Published online Sep 28, 2010. doi: 10.3748/wjg.v16.i36.4558
Revised: June 15, 2010
Accepted: June 22, 2010
Published online: September 28, 2010
AIM: To examine the detection rate of viable Mycobacterium avium subspecies paratuberculosis (MAP) in patients with inflammatory bowel disease [Crohn’s disease (CD) and ulcerative colitis (UC)].
METHODS: Thirty patients with CD (15 with at least one NOD2/CARD15 mutation), 29 with UC, and 10 with no inflammatory bowel disease (IBD). were tested for MAP by polymerase chain reaction (specific IS900 fragment) and blood culture.
RESULTS: MAP DNA was detected in all original blood samples and 8-wk blood cultures (CD, UC and non-IBD). Positive MAP DNA status was confirmed by dot blot assays. All 69 cultures were negative by acid-fast Ziehl-Neelsen staining. Viable MAP, in spheroplast form, was isolated from the 18-mo blood cultures of all 30 CD patients, one UC patient, and none of the non-IBD controls. No association was found between positive MAP cultures and use of immunosuppressive drugs or CD-associated single nucleotide polymorphisms.
CONCLUSION: MAP is widely present in our area and MAP DNA can be recovered from the blood of CD, UC and non-IBD patients. However, MAP spheroplasts were only found in CD patients.
-
Citation: Mendoza JL, San-Pedro A, Culebras E, Cíes R, Taxonera C, Lana R, Urcelay E, Torre FL, Picazo JJ, Díaz-Rubio M. High prevalence of viable
Mycobacterium avium subspeciesparatuberculosis in Crohn’s disease. World J Gastroenterol 2010; 16(36): 4558-4563 - URL: https://www.wjgnet.com/1007-9327/full/v16/i36/4558.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i36.4558
Crohn’s disease (CD) and ulcerative colitis (UC) are two related chronic remitting and relapsing inflammatory diseases of the gastrointestinal tract, commonly known as inflammatory bowel disease (IBD). Although the causes of IBD are unknown, it is thought that inflammation results from inappropriate chronic activation of the innate and adaptive mucosal immune systems in a genetically susceptible host, and that enteric microflora play a central role in initiation and maintenance of disease[1]. Some investigators have postulated that some mycobacterial infections are involved in development of CD, based on the similarities between CD and intestinal tuberculosis[2]. Mycobacterium avium subspecies paratuberculosis (MAP) has the specific ability to cause chronic bowel inflammation of a number of histopathological types in many animals, including primates[3,4]. Systematic review and meta-analysis of research from many laboratories have shown a significant and specific association between MAP infection and chronic bowel inflammation of the CD type in humans[5,6]. Recently, Naser et al[7] have found viable MAP in the peripheral blood from 50% of CD patients.
Several findings suggest that the presence of certain bacteria in the face of permissive NOD2/CARD15 mutations is necessary for development of CD and provides evidence for a pathogen-host interaction[8-10]. A recent study has demonstrated an association in IBD with a coding variant of ATG16L, IL-23R[11] and IRGM[12] genes, thereby implicating the autophagy pathway that is crucial in inhibiting Mycobacterium tuberculosis survival in infected macrophages[13].
The aim of this study was to examine, after stratifying CD patients based on the presence or absence of the well-established NOD2/CARD15 mutations, the culture detection rate of viable MAP in peripheral blood from patients with IBD (CD and UC) and healthy controls (non-IBD).
Sixty-nine subjects were recruited into the study: 30 CD patients in clinical remission (of whom 15 carried at least one NOD2/CARD15 mutant; these patients were matched one to one with patients with no NOD2/CARD15 mutants based on the following criteria: time since IBD diagnosis, age at diagnosis, disease location, behavior and prior surgery); 29 UC patients in clinical remission; and 10 healthy controls (non-IBD), members of the staff of the Department of Clinical Microbiology, Hospital Clinico San Carlos de Madrid, Spain. Diagnosis of CD and UC was based on standard clinical, radiographic, endoscopic, and histological criteria[14], and all patients were recruited at an IBD unit of a single referral center in Madrid, Spain. Phenotypic details were obtained by review of clinical records and personal interview with the patients.
The protocol was approved by the Ethics Committee of Hospital Clinico San Carlos, Madrid, and all patients were recruited into the study after giving informed consent.
Two venous blood samples (10 mL) were taken from all patients and controls and drawn into sterile K2-EDTA vacutainer tubes.
Genomic DNA extraction and blood cultures were done using culture methods previously reported by Naser et al[7], except that 10 mL of whole blood was used for each culture. Cultures were incubated at 37°C for 8 wk (bottle I) or 18 mo (bottle II).
Total DNA was prepared by two different methods. DNA was obtained from original samples and 8-wk cultures using the QIAamp DNA Blood Kit (Qiagen). Total DNA from 18-mo cultures was obtained using an EasyMag magnetic extractor (bioMerieux) according to the manufacturer’s recommendations.
Amplification of IS900 was conducted basically as described by Naser et al[7] with minor alterations: after the first round of nested polymerase chain reaction (PCR), amplicons were purified using the QIAquick PCR Purification Kit (Qiagen) and then diluted 1:100 with sterile water.
To confirm PCR results, DNA that had been extracted from original samples and 8-wk cultures was analyzed by dot blot hybridization using the DIG System (Roche Molecular Biochemicals). M. avium subs. paratuberculosis ATCC 43 544 was used as a positive control. One Mycobacterium fortuitum and one M. avium subs. avium were used as negative controls in dot blot assays.
Smears were prepared by placing one drop from 18-mo cultures on a microscope slide. Smears were heat-fixed and stained using the Ziehl-Neelsen and phenolic acridine orange techniques to detect mycobacterial bacilli and/or spheroplasts[15].
Genotyping of rs2241880 (ATG16L1), rs4958847 (IRGM), rs7517847 (IL23R) and NOD2/CARD15 polymorphisms was performed as previously described[16-18].
This was a case-control study. Numerical variables were summarized by the mean, median, and range. Nominal variables were summarized based on their frequency distribution.
The group of 30 CD patients consisted of 11 men and 19 women. Median age at diagnosis was 27 years (mean: 31, range: 14-48 years). Median follow-up duration was 7 years (mean: 8, range: 6-9 years). Table 1 shows the characteristics of the 30 CD and 29 UC patients enrolled into this study.
| Phenotypic characteristics | CD CARD15/NOD2 (+) (n = 15) | CD patients CARD15/NOD2 (-) (n = 15) | UC patients (n = 29) |
| Male/female | 5 (33.4)/10 (66.7) | 6 (49.1)/9 (50.9) | 15 (51.7)/14 (47.3) |
| Median age at diagnosis (range, yr) | 27 (14-48) | 27 (14-48) | |
| A1, < 40 | 14 (93.4) | 14 (93.4) | |
| A2, ≥ 40 | 1 (6.6) | 1 (6.6) | |
| Family history | 4 (26.7) | 3 (21.4) | 1 (3.4) |
| Disease behavior | |||
| Nonstricturing, nonpenetrating (B1) | 6 (40) | 6 (40) | |
| Stricturing (B2) | 3 (20) | 3 (20) | |
| Penetrating (B3) | 6 (40) | 6 (40) | |
| Perianal disease | 7 (46.6) | 7 (46.6) | |
| Disease location | |||
| Terminal ileum (L1) | 6 (40) | 6 (40) | |
| Colon (L2) | 3 (20) | 3 (20) | |
| Ileocolon (L3) | 5 (33.4) | 5 (33.4) | |
| Upper gastrointestinal (L4) | 1 (6.6) | 1 (6.6) | |
| Pancolitis | 15 (51.7) | ||
| Left side | 14 (48.3) | ||
| Treatment | |||
| Surgery | 6 (40) | 6 (40) | 1 (3.4) |
| Infliximab | 8 (53.3) | 6 (40) | 0 (0) |
| Immunosuppressants | 7 (46.6) | 7 (46.6) | 10 (34.5) |
| No immunosuppressive therapy | 5 (33.4) | 5 (33.4) | 19 (65.5) |
| Genotype characteristics | |||
| At least one CARD15/NOD2 mutation | 15 (100) | 0 (0) | 4 (13.8) |
| ATG16L1 (rs2241880) AA/GA/GG (n) | 0/9/5 | 1/9/4 | 2/11/13 |
| IRGM (rs4958847) AA/GA/GG (n) | 0/5/8 | 1/4/9 | 0/7/15 |
| IL23R (rs7517847) GG/TG/TT (n) | 2/6/3 | 2/5/7 | 3/10/13 |
MAP DNA was detected in all original blood samples. No PCR internal control was positive for MAP DNA, which indicated no laboratory contamination. Nucleotide sequencing of purified MAP DNA fragments was also positive in the second round of nested PCR, which confirmed amplification of the IS900 nucleotide sequence (Table 2).
After 8 wk incubation (bottle I), no mycobacterial growth was automatically detected in the 69 BACTEC MGTI cultures.
Dot blot assays confirmed the positive MAP status of all original blood samples and 8-wk cultures.
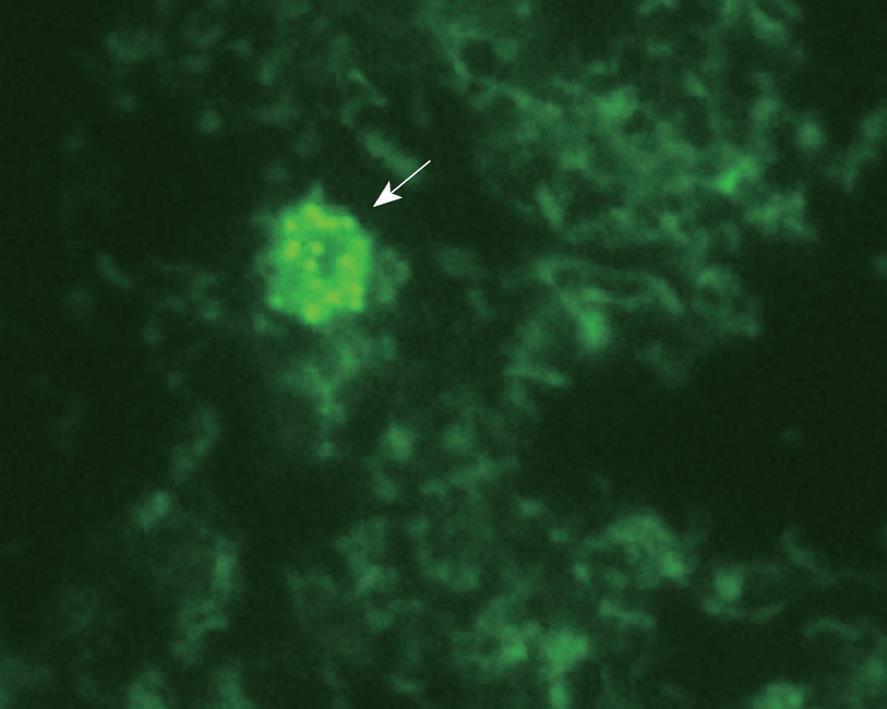
After 18 mo incubation (bottle II), no mycobacterial growth was automatically detected in the 69 BACTEC MGTI cultures. All 69 buffy coat cultures were negative by acid-fast Ziehl-Neelsen staining. However, all of the 30 18-mo cultures from CD patients were positive by phenolic acridine orange staining, which suggested the presence of wall-deficient cells or spheroplasts (Figure 1).
Thus, 18-mo blood cultures were MAP-positive in all CD patients. No association could be found between positive cultures and use of tumor necrosis factor (TNF)-α antibodies and thiopurine drugs. No correlation was seen between MAP-positive blood cultures and CARD15/NOD2, ATG16L1, IGRM or IL-23R CD-associated single nucleotide polymorphisms (SNPs).
In this study, nested IS900-specific PCR showed that MAP DNA is widespread in our environment. Original blood samples and 8-wk cultures from all CD and UC patients and non-IBD controls were PCR-positive. However, viable MAP spheroplasts (cell-wall-deficient forms) were only found in the 18-mo blood cultures from all CD and one UC patient, but in none of the non-IBD controls. The observation that MAP could be cultured from CD patients was not correlated with use of immunosuppressive drugs (TNF-α antibodies and thiopurine drugs), NOD2/CARD15 mutations, or other studied genes, which implicated the autophagy pathway of the innate immune system (ATG 16L1, IRGM, IL-23R).
The equal circulation of MAP DNA in patients with and without IBD lends support to the contention that environmental exposure to MAP is widespread, possibly from water, milk, or other sources[2]. Our group of subjects with no IBD was recruited from the staff of the Department of Microbiology, Hospital Clinico San Carlos de Madrid, Spain. These healthy subjects were most likely colonized by MAP, but none of them showed detection of viable MAP in 18-mo blood cultures. This result supported the findings in the study by Naser et al[7], where no viable MAP was subsequently cultured from any of the IS900-positive samples from healthy controls. The same occurred with the blood cultures from UC patients, as viable MAP was detected in only one of them. Thus, in our area, MAP is an ubiquitous environmental organism that does not usually cause disease unless the host is predisposed to infection, as occurs with other members of the M. avium complex.
Previous studies have shown that reliable and reproducible detection of MAP by PCR tests applied directly to DNA extracted from human tissue and other samples is extremely difficult. Use of suboptimal sample processing procedures results in false-negative results[19]. Results of MAP detection using nucleic-acid-based techniques have recently been reported in two meta-analyses that have suggested that there is adequate evidence for the presence of MAP in the bowel of CD patients, regardless of whether these patients are compared to subjects with no IBD or those with UC[5,20]. However, this association remains controversial and inconclusive. PCR data can be criticized on the grounds that the procedure assesses DNA that might come from live bacteria or merely be scattered debris from killed organisms, and therefore of questionable biological consequence[21].
The gold standard for detection of MAP is based on isolation of the organism using culture methods. However, this method is time-consuming because of the fastidious nature and slow growth, and pleomorphic, variably acid-fast and spheroplast-like organisms. Chiodini et al[15] have demonstrated that MAP strains isolated from CD tissue first appeared as cell-wall-deficient forms (spheroplasts), and have suggested that MAP is present in CD tissue in a spheroplast-like form[15,22]. We could not detect viable MAP by acid-fast Ziehl-Neelsen staining, but all cultures from CD patients were shown to contain spheroplasts. Naser et al[7] have reported that the MAP-positive cultures of the buffy coat were negative by acid-fast Ziehl-Neelsen staining during the early weeks of culture incubation but were positive by acridine orange (spheroplast), but this observation has not been confirmed by other studies[23-25]. Therefore, MAP spheroplasts might play a role in development of CD, as well as in paucibacillary forms of Johne’s disease in other species. It has not been determined whether the presence of MAP in CD is related to infection, colonization, or a defect in the intestinal barrier/microbial killing. We did not study MAP in intestinal tissue, nor explore the possibility of increased bowel permeability. However, detection of viable MAP in the blood of CD patients could be due to the inability of macrophages in CD to kill MAP[26].
No association was found between a positive MAP culture from the blood of CD patients and CARD15/NOD2, ATG16L1, IRGM or IL-23R CD-associated SNPs, but our sample was small and a type II error cannot be excluded.
The most irrefutable evidence that a microbial agent causes a disease is long-term remission of clinical manifestations and a change in the natural history of disease after clearance of infection. Recently, a large, well-designed, randomized, placebo-controlled trial of clarithromycin, rifabutin, and ethambutol failed to show a sustained response in CD patients, although a short-term benefit of antibiotics at 16 wk, additional to the effect of corticosteroid therapy, was reported[27]. A recent study[28] has shown that antimycobacterial and thiopurine drugs used in concert might have an interactive effect. The apparently bacteriostatic effects of 6-mercaptopurine on M. paratuberculosis renders the organism less susceptible to the bactericidal effects of antibiotics.
An argument against a role of MAP in CD is that if CD were a chronic mycobacterial infection, immunosuppressive therapies would be associated with increased rates and severity of mycobacterial disease, rather than with improvement[29]. We were not able to show any association between occurrence of MAP bacteremia and use of immunosuppressive drugs, because all CD patients showed positive MAP cultures. Viable MAP could not be cultured from UC patients who received immunosuppressive therapy. This might indicate that use of immunosuppressive therapy has no influence on the presence of viable MAP in blood.
In conclusion, MAP is widely present in our area and MAP DNA can be recovered from the blood of CD and UC and healthy controls. Spheroplasts were only found in the blood cultures from CD patients. However, the pathogenetic role of MAP remains controversial and inconclusive. However, even if MAP is not causally related to CD, the presence of viable MAP in the blood might have secondary clinical implications.
The hypothesis postulating that Mycobacterium avium paratuberculosis (MAP) is the cause of Crohn’s disease (CD) has been circulating for many years. Advances in molecular techniques, such as PCR and culture methods, have allowed researchers to demonstrate an association between MAP and CD.
MAP is a recurrent candidate as the cause of CD for several reasons: MAP induces epidemic chronic colitis in cattle and other species, including primates; it is reportedly detectable in the intestinal tissues and blood of many CD patients; MAP antibodies are often associated to the disease; and in some cases, antimycobacterial drugs improve the disease. In this study, authors demonstrated that MAP spheroplasts were cultured from the peripheral blood of CD patients only, but not from patients with ulcerative colitis (UC) or normal controls.
MAP is widely present in Spain, and MAP DNA may be recovered from the blood of CD patients, UC patients, and healthy controls, but in this study MAP spheroplasts were only found in the 18-mo blood cultures from all CD patients. The observation that MAP could be cultured from CD patients was not correlated with the use of immunosuppressive drugs or mutations associated with CD patients.
The status of viable MAP spheroplasts might play a role in development of CD, as well as in paucibacillary forms of Johne’s disease in other species.
CD is a chronic remitting and relapsing inflammatory disease of the gastrointestinal tract. MAP is a bacterium that is a member of the M. avium complex. M. avium strains are widely distributed in the environment and also occur in normal animal and human intestines. Spheroplasts are cell-wall-deficient forms of MAP.
This is a small case-control study that attempted to correlate the presence of MAP with inflammatory bowel disease. Although this is not an entirely original study and the number of patients is relatively small, the authors report some interesting findings.
Peer reviewers: Kiron Moy Das, MD, Department of GI/Hepatology, UMDNJ-Robert Wood Johnson Medical School, 1 Robert Wood Johnson Place, MEB 478, New Brunswick, NJ 08903, United States; Elias A Kouroumalis, Professor, Department of Gastroenterology, University of Crete, Medical School, Heraklion, Crete, 71110, Greece
S- Editor Wang YR L- Editor Kerr C E- Editor Lin YP
| 1. | Goyette P, Labbé C, Trinh TT, Xavier RJ, Rioux JD. Molecular pathogenesis of inflammatory bowel disease: genotypes, phenotypes and personalized medicine. Ann Med. 2007;39:177-199. |
| 2. | Greenstein RJ. Is Crohn's disease caused by a mycobacterium? Comparisons with leprosy, tuberculosis, and Johne's disease. Lancet Infect Dis. 2003;3:507-514. |
| 3. | Chacon O, Bermudez LE, Barletta RG. Johne's disease, inflammatory bowel disease, and Mycobacterium paratuberculosis. Annu Rev Microbiol. 2004;58:329-363. |
| 4. | McClure HM, Chiodini RJ, Anderson DC, Swenson RB, Thayer WR, Coutu JA. Mycobacterium paratuberculosis infection in a colony of stumptail macaques (Macaca arctoides). J Infect Dis. 1987;155:1011-1019. |
| 5. | Feller M, Huwiler K, Stephan R, Altpeter E, Shang A, Furrer H, Pfyffer GE, Jemmi T, Baumgartner A, Egger M. Mycobacterium avium subspecies paratuberculosis and Crohn's disease: a systematic review and meta-analysis. Lancet Infect Dis. 2007;7:607-613. |
| 6. | Mendoza JL, Lana R, Díaz-Rubio M. Mycobacterium avium subspecies paratuberculosis and its relationship with Crohn's disease. World J Gastroenterol. 2009;15:417-422. |
| 7. | Naser SA, Ghobrial G, Romero C, Valentine JF. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn's disease. Lancet. 2004;364:1039-1044. |
| 8. | Hampe J, Cuthbert A, Croucher PJ, Mirza MM, Mascheretti S, Fisher S, Frenzel H, King K, Hasselmeyer A, MacPherson AJ. Association between insertion mutation in NOD2 gene and Crohn's disease in German and British populations. Lancet. 2001;357:1925-1928. |
| 9. | Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599-603. |
| 10. | Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603-606. |
| 11. | Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461-1463. |
| 12. | Parkes M, Barrett JC, Prescott NJ, Tremelling M, Anderson CA, Fisher SA, Roberts RG, Nimmo ER, Cummings FR, Soars D. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn's disease susceptibility. Nat Genet. 2007;39:830-832. |
| 13. | Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753-766. |
| 14. | Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2-6; discussion 16-19. |
| 15. | Chiodini RJ, Van Kruiningen HJ, Thayer WR, Coutu JA. Spheroplastic phase of mycobacteria isolated from patients with Crohn's disease. J Clin Microbiol. 1986;24:357-363. |
| 16. | Márquez A, Mendoza JL, Taxonera C, Díaz-Rubio M, De La Concha EG, Urcelay E, Martínez A. IL23R and IL12B polymorphisms in Spanish IBD patients: no evidence of interaction. Inflamm Bowel Dis. 2008;14:1192-1196. |
| 17. | Mendoza JL, Murillo LS, Fernández L, Peña AS, Lana R, Urcelay E, Cruz-Santamaría DM, de la Concha EG, Díaz-Rubio M, García-Paredes J. Prevalence of mutations of the NOD2/CARD15 gene and relation to phenotype in Spanish patients with Crohn disease. Scand J Gastroenterol. 2003;38:1235-1240. |
| 18. | Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, Albrecht M, Mayr G, De La Vega FM, Briggs J. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39:207-211. |
| 19. | Bull TJ, McMinn EJ, Sidi-Boumedine K, Skull A, Durkin D, Neild P, Rhodes G, Pickup R, Hermon-Taylor J. Detection and verification of Mycobacterium avium subsp. paratuberculosis in fresh ileocolonic mucosal biopsy specimens from individuals with and without Crohn's disease. J Clin Microbiol. 2003;41:2915-2923. |
| 20. | Abubakar I, Myhill D, Aliyu SH, Hunter PR. Detection of Mycobacterium avium subspecies paratuberculosis from patients with Crohn's disease using nucleic acid-based techniques: a systematic review and meta-analysis. Inflamm Bowel Dis. 2008;14:401-410. |
| 21. | Chamberlin WM, Naser SA. Integrating theories of the etiology of Crohn's disease. On the etiology of Crohn's disease: questioning the hypotheses. Med Sci Monit. 2006;12:RA27-RA33. |
| 22. | Wall S, Kunze ZM, Saboor S, Soufleri I, Seechurn P, Chiodini R, McFadden JJ. Identification of spheroplast-like agents isolated from tissues of patients with Crohn's disease and control tissues by polymerase chain reaction. J Clin Microbiol. 1993;31:1241-1245. |
| 23. | Lozano-Leon A, Barreiro-de Acosta M, Domínguez-Munoz JE. Absence of Mycobacterium avium subspecies paratuberculosis in Crohn's disease patients. Inflamm Bowel Dis. 2006;12:1190-1192. |
| 24. | Freeman H, Noble M. Lack of evidence for Mycobacterium avium subspecies paratuberculosis in Crohn's disease. Inflamm Bowel Dis. 2005;11:782-783. |
| 25. | Sartor RB, Blumberg RS, Braun J, Elson CO, Mayer LF. CCFA microbial-host interactions workshop: highlights and key observations. Inflamm Bowel Dis. 2007;13:600-619. |
| 26. | Ferwerda G, Kullberg BJ, de Jong DJ, Girardin SE, Langenberg DM, van Crevel R, Ottenhoff TH, Van der Meer JW, Netea MG. Mycobacterium paratuberculosis is recognized by Toll-like receptors and NOD2. J Leukoc Biol. 2007;82:1011-1018. |
| 27. | Selby W, Pavli P, Crotty B, Florin T, Radford-Smith G, Gibson P, Mitchell B, Connell W, Read R, Merrett M. Two-year combination antibiotic therapy with clarithromycin, rifabutin, and clofazimine for Crohn's disease. Gastroenterology. 2007;132:2313-2319. |
| 28. | Shin SJ, Collins MT. Thiopurine drugs azathioprine and 6-mercaptopurine inhibit Mycobacterium paratuberculosis growth in vitro. Antimicrob Agents Chemother. 2008;52:418-426. |