Published online Jun 21, 2010. doi: 10.3748/wjg.v16.i23.2949
Revised: March 10, 2010
Accepted: March 17, 2010
Published online: June 21, 2010
AIM: To clarify the association between CYP2E1 PstI/RsaI polymorphism and susceptibility to colorectal cancer.
METHODS: A meta-analysis based on 10 eligible case-control studies involving 4979 cases and 6012 controls was carried out to summarize the data on the association between CYP2E1 RsaI/PstI polymorphism and colorectal cancer risk.
RESULTS: In comparison of the homozygote c2c2 and c2 carriers (c1c2 + c2c2) and the homozygous wild-type genotype (c1c1), no association was found between CYP2E1 RsaI/PstI polymorphism and colorectal cancer risk [odds ratio (OR) = 1.24 (95% CI: 0.93-1.66) for c2c2; OR = 1.02 (95% CI: 0.88-1.19) for c2 carriers]. In stratified analysis, Caucasians with c2c2 homozygote appeared to have an increased risk of colorectal cancer (OR = 2.67, 95% CI: 1.03-6.89, P = 0.043), no significant associations were found in other groups.
CONCLUSION: c2c2 homozygote of CYP2E1 PstI/RsaI polymorphism may be associated with the increased risk of colorectal cancer in Caucasians, which needs further investigations.
-
Citation: Zhou GW, Hu J, Li Q.
CYP2E1 PstI/RsaI polymorphism and colorectal cancer risk: A meta-analysis. World J Gastroenterol 2010; 16(23): 2949-2953 - URL: https://www.wjgnet.com/1007-9327/full/v16/i23/2949.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i23.2949
Colorectal cancer is one of the most common cancers in developed countries and getting more and more attentions in developing countries for its morbidity and mortality. The definite mechanism of its development is still unknown, but both environmental factors and genetic susceptibility are believed to contribute to the onset of colorectal cancer[1,2].
Cytochrome P450 2E1 (CYP2E1), a member of the cytochrome P-450 superfamily, is a naturally ethanol-inducible enzyme and primarily responsible for the metabolic activation of many low molecular weight carcinogens[3,4], including certain nitrosamines believed to participate in the carcinogenesis of digestive tract[5]. Based on the biological significance of CYP2E1, a hypothesis has been proposed that CYP2E1 polymorphisms may be associated with cancer susceptibility[6]. Of the many known genetic polymorphisms in the CYP2E1 gene, two point mutations in the 5’-flanking region (PstI, RsaI), which are in close linkage disequilibrium, have drawn much interest as it is known to alter the transcriptional activity of the gene[7]. Three genotypes consisting of wild-type allele (c1) and variant-type allele (c2) of PstI/RsaI polymorphism are defined as homozygous wild-type genotype (c1c1), heterozygote (c1c2) and rare homozygote (c2c2), respectively[8].
Recently, meta-analyses showed that c2 allele carriers had a significantly lower risk of lung cancer in the Asian population, but a higher risk of gastric cancer. However, the conclusion regarding colorectal cancer remains to be established. Though several studies focusing on PstI/RsaI polymorphism and susceptibility to colorectal cancer have been conducted, the results are inconsistent due to the small sample size of individual study or other factors such as race, diet and tumor location[9-18]. For better understanding this relationship, a meta-analysis was performed.
Inclusion criteria was defined as follows: (1) articles evaluating the association between CYP2E1 PstI/RsaI polymorphism and colorectal cancer risk; (2) studies designed as case-control; (3) sufficient data available to estimate an odds ratio (OR) with its 95% CI.
A literature search of Medline and Embase (updated to January 1, 2010) was conducted using the following terms: ‘cytochrome p450 2E1/IIE1’ or ‘CYP2E1/IIE1’, ‘colorectal’ and ‘cancer’ or ‘adencarcinoma’, without restriction on language. The retrieved literatures were then read in their entirety to assess their appropriateness for the inclusion in this meta-analysis by the two authors (Zhou GW, Hu J) independently. The reference lists of reviews and retrieved articles were searched simultaneously to find additional eligible studies. If more than one article was published by the same author using the same case series, we selected the study with the largest size of samples. If there was an disagreement, it was resolved through discussion between the two authors.
The following variables were extracted from each study if available: first author’s surname, publication year, country, ethnicity, source of controls (hospital-based or population-based), matching criteria, number of cases and controls, and number of cases and controls in different PstI/RsaI genotypes.
The strength of association between CYP2E1 PstI/RsaI polymorphism and colorectal cancer risk was assessed by odds ratio (OR) with the corresponding 95% CI. The pooled OR was calculated by a fixed-effects model (the Mantel-Haenszel method)[19] or a random-effects model (the DerSimonian and Laird method)[20] according to the heterogeneity. Heterogeneity among studies was checked by the Q test[21] and P < 0.10 was considered statistically significant. The ORs of colorectal cancer associated with c2 allele carriers (c1c2 + c2c2) and homozygous c2 genotype (c2c2) for the CYP2E1 PstI/RsaI polymorphism were estimated using the homozygous wild-type (c1c1) as the reference group. Influence analysis was performed by omitting an individual study each time to find potential outliers to the pooled OR[22]; sensitivity analysis was also performed, if necessary, by excluding the Hardy-Weinberg equilibrium (HWE)-violating studies to check the robustness of the result. Departure from HWE was detected in the control populations, but a deviation from HWE was allowed in mixed control populations. Subgroup analyses for different ethnicities (Asian, Caucasian and mixed population) and cancer locations (colon cancer, rectum cancer) were conducted. The possible publication bias was examined visually in a funnel plot of log[OR] against its standard error (SE), and the degree of asymmetry was tested by Egger’s test (P < 0.05 was considered a significant publication bias)[23]. Meta-analysis was performed using the STATA version 10.0 software.
A total of 13 publications met the inclusion criteria. Of these studies, two studies[24,25] were excluded as another two included studies[14,16] were based on the same population with a larger sample size. With 14 more cases but less information on the relationship studied in this paper, Chen’s study[26] published in 2005 overlapped with Yu’s work[11], and it was excluded after discussion. As a result, a total of 10 publications[9-18] containing 4979 cases and 6012 controls were included into this meta-analysis. Table 1 lists the main characteristics of these studies. Of these publications, only one was not published in English but in Chinese[11]. The sample sizes ranged from 419 to 2144. All of the cases were histologically confirmed as colorectal cancer. Controls were mainly healthy populations, and matched with age, sex, or cancer-free. There were three groups of Asians, six groups of Caucasians and one group of mixed populations. Four studies[10,11,16,18] provided information on PstI/RsaI polymorphism status associated with tumor locations classified as colon cancer and rectum cancer. Four studies[10,15,16,18] investigated the significance of PstI/RsaI polymorphism interacting with environmental factors with regard to susceptibility to colorectal cancer. Genotype distributions in the controls of all studies were in agreement with HWE except for one study with mixed control populations[10], without conducting sensitivity analysis by excluding the HWE-violating studies.
| Author, yr | Ethnicity (country) | Source of controls | Matching criteria | Sample size (case/control) | Genotype (case/controls) | HWE | ||
| c1c1 | c2c2 | c1c2 + c2c2 | ||||||
| Butler et al[9], 2001 | Caucasian (Australian) | Population-based | Age, sex | 219/200 | 147/194 | NA/NA | 2/6 | NA |
| Le Marchand et al[10], 2002 | Mixed (Japanese, Caucasian, Hawaiian) | Population-based | Age, sex, ethnicity | 521/639 | 384/449 | 21/26 | 137/190 | No1 |
| Yu et al[11], 2004 | Asian (Chinese ) | Population-based | Cancer-free | 126/343 | 69/209 | 3/8 | 57/129 | Yes |
| Landi et al[12], 2005 | Caucasian (Spanish) | Hospital-based | Cancer-free | 377/326 | 323/283 | 1/1 | 18/16 | Yes |
| van der Logt et al[13], 2006 | Caucasian (Netherlander) | Population-based | Age > 18-yr, without (family) history of CRC | 371/415 | 333/389 | 1/2 | 24/23 | Yes |
| Kiss et al[14], 2007 | Caucasian (Hungarian) | Both | Age, sex, red meat intake, smoking | 500/500 | 428/456 | 7/2 | 72/44 | Yes |
| Küry et al[15], 2007 | Caucasian (French) | Population-based | Age, sex | 1023/1121 | 940/1027 | 6/1 | 73/91 | Yes |
| Gao et al[16], 2007 | Asian (Chinese ) | Population-based | Age, sex, ethnicity | 315/439 | 185/266 | 22/13 | 128/167 | Yes |
| Cotterchio et al[17], 2008 | Caucasian (Canadian ) | Population-based | Age, sex | 842/1251 | 784/1162 | 0/0 | 48/85 | Yes |
| Morita et al[18], 2009 | Asian (Japanese ) | Population-based | Sex, smoking, red meat intake, residence area | 685/778 | 412/455 | 36/44 | 273/323 | Yes |
Table 2 lists the main results of this meta-analysis. When the homozygote c2c2 and c2 carriers (c2c2 + c1c2) were compared with the homozygous wild-type genotype (c1c1), the pooled ORs for all the 10 studies were 1.24 (95% CI: 0.93-1.66, P = 0.148) and 1.02 (95% CI: 0.88-1.19, P = 0.780), respectively. In the subgroup analysis of ethnicity, Caucasians with c2c2 homozygote appeared to have an increased risk of colorectal cancer (OR = 2.67, 95% CI: 1.03-6.89, P = 0.043), while no significant association was found among Asians and mixed populations. In the subgroup analysis of cancer locations, no significant associations were discovered in both colon cancer and rectum cancer.
| Analysis | Number of cases/controls | c1c2 + c2c2 vs c1c1 | c2c2 vs c1c1 | ||
| OR (95% CI) | P/Ph | OR (95% CI) | P/Ph | ||
| Overall | 4979/6012 | 1.02 (0.88, 1.19) | 0.780/0.094 | 1.24 (0.93, 1.66) | 0.148/0.148 |
| Subgroup analysis for ethnicity | |||||
| Asian | 1126/1560 | 1.03 (0.88, 1.21) | 0.707/0.274 | 1.35 (0.66, 2.75) | 0.414/0.072 |
| Caucasian | 3332/3813 | 1.05 (0.78, 1.41) | 0.747/0.066 | 2.67 (1.03, 6.89) | 0.043/0.389 |
| Mixed | 521/639 | 0.84 (0.65, 1.09) | 0.196/NA | 0.94 (0.52, 1.71) | 0.850/NA |
| Subgroup analysis for tumor location | |||||
| Colon cancer | 907/2188 | 1.09 (0.83, 1.43) | 0.522/0.082 | 1.22 (0.85, 1.77) | 0.279/0.809 |
| Rectum cancer | 739/2188 | 0.89 (0.75, 1.06) | 0.206/0.241 | 0.97 (0.38, 2.43) | 0.941/0.014 |
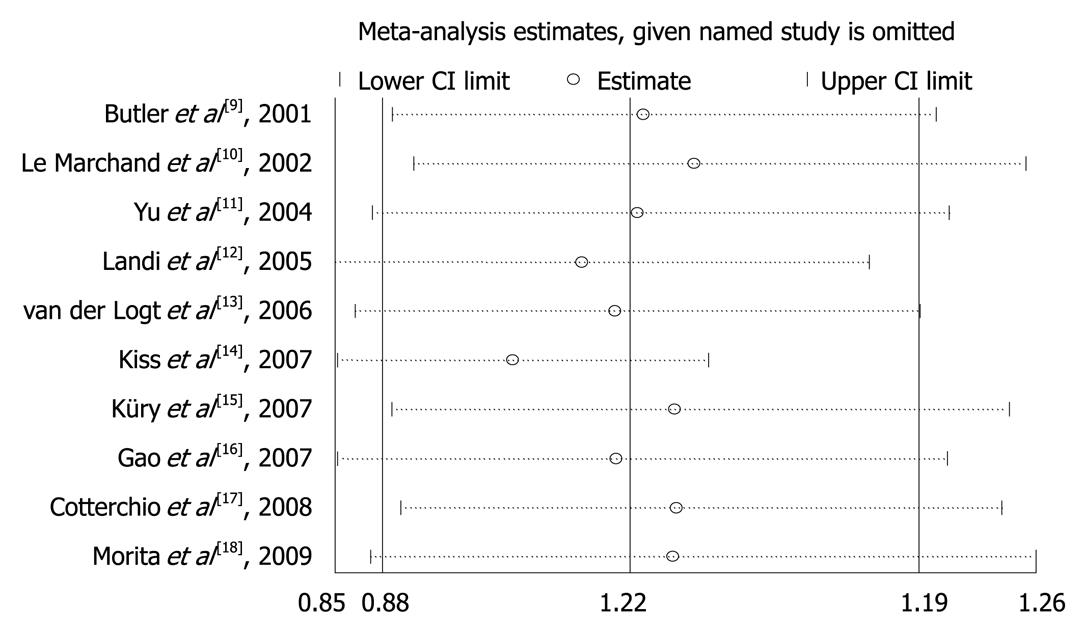
Influence analysis was performed to assess the effects of each individual study on the pooled OR in each analysis, by removing an individual study each time. The results suggested that no individual study significantly affected the pooled ORs. Figure 1 shows the result of influence analysis for c1c2 + c2c2 vs c1c1.
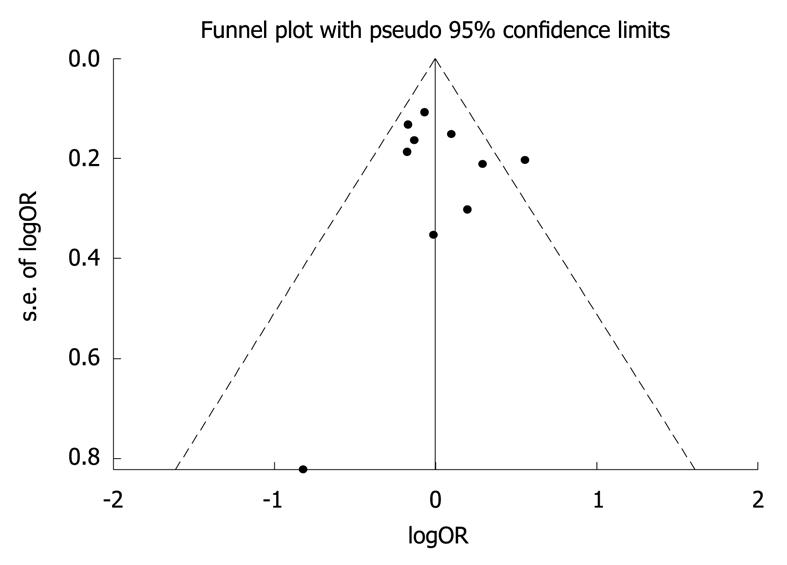
Funnel plot and Egger’s test were performed to assess the publication bias. The shape of the funnel plot did not indicate any evidence of obvious asymmetry (Figure 2) and no publication bias was found in the Egger’s test P = 0.647).
The relationship between CYP2E1 PstI/RsaI polymorphism and colorectal cancer susceptibility has been studied in several researches[9-18], and the results are controversial. Kiss and his colleagues[14] reported that c2 variant allele significantly increased the risk of colorectal cancer, while Gao et al[16] suggested that only c2c2 homozygote was associated with colorectal cancer, and no significant association was found in other studies[10,13]. This is the first systematic study of the meta-analysis of this relationship.
Ten case-control studies were included in this meta-analysis, involving 4979 cases and 6012 controls. The results strongly suggested that there was no significant association between CYP2E1 PstI/RsaI polymorphism and colorectal cancer susceptibility. By sequentially removing individual studies during influence analysis, it was confirmed that all results were not materially altered by an individual study. In addition, no significant publication bias was found.
Analysis based on the ethnic subgroup showed that Caucasians with c2c2 homozygote had an increased risk of colorectal cancer. This may be explained by various frequency distributions of PstI/RsaIc2 allele[27] and different living habits and environment in different ethnicity. However, no study regarding African populations was done.
Sensitivity of colon and rectum tissue to carcinogens is inconsistent. In contrast to studies involving rectal cancer, animal studies suggested that nitrosamines may have no correlation with the development of colon cancer[28]. Based on this phenomenon, it is proposed that differences may exist between colon and rectal cancer susceptibility with respect to PstI/RsaI polymorphism. Morita et al[18] found that c2 allele may significantly reduce the risk of rectal cancer, while in a similar study, Gao’s results[16] were completely the opposite. On the other hand, no association was revealed between c2 allele and colon cancer in these two studies. But Yu et al[11] suggested that c2 allele may increase the susceptibility to colon cancer. In this meta-analysis, a stratified analysis was performed by tumor location due to these conflicting results, and no association was revealed in neither the colon cancer group nor the rectal cancer group (Table 2). A very noteworthy point was that subjects investigated in this stratified analysis were mainly Asians, and studies on Caucasians are needed.
Although the result of this meta-analysis is suggestive, some limitations still exist. Firstly, heterogeneity among the studies, resulting from different defined controls or some other factors, may influence the results of this analysis. In some studies, the controls were selected randomly from a healthy population, and in other studies from hospital-based cancer-free patients. Furthermore, the matching criteria of the control group differed across studies in age and gender. The variant risks of colorectal cancer in these different populations may affect the results. Secondly, OR value was obtained without correction. More accurate OR should be corrected by age, gender and other factors. Thirdly, no African population was included in these ten studies, and the frequency distribution of CYP2E1 PstI/RsaI genotype in Africans was significantly different from that in Caucasians and Asians[27]. Therefore, the conclusion of this study is not applicable to the African population, and further investigations are required. Fourthly, the relationship between CYP2E1 gene polymorphism and colorectal cancer risk was analyzed without consideration of gene-environment interactions. Although evidence supported that c2 allele combined with smoking, drinking and consumption of red meat intake can significantly increase the susceptibility to colorectal cancer[10,15,16,18], this study failed to draw any concrete conclusions due to the different classification criteria of environmental factors in these studies. In addition, there has been no study on the association between PstI/RsaI polymorphism gene-gene interactions and colorectal cancer risk, and this should be further investigated.
Cytochrome P450 2E1 (CYP2E1) is a member of the cytochrome P-450 superfamily, and its gene polymorphisms are supposed to be associated with cancer susceptibility. Several studies focusing on CYP2E1 PstI/RsaI polymorphism and colorectal cancer susceptibility have been conducted.
The relationship between CYP2E1 PstI/RsaI polymorphism and cancer susceptibility has been studied in different tumor types. Recently, meta-analyses showed that c2 allele carriers had a significantly lower risk of lung cancer in Asian population but a higher risk of gastric cancer. However, the relationship with colorectal cancer remains controversial and no meta-analysis has been conducted.
This is the first meta-analysis which systemically studied the relationship between CYP2E1 PstI/RsaI polymorphism and colorectal cancer susceptibility, and suggested that Caucasians with c2c2 homozygote may have a higher risk of colorectal cancer.
This study provided a potential biomarker for identifying high-risk populations of colorectal cancer in Caucasians.
The article is meritoriously and methodologically well written. The presentation is rigorous and the approach used is robust.
Peer reviewer: Gianpiero Gravante, MD, BsC, MBBS, Department of Hepatobiliary and Pancreatic Surgery, Leicester General Hospital, Flat 38, Room 8, Hospital Close, Leicester, LE5 4WU, United Kingdom
S- Editor Tian L L- Editor Ma JY E- Editor Ma WH
| 1. | Seitz HK, Maurer B, Stickel F. Alcohol consumption and cancer of the gastrointestinal tract. Dig Dis. 2005;23:297-303. |
| 2. | Reszka E, Wasowicz W, Gromadzinska J. Genetic polymorphism of xenobiotic metabolising enzymes, diet and cancer susceptibility. Br J Nutr. 2006;96:609-619. |
| 3. | Guengerich FP, Kim DH, Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol. 1991;4:168-179. |
| 4. | Yamazaki H, Inui Y, Yun CH, Guengerich FP, Shimada T. Cytochrome P450 2E1 and 2A6 enzymes as major catalysts for metabolic activation of N-nitrosodialkylamines and tobacco-related nitrosamines in human liver microsomes. Carcinogenesis. 1992;13:1789-1794. |
| 5. | Montesano R, Hall J. Environmental causes of human cancers. Eur J Cancer. 2001;37 Suppl 8:S67-S87. |
| 6. | Agundez JA. Cytochrome P450 gene polymorphism and cancer. Curr Drug Metab. 2004;5:211-224. |
| 7. | Hayashi S, Watanabe J, Kawajiri K. Genetic polymorphisms in the 5’-flanking region change transcriptional regulation of the human cytochrome P450IIE1 gene. J Biochem. 1991;110:559-565. |
| 8. | Carrière V, Berthou F, Baird S, Belloc C, Beaune P, de Waziers I. Human cytochrome P450 2E1 (CYP2E1): from genotype to phenotype. Pharmacogenetics. 1996;6:203-211. |
| 9. | Butler WJ, Ryan P, Roberts-Thomson IC. Metabolic genotypes and risk for colorectal cancer. J Gastroenterol Hepatol. 2001;16:631-635. |
| 10. | Le Marchand L, Donlon T, Seifried A, Wilkens LR. Red meat intake, CYP2E1 genetic polymorphisms, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:1019-1024. |
| 11. | Yu WP, Chen K, Ma XY, Yao KY, Jiang QT, Zou Y, Zhou HG. [Genetic polymorphism in cytochrome P450 2E1, salted food and colorectal cancer susceptibility: a case-control study]. Zhonghua Yufang Yixue Zazhi. 2004;38:162-166. |
| 12. | Landi S, Gemignani F, Moreno V, Gioia-Patricola L, Chabrier A, Guino E, Navarro M, de Oca J, Capellà G, Canzian F. A comprehensive analysis of phase I and phase II metabolism gene polymorphisms and risk of colorectal cancer. Pharmacogenet Genomics. 2005;15:535-546. |
| 13. | van der Logt EM, Bergevoet SM, Roelofs HM, Te Morsche RH, Dijk Y, Wobbes T, Nagengast FM, Peters WH. Role of epoxide hydrolase, NAD(P)H:quinone oxidoreductase, cytochrome P450 2E1 or alcohol dehydrogenase genotypes in susceptibility to colorectal cancer. Mutat Res. 2006;593:39-49. |
| 14. | Kiss I, Orsós Z, Gombos K, Bogner B, Csejtei A, Tibold A, Varga Z, Pázsit E, Magda I, Zölyomi A. Association between allelic polymorphisms of metabolizing enzymes (CYP 1A1, CYP 1A2, CYP 2E1, mEH) and occurrence of colorectal cancer in Hungary. Anticancer Res. 2007;27:2931-2937. |
| 15. | Küry S, Buecher B, Robiou-du-Pont S, Scoul C, Sébille V, Colman H, Le Houérou C, Le Neel T, Bourdon J, Faroux R. Combinations of cytochrome P450 gene polymorphisms enhancing the risk for sporadic colorectal cancer related to red meat consumption. Cancer Epidemiol Biomarkers Prev. 2007;16:1460-1467. |
| 16. | Gao CM, Takezaki T, Wu JZ, Chen MB, Liu YT, Ding JH, Sugimura H, Cao J, Hamajima N, Tajima K. CYP2E1 Rsa I polymorphism impacts on risk of colorectal cancer association with smoking and alcohol drinking. World J Gastroenterol. 2007;13:5725-5730. |
| 17. | Cotterchio M, Boucher BA, Manno M, Gallinger S, Okey AB, Harper PA. Red meat intake, doneness, polymorphisms in genes that encode carcinogen-metabolizing enzymes, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:3098-3107. |
| 18. | Morita M, Le Marchand L, Kono S, Yin G, Toyomura K, Nagano J, Mizoue T, Mibu R, Tanaka M, Kakeji Y. Genetic polymorphisms of CYP2E1 and risk of colorectal cancer: the Fukuoka Colorectal Cancer Study. Cancer Epidemiol Biomarkers Prev. 2009;18:235-241. |
| 19. | Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719-748. |
| 20. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. |
| 21. | Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101-129. |
| 22. | Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull. 1999;47:15-17. |
| 23. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. |
| 24. | Kiss I, Sándor J, Pajkos G, Bogner B, Hegedüs G, Ember I. Colorectal cancer risk in relation to genetic polymorphism of cytochrome P450 1A1, 2E1, and glutathione-S-transferase M1 enzymes. Anticancer Res. 2000;20:519-522. |
| 25. | Chen MB, Gao CM, Wu JZ, Cao HX, Fu QH, Ding JH, Liu YT, Li SP, Su P, Toshiro T. Polymorphisms of CYP2E1 Rsa I and susceptibility of colorectal cancer. Zhongguo Zhongliu Fangzhi Zazhi. 2007;14:409-411. |
| 26. | Chen K, Jin MJ, Fan CH, Song L, Jiang QT, Yu WP, Ma XY, Yao KY. [A case-control study on the association between genetic polymorphisms of metabolic enzymes and the risk of colorectal cancer]. Zhonghua Liuxingbingxue Zazhi. 2005;26:659-664. |
| 27. | Ulusoy G, Arinç E, Adali O. Genotype and allele frequencies of polymorphic CYP2E1 in the Turkish population. Arch Toxicol. 2007;81:711-718. |
| 28. | Parnaud G, Pignatelli B, Peiffer G, Taché S, Corpet DE. Endogenous N-nitroso compounds, and their precursors, present in bacon, do not initiate or promote aberrant crypt foci in the colon of rats. Nutr Cancer. 2000;38:74-80. |