicobacter pylori (H. pylori) and anti-inflammation in vivo effects of two lactobacillus strains from human stomach.
METHODS: Forty H. pylori infected Balb/c mice were randomly divided into 4 groups: proton pump inhibitor and antibiotics triple treated group, Lactobacillus fermenti (L. fermenti) treated group, Lactobacillus acidophilus treated group and normal saline control group. Ten uninfected mice were also included as blank control group. The infection of H. pylori was detected by rapid urease tests, Giemsa staining and bacterial culture. The colonization of H. pylori was assessed in bacterial density score and gastric inflammation was assessed in histological score. The colonization of L. fermenti was performed by fluorescent probe.
RESULTS: Histopathologic evaluation showed significant release of mucosal inflammation in gastric antrum and gastric body in lactobacillus treated groups and triple treated group. H. pylori eradication rate in both lactobacillus treated groups and triple treated group were higher than normal saline control group. Lactobacillus treated groups and triple treated group showed significant decrease of H. pylori bacterial density.
CONCLUSION: Both lactobacillus strains have a significant anti-H. pylori activity; L. fermenti displays more efficient antagonistic activity in vivo against H. pylori infection.
-
Citation: Cui Y, Wang CL, Liu XW, Wang XH, Chen LL, Zhao X, Fu N, Lu FG. Two stomach-originated
lactobacillus strains improveHelicobacter pylori infected murine gastritis. World J Gastroenterol 2010; 16(4): 445-452 - URL: https://www.wjgnet.com/1007-9327/full/v16/i4/445.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i4.445
Helicobacter pylori (H. pylori) is a spiral-shaped, Gram-negative microaerophilic stomach rod that infects over 50% of the population around the world[1-3], which is considered to be the most important etiological agent of chronic gastritis, peptic ulcer, gastric cancer and mucosa-associated lymphoid tissue[1,4,5]. So far, the eradication of H. pylori depends on the combination of antibiotics and acid suppression drugs, with an efficiency of 80%-90%. Unexpectedly, symptoms of 10%-20% patients remain unimproved or reinfected due to the incomplete eradication or antibiotics resistance[5,6]. Moreover, the side effect of using multi-antibiotics is obvious, such as antibiotic associated diarrhea, enteric dysbacteriosis, pseudo-menbraneouscolitis, and so on, which lower the curative effect and treatment compliance. Thus attention has been drawn to seek for any alternative method which can eradicate or inhibit H. pylori infection efficiently without antibiotics associated side effects.
As mentioned above, over 50% of the population are infected by H. pylori, while only a few of them suffer from H. pylori associated disease. Some published data showed that when the density of H. pylori in gastric antrum drops to less than 105, it cannot lead to the formation of gastric and duodenum ulcer[7,8]. It is believed that the severity and activity of gastritis is related to the number of bacteria in stomach.
Recently, some reports have suggested that exogenous lactobacilli have some inhibitory effects on H. pylori infection[9,10], and the colonization of H. pylori has a close relationship with the number of lactobacilli. It is also reported that H. pylori colonization can be inhibited in lactobacillus strain L. salivarius-fed mice[11,12]. Some studies showed that probiotics isolated from dairy products or human feces have suppressive effects on H. pylori infection[9,13]. Similar results from some clinical trials also support the inhibitory effects of some microbial ecological agents on H. pylori associated diseases[14,15].
However, results of clinical trials from some separate groups did not make a definite conclusion. Some studies showed that it is effective to prevent and treat H. pylori infected diseases by administering lactobacillus or fermented dairy products[13,16,17]. Some believe that probiotics have no effect in treating H. pylori infected diseases because they are live microbes, and their living environment may change depending on what kinds of food the host takes[18]. The possibilities include inappropriate methods to assess the anti-H. pylori activity, different strains of probiotics and different origins of strains[12,19-21]. Therefore, more detailed studies on more stomach originated probiotic strains will help draw a more definite conclusion.
In the present study, we isolated two stomach originated lactobacillus strains, and screened their potential anti-H. pylori activity and anti-inflammatory effects on mouse model of H. pylori-associated Balb/c gastritis. We found that lactobacillus strain Lactobacillus fermenti (L. fermenti) could adhere to gastric epithelium and displayed antagonistic activity in vivo against H. pylori infection, thus significantly improving the H. pylori-associated Balb/c gastritis in mice.
H. pylori standard strain SS1 (Sydney strain 1) was kindly provided by the Infectious Disease Institute of the Chinese Center for Disease Control. The two lactobacillus strains L. fermenti (CCTCC M 206110) and Lactobacillus acidophilus (L. acidophilus) (strain LC) were isolated by us from gastric biopsy materials of patients who received endoscopic examination in the Gastroenterology Department of the Second Xiangya Hospital, Hunan, China.
H. pylori was resuscitated and inoculated into brain heart infusion broth containing 10% sheep blood, incubated under microaerophilic conditions (5% O2, 85% N2, 10% CO2) at 37°C for 3-5 d. Both lactobacillus strains were cultured in Man-Rogosa-Sharpe (MRS) broth, and incubated in anaerobic box at 37°C for 48 h. The concentration of bacteria was regulated to 1 × 109 CFU/mL by turbidimetry.
Sixty specific-pathogen-free 6-8-wk-old Balb/c mice (a male to female ratio of 1:1) were obtained from the Central Animal Facility of Wuhan. They were housed according to relevant Chinese national legislation, fed a commercial diet, and were given water and libitum, except as otherwise stated. H. pylori infections by the SS1 strain were induced as follows: freshly prepared aliquots (0.4 mL of 1 × 109 CFU/mL) of H. pylori SS1 strain in brain heart infusion broth were administered to 50 mice via orogastric inoculation 3 times a week (days 1, 3 and 5) at a 1-d interval between inoculations for 2 wk. Accordingly, the other 10 non-infected control animals were inoculated with the same volume of plain brain heart infusion broth. At the end of the 1st and 2nd wk, 5 animals receiving H. pylori strain SS1 were sacrificed by carbon dioxide inhalation, and tests were made to confirm whether they were infected with SS1 strain successfully.
The following groups of animals were included in the study: H. pylori-infected mice treated by L. fermenti (L. fermenti group, n = 10), H. pylori-infected mice treated by L. acidophilus (L. acidophilus group, n = 10), H. pylori-infected mice treated by proton pump inhibitor (PPI) and antibiotics (triple group, n = 10). The control groups were H. pylori-infected mice treated by normal saline solution (NS group, n = 10). The non-infected mice (blank control group, n = 10) were also included. The quantity of lactobacillus was regulated to 0.5 mL of 1 × 109 CFU/mL per mouse for L. fermenti and L. acidophilus groups, and PPI and antibiotics were 0.25 mL of 0.4 mg/mL pantoprazole (Zhongmei East China Pharmaceutical Group Corporation in Hangzhou, 060923), 0.25 mL of 20 mg/mL clarithromycin (Huiren Pharmaceutical Group Corporation in Jiangxi, 0510019), and 0.1 mL of 50 mg/mL ampicillin (North China Pharmaceutical Group Corporation, 060307) per mouse for the triple group. The treatment lasted 10 d, and all animals were sacrificed by carbon dioxide inhalation 4 wk later.
All the methods for the assessment of H. pylori colonization and identification in gastric samples as well as evaluation of gastritis will be described in detail below.
The stomach of each mouse was removed and dissected longitudinally, the antrum and body were divided and treated separately. One-third of each longitudinal strip was fixed in 10% formalin-buffered saline, embedded in paraffin, and processed for histopathologic analysis. The other two strips were used for rapid urease test and H. pylori culture, respectively. Four sections were cut from each paraffin block specimen. Two sections of them were stained with hematoxylin-eosin (HE) for evaluation of gastric inflammation, the other 2 sections were used for Giemsa staining for the assessment of H. pylori colonization. The bacterial density was scored from 0 to 4 as follows: Score 0, no bacteria; score 1, 1-2 bacteria on average/crypt; score 2, 3-10 bacteria on average/crypt; score 3, 11-20 bacteria on average/crypt; and score 4, > 20 bacteria on average/crypt[22]. The pathologic characteristics were graded for the degree of neutrophil and mononuclear cell infiltration in the antrum and body as follows: Score 1, mildly multifocal; score 2, mildly widespread or moderately multifocal; score 3, mildly widespread and moderately multifocal or severely multifocal; score 4, moderately widespread; score 5, moderately widespread and severely multifocal; and score 6, severely widespread[8]. Histopathologic evaluation was performed with no prior knowledge of the identity of the samples by the histopathologist.
Urease activity was determined by a method based on the commercial rapid urease test (Sanqiang Biochemical Industry Corporation in Fujian, China) with a sensitivity of 102 bacteria. Following the instruction of the product, each strip of stomach antrum and body was homogenized and placed in 1 mL of the reaction solution [1 g of urea/mL (wt/vol) containing 850 μg phenol red/mL (wt/vol) as a pH indicator]. The solution became pink or red or dark red within 5 min as positive result, still yellow as negative.
Each sample of stomach antrum and body was homogenized and suspended in 500 μL brain heart infusion broth containing 10% sheep blood, and 100 μL was inoculated onto brain heart infusion agar plates and cultured under microaerophilic conditions at 37°C for 5-7 d. Gram’s staining and urease activity were detected.
When at least two of the three tests of rapid urease test, H. pylori culture and Giemsa staining for the assessment of H. pylori colonization, appeared positive, H. pylori infection could be diagnosed.
When the three tests of rapid urease test, H. pylori culture and Giemsa staining of gastric mucosa samples were all negative, the eradication was confirmed to be successful.
A 100 µmol/L stock solution of fluorescence-labeled molecular probe (cFDA-SE, Invitrogen Corporation) was prepared, which was first dissolved in 90 μL dimethyl sulfoxide and then further diluted in ethanol (1 mL; reagent grade). This solution was then filter sterilized (0.2-μm-pore-size Acrodisc filter; Gelman) before being aliquoted and stored at -20°C.
L. fermenti was grown overnight at 37°C in MRS broth. The bacterial culture was centrifuged at 3000 r/min for 10 min, and the pellet was washed twice in sterile phosphate-buffered saline (PBS). The concentration of bacteria was regulated to 1 × 1010 CFU/mL prior to labeling with 50 μm cFDA-SE at 37°C for 20 min. Fluorescent labeling was terminated by pelleting the bacteria, washing twice in PBS to remove excessive cFDA-SE, and resuspending the pellet in PBS.
A group of 30 mice were administered orally with approximately 109 cFDA-SE-labeled L. fermenti by orogastric intubation. The other group of 6 mice that had been orally fed with sterile PBS served as controls. The food and water intake for the experimental and control mice was measured daily. Groups of 6 mice each were sacrificed at 2, 4, 8, 12 and 24 h after cFDA-SE. The stomach and duodenum of each mouse was divided and dissected longitudinally, any visible residual food particles were removed. Both specimens were examined for the adherence of cFDA-SE-labeled L. fermenti. PBS 150 μL was added to each 1.0 cm of the tissue and microbes from the mucosal surface were dislodged by a plunger from a syringe (1.0 mL), then fixed with formaldehyde (0.75%, vol/vol) prior to the detection under fluorescence microscope (Nikon E80, Japan).
Enumeration of cFDA-SE-labeled L. fermenti was conducted under a fluorescent microscope (Nikon E80, Japan) at a 488-nm excitation wavelength. Upon excitation at 488 nm under the fluorescent microscope, cFDA-SE gave a maximal emission signal in the green at 518 nm.
Statistical analyses were performed using SPSS for Windows, version 13.0. Results were expressed as measurement data and enumeration data. For statistical comparisons, t test and χ2 test were performed and P < 0.05 was considered statistically significant.
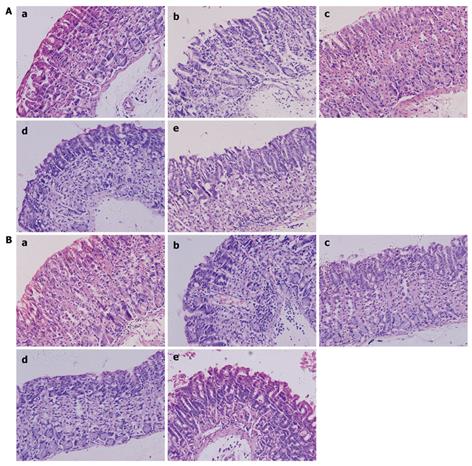
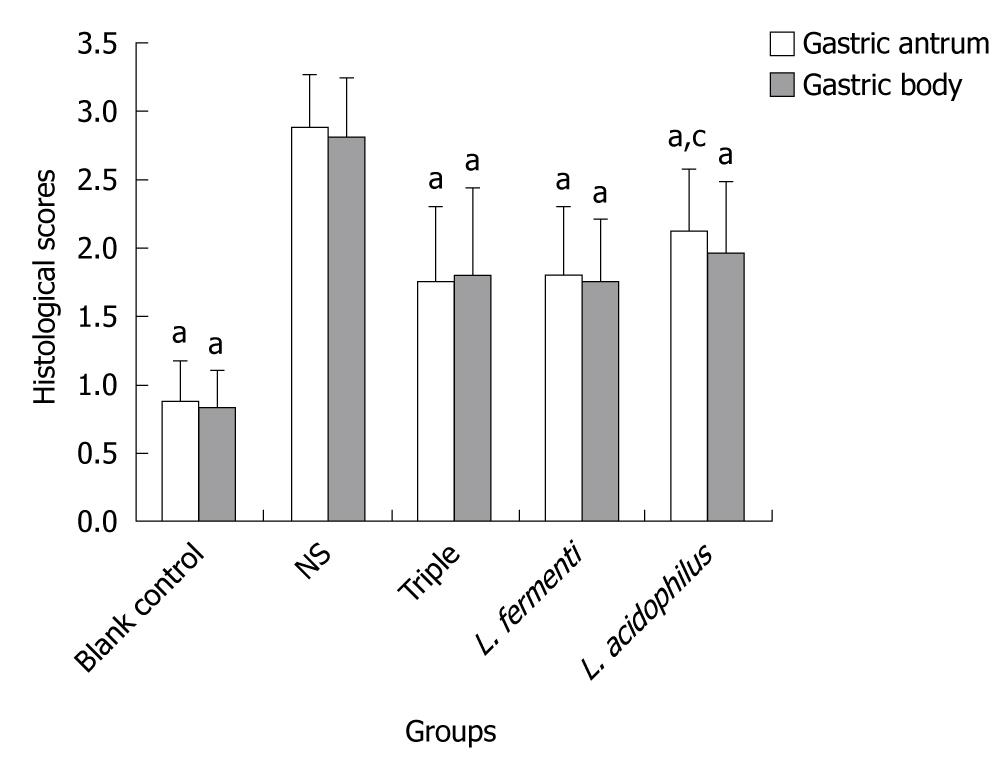
Histopathologic evaluation revealed significant improvement of mucosal inflammation in gastric antrum and gastric body both in lactobacillus treated as well as triple treated groups. The score of pathologic characteristics of the gastric mucosa also showed obvious decrease. The score of L. fermenti treated group was significantly lower than normal saline control group, and almost similar as triple treated group and blank control group. The score of L. acidophilus treated group was also lower than normal saline control group and close to triple treated group. The score of L. acidophilus group in gastric body was similar to the blank control group, while it was higher in gastric antrum (Figures 1 and 2).
The H. pylori infection rate in normal saline control group indicated that the model was made successfully while the rate of H. pylori infection in blank control group suggested that there was no pollution in environment and diet. We found a significant increase of H. pylori eradication rate in lactobacillus treated groups and triple treated group. The eradication rate of H. pylori in L. fermenti treated group was higher than the normal saline control group and close to that in PPI triple treated group. The eradication rate of H. pylori in L. acidophilus treated group was higher than the normal saline control group, but without significant statistical difference (Table 1).
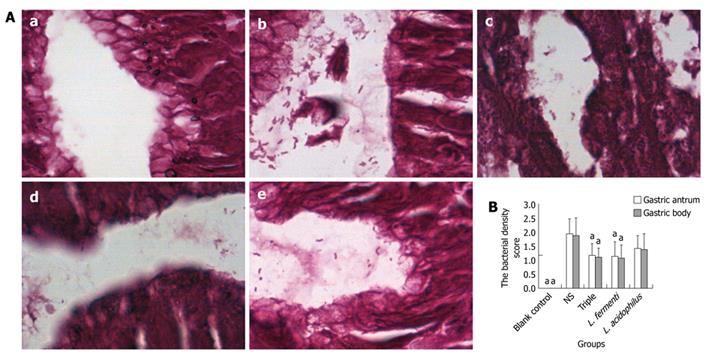
Lactobacillus treated groups and triple treated group showed significant decrease of H. pylori bacterial density. In L. fermenti treated group, the bacterial density was lower than the normal saline control group and close to PPI triple treated group. The bacterial density in L. acidophilus treated group is lower than the normal saline control group, however it was statistically insignificant (Figure 3).
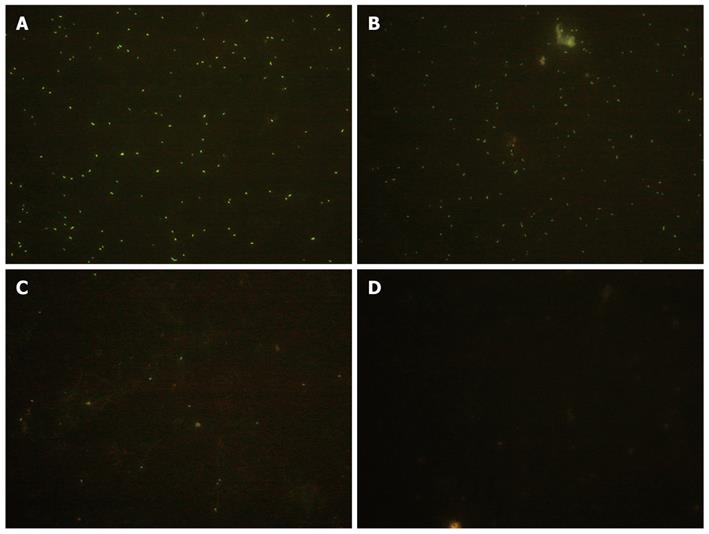
The fluorescence intensity indicated the colonization of lactobacillus at various time points after orogastric intubation. The bacteria were not uniformly labeled by cFDA-SE, probably due to the physiological status of the bacteria at the time of incubation with cFDA-SE (Figure 4).
H. pylori is a spiral-shaped, gram-negative organism that commonly causes chronic infections and is usually acquired in childhood[23]. Patients with complications (e.g. gastritis, ulcer and malignancy) should have the organism eradicated. So far, H. pylori can be typically eradicated using a combination of certain antibacterial agents and antacid treatment, which resulted in dramatic reduction in ulcer recurrence and associated complications. The efficacy of recommended eradication regimens is approximately 80%. In our study, standard H. pylori SS1 strain was used to colonize the stomach of Balb/c mouse, with an infection rate of 80.0% in gastric antrum and gastric body. This leads to the development of gastric mucosa inflammation closely mimicking human H. pylori associated gastritis. The eradication efficacy of H. pylori in our study was around 70.0% based on typical triple therapy, which proved that the mouse model of H. pylori infection was made successfully and could be established as an animal model to assess anti-H. pylori activities of potential therapeutic agents.
A recent clinical trials demonstrated that the addition of Will yogurt to triple therapy increased the H. pylori eradication rate by per-protocol (PP) analysis[14]. Another clinical study showed that some probiotics, such as L. johnsonii LA1, could be used as an adjuvant of antibiotic-antacid treatment to prevent the reemergence of H. pylori infection in humans[19]. It indicated that the addition of probiotics to PPI-based triple therapy could increase the likelihood of successful H. pylori eradication.
In our study, histopathologic changes on H. pylori SS1 mouse model showed a significant release of mucosal inflammation in both gastric antrum and gastric body in lactobacillus strain L. fermenti and L. acidophilus treated groups and triple treated group. While the histological score of L. acidophilus treated group was higher than L. fermenti treated group and triple treated group. It showed that oral administration of L. fermenti strain could alleviate the gastric inflammation in H. pylori-infected Balb/c mouse model, with less significance in strain L. acidophilus treated group.
Accordingly, lactobacillus treated groups and triple treated group showed a significant decrease of H. pylori bacterial density, with an eradication rate of H. pylori 70.0% in gastric antrum and 60.0% in gastric body in L. fermenti treated group, which was obviously higher than the normal saline control group and close to that in the triple treated group. The eradication rate of H. pylori in L. acidophilus treated group was 50.0% in gastric antrum and gastric body, being higher than the normal saline control group, but without statistical difference. It indicates that different lactobacillus strains have various anti-H. pylori effects due to different H. pylori density and H. pylori eradication rate.
Our data from the SS1 mouse model with reference to H. pylori colonization were in agreement with the histopathological changes among different groups. L. fermenti can inhibit the colonization of H. pylori in gastric mucosa so as to improve its inflammatory injury. In the L. fermenti strain group, we observed a significant reduction of H. pylori colonization in the gastric mucosa throughout the entire observation period. Continuous administration of L. acidophilus did not reduce the H. pylori colonizing numbers over this experimental period. These indicate that some strains of lactobacillus can colonize in gastric mucosa and exhibit anti-H. pylori colonization. These strains due to their differences in species and specificity can lead to different anti-H. pylori activities. Detection of living bacteria under fluorescent microscope provided evidence of colonization of the stomach and duodenum by L. fermenti. The appearance of L. fermenti supports the idea that they come from the supplement.
As it is known, lactobacilli are components of the normal intestinal flora of healthy humans which exert antagonistic activities against pathogens[7,9,10,16]. In particular, it has been reported that the primary microorganisms associated with the stomach belong to the genus lactobacillus. Lactobacillus earn particular capacity to survive and develop in an acidic environment and live as an indigenous bacterium in gastric mucosa, which can effectively inhibit the colonization of H. pylori[11,12,15,21]. Our study also adds evidence that H. pylori can live together with other bacteria and maintain a dynamic equilibrium state. In another word, it is rational to prevent and control H. pylori infection by regulating the balance of flora in stomach. Thus lactobacillus can be a choice to replace antibiotics or as an adjuvant to antibiotics in treating H. pylori- infected diseases.
In conclusion, both lactobacillus strain L. fermenti and L. acidophilus, which are isolated from human gastric mucosa, showed significant anti-H. pylori activity, while strain L. fermenti displayed more efficient antagonistic activity in vivo whose efficacy is close to the standard triple therapy, thus significantly improving the H. pylori-associated Balb/c gastritis. It would be of great interest to further explore the role of such probiotic strains in the complex regulation of anti-H. pylori activities and screen for more efficient clinical potential agents.
Helicobacter pylori (H. pylori) are considered to be the most important etiological agents of chronic gastritis. The eradication of H. pylori depends on the combination of antibiotics and acid suppression drugs. Unfortunately, the side effects of antibiotics reduce the curative effect and treatment compliance. Probiotics provides an alternative method which can inhibit H. pylori infection efficiently without antibiotics associated side effects.
Lactobacilli earn particular capacity to survive and develop in an acidic environment and live as an indigenous bacterium in gastric mucosa, which can effectively inhibit the colonization of H. pylori. Different strains display different effects on H. pylori infected gastritis.
The study showed that H. pylori has some interactions with other microbes in stomach. They live together and maintain a dynamic equilibrium state. Both the strains of lactobacillus isolated from human gastric mucosa showed significant anti-H. pylori activity while Lactobacillus fermenti (L. fermenti) displayed more efficient antagonistic activity in vivo. This study also provides evidences of colonization of the stomach and duodenum by L. fermenti.
The study provides a new clue for the therapy of H. pylori associated diseases, which could be prevented and treated by regulating the balance of flora in stomach. Thus lactobacillus can be a choice to replace antibiotics or as an adjuvant to antibiotics in treating H. pylori-infected diseases.
In this paper, the authors investigated the potential effects of anti-H. pylori activity and anti-inflammation in vivo of two lactobacillus strains from stomach. And they found that both lactobacillus strains showed significant anti-H. pylori activity, and L. fermenti displayed more efficient antagonistic activity in vivo against H. pylori infection. This paper gave us a new point of view on the interaction of gastric microbiology and also provided a new clue for H. pylori treatment.
Peer reviewer: Yuan Yuan, Professor, Cancer Institute of China Medical University, 155 North Nanjing Street, Heping District, Shenyang 110001, Liaoning Province, China
S- Editor Wang JL L- Editor Ma JY E- Editor Zheng XM
| 1. | Romshoo GJ, Malik GM, Bhat MY, Rather AR, Basu JA, Qureshi KA. Helicobacter pylori associated antral gastritis in peptic ulcer disease patients and normal healthy population of kashmir, India. Diagn Ther Endosc. 1998;4:135-139. |
| 2. | Lehours P, Yilmaz O. Epidemiology of Helicobacter pylori infection. Helicobacter. 2007;12 Suppl 1:1-3. |
| 3. | Bruce MG, Maaroos HI. Epidemiology of Helicobacter pylori infection. Helicobacter. 2008;13 Suppl 1:1-6. |
| 4. | Dubois A, Borén T. Helicobacter pylori is invasive and it may be a facultative intracellular organism. Cell Microbiol. 2007;9:1108-1116. |
| 5. | Ziemniak W. Efficacy of Helicobacter pylori eradication taking into account its resistance to antibiotics. J Physiol Pharmacol. 2006;57 Suppl 3:123-141. |
| 6. | Silva FM, Eisig JN, Teixeira AC, Barbuti RC, Navarro-Rodriguez T, Mattar R. Short-term triple therapy with azithromycin for Helicobacter pylori eradication: low cost, high compliance, but low efficacy. BMC Gastroenterol. 2008;8:20. |
| 7. | Lesbros-Pantoflickova D, Corthésy-Theulaz I, Blum AL. Helicobacter pylori and probiotics. J Nutr. 2007;137:812S-818S. |
| 8. | Nolan KJ, McGee DJ, Mitchell HM, Kolesnikow T, Harro JM, O'Rourke J, Wilson JE, Danon SJ, Moss ND, Mobley HL. In vivo behavior of a Helicobacter pylori SS1 nixA mutant with reduced urease activity. Infect Immun. 2002;70:685-691. |
| 9. | Wang KY, Li SN, Liu CS, Perng DS, Su YC, Wu DC, Jan CM, Lai CH, Wang TN, Wang WM. Effects of ingesting Lactobacillus- and Bifidobacterium-containing yogurt in subjects with colonized Helicobacter pylori. Am J Clin Nutr. 2004;80:737-741. |
| 10. | Heczko PB, Strus M, Kochan P. Critical evaluation of probiotic activity of lactic acid bacteria and their effects. J Physiol Pharmacol. 2006;57 Suppl 9:5-12. |
| 11. | Johnson-Henry KC, Mitchell DJ, Avitzur Y, Galindo-Mata E, Jones NL, Sherman PM. Probiotics reduce bacterial colonization and gastric inflammation in H. pylori-infected mice. Dig Dis Sci. 2004;49:1095-1102. |
| 12. | Ryan KA, Daly P, Li Y, Hooton C, O'Toole PW. Strain-specific inhibition of Helicobacter pylori by Lactobacillus salivarius and other lactobacilli. J Antimicrob Chemother. 2008;61:831-834. |
| 13. | Uchida M, Kurakazu K. Yogurt containing Lactobacillus gasseri OLL2716 exerts gastroprotective action against [correction of agaisnt] acute gastric lesion and antral ulcer in rats. J Pharmacol Sci. 2004;96:84-90. |
| 14. | Kim MN, Kim N, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, Lee DH, Kim JS, Jung HC. The effects of probiotics on PPI-triple therapy for Helicobacter pylori eradication. Helicobacter. 2008;13:261-268. |
| 15. | Valeur N, Engel P, Carbajal N, Connolly E, Ladefoged K. Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Appl Environ Microbiol. 2004;70:1176-1181. |
| 16. | Adolfsson O, Meydani SN, Russell RM. Yogurt and gut function. Am J Clin Nutr. 2004;80:245-256. |
| 17. | Sýkora J, Valecková K, Amlerová J, Siala K, Dedek P, Watkins S, Varvarovská J, Stozický F, Pazdiora P, Schwarz J. Effects of a specially designed fermented milk product containing probiotic Lactobacillus casei DN-114 001 and the eradication of H. pylori in children: a prospective randomized double-blind study. J Clin Gastroenterol. 2005;39:692-698. |
| 18. | Tan MP, Kaparakis M, Galic M, Pedersen J, Pearse M, Wijburg OL, Janssen PH, Strugnell RA. Chronic Helicobacter pylori infection does not significantly alter the microbiota of the murine stomach. Appl Environ Microbiol. 2007;73:1010-1013. |
| 19. | Sgouras DN, Panayotopoulou EG, Martinez-Gonzalez B, Petraki K, Michopoulos S, Mentis A. Lactobacillus johnsonii La1 attenuates Helicobacter pylori-associated gastritis and reduces levels of proinflammatory chemokines in C57BL/6 mice. Clin Diagn Lab Immunol. 2005;12:1378-1386. |
| 20. | Coconnier MH, Lievin V, Hemery E, Servin AL. Antagonistic activity against Helicobacter infection in vitro and in vivo by the human Lactobacillus acidophilus strain LB. Appl Environ Microbiol. 1998;64:4573-4580. |
| 21. | Sgouras D, Maragkoudakis P, Petraki K, Martinez-Gonzalez B, Eriotou E, Michopoulos S, Kalantzopoulos G, Tsakalidou E, Mentis A. In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus casei strain Shirota. Appl Environ Microbiol. 2004;70:518-526. |
| 22. | Sutton P, Danon SJ, Walker M, Thompson LJ, Wilson J, Kosaka T, Lee A. Post-immunisation gastritis and Helicobacter infection in the mouse: a long term study. Gut. 2001;49:467-473. |
| 23. | Tam YH, Yeung CK, Lee KH, Sihoe JD, Chan KW, Cheung ST, Mou JW. A population-based study of Helicobacter pylori infection in Chinese children resident in Hong Kong: prevalence and potential risk factors. Helicobacter. 2008;13:219-224. |