Published online Nov 7, 2005. doi: 10.3748/wjg.v11.i41.6549
Revised: April 15, 2005
Accepted: April 18, 2005
Published online: November 7, 2005
AIM: To investigate the influence of IL-1B-511 gene polymorphism on IL-1B mRNA expression and gastric acid output in individual with or without Helicobacter pylori (H pylori) infection.
METHODS: IL-1B mRNA expression and gastric acid secretion in 117 health volunteers were assayed using semi-quantitative RT-PCR and gastric juice assay, respectively. Pepsinogen (PG) I and II of 255 subjects (including 117 health volunteers) were also examined.
RESULTS: T/T genotype individuals with H pylori infection had a more decreased PG I/II ratio. In gastric antrum mucosa, the individuals with H pylori infection had higher IL-1B expression than those without H pylori infection, but there was no obvious difference among each genotype. In gastric corpus, the individuals with H pylori infection had a significantly higher IL-1B expression than those without H pylori infection. IL-1B-511T/T genotype was markedly higher as compared with the other two genotypes. Both maximal acid output and basic acid output were similar among each genotype in IL-1B-511 gene locus, regardless of H pylori infection.
CONCLUSION: IL-1B-511 T allele does not decrease gastric acid output, although it has a stimulated influence on IL-1B expression. Consequently, the pathway, through which IL-1B plays a central role in gastric cancer development, might not depend on low acid, but on the other regulation mechanisms.
- Citation: Hu S, Song QB, Yao PF, Hu QL, Hu PJ, Zeng ZR, Pang RP. No relationship between IL-1B gene polymorphism and gastric acid secretion in younger healthy volunteers. World J Gastroenterol 2005; 11(41): 6549-6553
- URL: https://www.wjgnet.com/1007-9327/full/v11/i41/6549.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i41.6549
Gastric cancer development is a multifactor and multi-step process. Clinical and epidemiological studies have suggested that environmental effects and dietary habits, such as smoking, alcohol consumption, low intake of fruits or vegetables and Helicobacter pylori (H pylori) infection, were the primary causes for the occurrence of carcinogenesis[1,2]. Furthermore, decades of researches have built up a variety of evidence that genetic risk factors also play an important role in cancer development[3]. Recently, through a case-control study, El-Omar et al[4] discovered that there was a strong relationship between IL-1 gene polymorphism and gastric cancer in the Polish population. The association between IL-1B-511 T allele and gastric carcinoma or atrophic gastritis was discovered by Machado et al[5] and Furuta et al[6] respectively. The results of several studies are in agreement with ours on Chinese population[7].
The epidemiological studies mentioned above were based on a hypothesis that IL-1B-511 and -31 gene polymorphisms contributed to stomach cancer development through T allele upregulating IL-1B mRNA expression, and then inhibiting directly gastric acid secretion. Low gastric acid was reported as a risk factor for cancer, because it resulted in a change of the colonized place of H pylori from gastric antrum to the corpus[8,9]; unfortunately, corpus-predominant gastritis with bacterial overgrowth was at increased risk of atrophic gastritis (with several biomarkers, such as pepsinogen (PG)I, II or I/II ratio)[6,10] and even gastric cancer. Both increased intragastric pH value and H pylori infection could significantly enhance N-nitroso compounds concentration, which is a putative promoter of carcinogenesis and tumor progression[11]. Svendsen et al[12] discovered 5 gastric cancer patients among 114 patients with low gastric acid secretion in a long-term follow-up (mean 8.4 years) study. However, this model was only supported by animal studies[4,13]. It is unknown now whether IL-1B gene polymorphism increased IL-1β protein level and resulted in human hypochlorhydria and atrophic gastritis in vivo. In the present study, we investigated the effects of IL-1B-511 genetic polymorphism on IL-1β mRNA expression and gastric acid secretion in the gastric mucosa of human beings with or without H pylori infection.
A total of 255 students (121 females and 132 males) from the Sun Yat-Sen University of Medical Sciences were enrolled in this study (Table 1). All subjects belonged to the ethnic group of Han and their age ranged from 19 to 24 years (mean 21.4±1.5 years). None of them had the histories of systemic lupus erythematosis, diabetes mellitus, rheumatoid arthritis, and inflammatory bowel disease. None of the subjects had received treatment for H pylori infection. Subjects with a family history of gastric cancer were also excluded.
| Loci | Genotype | H pylori+ | H pylori– | Total |
| (n=96) | (n=159) | (n=255) | ||
| IL-1B-511 | C/C | 34 | 63 | 97 |
| C/T | 46 | 75 | 121 | |
| T/T | 16 | 211 | 37 (14.1%) | |
| IL-1B-31 | C/C | 72 | 120 | 192 |
| C/T | 22 | 37 | 59 | |
| T/T | 2 | 22 | 4 (1.6%) |
After genotyping of IL-1B gene and test of H pylori antibody IgG, 117 subjects were randomly selected for the second step study on IL-1B-511 locus, but IL-1B-31 T/T genotype frequency is too low to be researched (Tables 1 and 2). Three biopsy specimens were collected from the antrum (two specimens for RNA extraction and another one for urease test) and two biopsy specimens from corpus for RNA extraction. In each genotypical group, no statistical difference in H pylori prevalence, sex, and age was observed (Table 2).
DNA was isolated from peripheral blood using the NaI method[14]. Briefly, heparinized whole blood (100 mL) was added to twofold volume of 6 mol/L NaI and fourfold volume of chloroform:isoamyl alcohol (24:1) and centrifuged at 5 000 r/min for 5 min. The aqueous layer was removed and isopropanol was added to the pellet to deposit DNA (centrifugation at 5 000 r/min for 5 min). Extracted DNA was rinsed 2-3 times with 700 mL/L alcohol and resuspended in 40 µL TE buffer (pH 8.0).
Polymorphism of IL-1B-511 and -31 that encodes IL-1B was genotyped by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) A fragment containing the AvaI polymorphic site at position -511 of the IL-1B gene was amplified using PCR. The oligonucleotides, 5’-GCCTGAACCCTGCATACCGT-3’ and 5’-GCCAATAGCCCTCCCTTCT-3’, flanking this region were used as primers. AluI polymorphic site at position -31 was amplified using primers: 5’-AGAAGCTTCCACCAATACTC-3’ and 5’-ACCACCTAGTTGTAAGGAAG-3’. PCR was carried out as described previously by Zeng et al[7] PCR fragments were separated by electrophoresis on 30 g/L agarose with ethidium bromide staining. The C allele was designated, if two bands of 92 and 63 bp were obtained, and T allele was designated, if a single band of the undigested 155 bp was obtained. The genotype was designated as follows: C/C, 2 bands of 92 and 63 bp; C/T, 3 bands of 155, 92, and 63 bp; T/T, a single band of 155 bp.
The biopsy specimens (5-10 mg) were mixed with 1 000 µL TRIzol (Invitrogen) and then homogenized for RNA extraction. RNA was resuspended in 40 µL TE buffer (RNase-free).
RNA solution (5 µL) was diluted 10-fold and A260/280 ratio was examined. The total RNA was calculated as follows: RNAtotal=A260×40×N.
IL-1B amplification was performed using the primers: IL-1B primers, P1 5’-gatgaagtgctccttccaggac-’3, P2 5’-tggagcaacaagtggtgttctcca-’3 (480 bp); and GAPDH primers, P1 5’-cacagtccatgccatcactg-’3, P2 5’-tactccttggaggccatgtg-’3 (480 bp).
RT-PCR amplification was performed in a volume of 50 µL containing 2× AccessQuickTM Master mixture (Promega Company). The final PCR aliquot (10 µL) was analyzed by electrophoresis on 30 g/L agarose with ethidium bromide staining.
Gastric acid secretion was detected in 117 subjects following pentagastrin injection. For this, all subjects received no medication (e.g. antiacid, etc.) for 24 h and no food for 12 h before the test. On the morning of the test, a tube was passed into the stomach through the nose. The tube was securely fastened and subjects were made to lie on their left-side. The gastric juices were then collected by applying continuous suction (at 30-50 mmHg below atmospheric pressure) to the tube.
Hardy-Weinberg equilibrium at individual loci was assessed using χ2 test in the statistics program SPSS (version 12.0, Chicago, IL, USA). Comparison of genotype frequencies between cases and controls was assessed by χ2 test. ANOVA or t-test was used for analysis of means. All P values were two-sided and considered statistically significant at P<0.05.
In H pylori-positive cases, T/T genotype individuals had markedly decreased PGI/II ratio as compared with the other genotypes (F = 3.7, P = 0.03). On the contrary, no significant difference in PGI/II ratio was observed among the genotypical groups without H pylori infection. PGI level was similar in the three genotypes of infected subjects or non-infected subjects, although PGI level was lower in H pylori-positive subjects than that in negative subjects (Table 3).
| IL-1B-511 genotype | ||||
| C/C (n = 97) | C/T (n = 121) | T/T (n = 37) | ||
| H pylori+ | PGI | 38.5 ± 6.7 | 40.3 ± 7.2 | 38.0 ± 6.7 |
| (n=96) | PGI/II | 4.9 ± 0.4 | 5.0 ± 0.4 | 4.7 ± 0.31 |
| H pylori– | PGI | 23.6 ± 4.3 | 24.0 ± 5.0 | 23.5 ± 3.5 |
| (n=159) | PGI/II | 5.0 ± 0.3 | 4.9 ± 0.3 | 4.9 ± 0.4 |
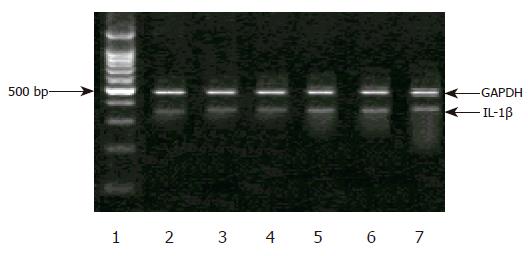
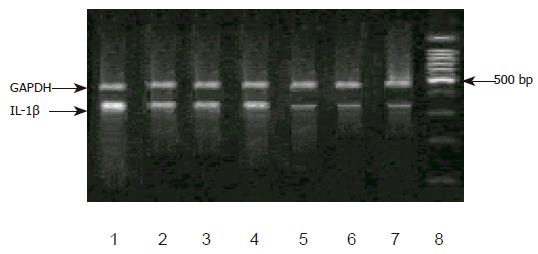
In H pylori-negative individuals, the levels of IL-1B mRNA expression were markedly decreased in the gastric antrum, and similar among each genotype. However, in H pylori-positive subjects, IL-1β mRNA expression was significantly increased, but there was no difference among C/C, C/T, or T/T genotype (Figures 1 and 2).
Furthermore, in the individuals without H pylori-infected corpus, no significant difference was detected in the expression of IL-1β mRNA among the genotype in IL-1B-511 single nucleotide polymorphism. In H pylori infection corpus, however, the level of IL-1β mRNA was higher. Individuals with T/T genotype had remarkably increased IL-1B mRNA than those with C/C or C/T genotype. No difference in IL-1B mRNA levels was observed between C/C and C/T genotypes. Meantime, we discovered a carrier of both IL-1B-511 T/T and -31 T/T genotypes, which had the highest IL-1B gene expression among all the subjects (Figures 1 and 2).
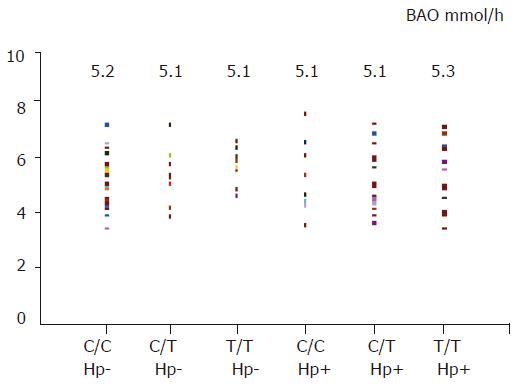
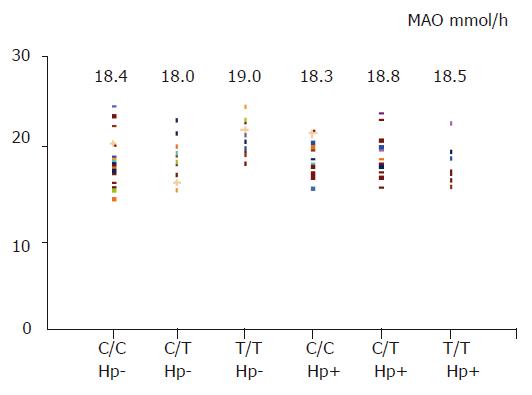
In basic condition (basic acid output), gastric acid secretion was similar between H pylori-negative and -positive subjects (5.1±1.0 vs 5.2±1.1 mmol/h, t = 0.48), and also among three different genotypes (six groups comparison, F = 0.2, Figure 3). After a pentagastrin stimulation, there was no significant difference for maximal acid output between H pylori-negative and -positive subjects (18.3±2.3 vs 18.4±2.0 mmol/h, t = 0.1), and among three different genotypes (six groups comparison, F = 0.7, Figure 4).
pH values of basic gastric juice and stimulated gastric fluid were 4.8±1.1 and 1.4±0.4, respectively. There was a slight increased pH value in H pylori infection subjects. Nevertheless, the individuals with T/T genotype did not show a much weaker ability to secrete hydrogen ion (Figure 5, F = 0.13 and 0.35).
Several studies revealed that hypochlorhydria induced by overexpression of IL-1B was regarded as a bridge to link IL-1 gene polymorphism to gastric cancer[4-7]. The ability of mucosa to secrete gastric acid has been considered as a critical factor to decide clinical outcomes and bacterial description of H pylori infection[14,15]. H pylori implanted predominantly in the antrum mucosa, a place with a higher pH value, contrarily increased acid environment of corpus mucosa that resulted from a large number of B lymphocytes provides a habitat for H pylori to grow. However, for some special individuals with genetic hypochlorhydria, H pylori has a chance to migrate from antrum to the corpus. As a result, a large ulcer area resulted in a decreased acid level, which accumulates gastric atrophy or stomach carcinoma development[9]. Schepp et al[16] reported that rat parietal cells express IL-1 receptors mediating inhibition of H+ production. The antisecretory effect of IL-1B may contribute to hypoacidity secondary to acute H pylori infection or during chronic colonization by H pylori preferring the fundic mucosa. Another study reported that the efficacy of IL-1B to inhibit parietal cell H+ production was 100-fold and 6 000-fold higher as compared to proton pump inhibitors and H2 antagonists, respectively[17]. Recently, it has been identified that IL-1B exerts a potent function of gastric acid secretion through the other molecules and protein kinase pathway[18,19].
Until recently, however, no research has provided unambiguous and direct proofs suggesting that lower acid output resulted from higher IL-1B level in the human body except from a rodent model.
In this study, we discovered that T/T genotype individuals infected with H pylori had a more decreased PGI/II ratio as compared with the other genotypes, which is partly consistent with the results reported by Furuta et al[6] suggesting that IL-1B-511 gene polymorphism might be a risk factor for gastric carcinoma. IL-1B mRNA expression in subjects without H pylori infection was not remarkable, and was similar in each genotype, showing that there might be a very low level of IL-1B mRNA expression in normal mucosa. In contrast, the results are more complex in H pylori-positive individuals. There was a remarkable upregulation in the antrum, but no relationship with genotype was observed. In the corpus mucosa, however, the level of IL-1B mRNA was markedly higher than that in the antrum mucosa. Furthermore, IL-1B-511 T/T genotype individual had a mild increased IL-1B mRNA level as compared with C/T and C/C genotype. So, these results indicated that this mutation of IL-1B-511 C→T might upregulate IL-1B mRNA expression in corpus but not in the antrum, as these results are consistent to the results reported by Hwang et al[20].
The association of IL-1B-31 locus and IL-1B expression is not investigated because genetically mutated subject is too small (about 1%) to be collected. Fortunately, we found one female subject with H pylori positive and IL-1B-511 T/T and -31 T/T genotypes simultaneously had a markedly increased IL-1B mRNA level in corpus but not in the antrum. These results suggested a cooperative effect between -511 and -31 locus in IL-1B mRNA expression. These findings are also in agreement with the previous results reported by El-Omar et al[4] and Hwang et al[20].
We discovered that in basic or pentagastrin stimulation condition, gastric acid secretion was similar between H pylori-negative and -positive subjects. Acid secretion function did not show a marked heterogeneity among three different genotypes of IL-1B-511. This data denoted that higher IL-1B level does not make a strong impact on gastric acid output. So, previous hypothesis that H pylori infection leads to IL-1B mRNA overexpression in IL-1B-511 T/T genotype, thereby directly inhibits gastric acid secretion is impossible, at least in vivo, in low gastric cancer prevalence region.
Hence, we inferred that the other pathways (such as chronic inflammatory process[21]), but not low acid secretion regulated by IL-1B (a pre-inflammatory factor), lead to mucosal atrophy. Our results, also taking into consideration the reports of several studies, suggested that IL-1B gene polymorphism was a susceptibility factor to gastric carcinoma in population-based studies. Furthermore, Lundell et al[21,22] have reported that acid-suppressive therapy maintained for 3 years facilitates neither the development of gastric glandular atrophy of the corpus mucosa nor the occurrence of intestinal metaplasia in H pylori-infected GERD patients. Fox et al[23] argued that coca leaf chewers (added with slaked lime or ash) did not have a higher prevalence of H pylori infection, or a higher rate of progression to gastric atrophy. So, we propose a new hypothesis that IL-1B gene polymorphism acts as a gastric cancer risk factor via upregulating IL-1B expression but downregulating acid secretion.
The individuals included in our study had a strong physiological captivity of acid output in the gastric mucosa as their age ranged from 19 to 24 years. This might be the reason that acid secretion levels were not seen significantly different between upregulated IL-1B mRNA and unchanged IL-1B mRNA groups. But using younger population in the present study is not unreasonable. As we know H pylori infection and genetic background play crucial roles in carcinogenesis beginning from early age. Further studies, however, should focus on larger age-range subjects or on other races to identify our results.
Science Editor Kumar M and Guo SY Language Editor Elsevier HK
| 1. | Stadtländer CT, Waterbor JW. Molecular epidemiology, pathogenesis and prevention of gastric cancer. Carcinogenesis. 1999;20:2195-2208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 133] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 470] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 3. | Rhyu MG. Genetic events underlying morphological complexity of gastric carcinoma. J Korean Med Sci. 1998;13:339-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1674] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 5. | Machado JC, Pharoah P, Sousa S, Carvalho R, Oliveira C, Figueiredo C, Amorim A, Seruca R, Caldas C, Carneiro F. Interleukin 1B and interleukin 1RN polymorphisms are associated with increased risk of gastric carcinoma. Gastroenterology. 2001;121:823-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 309] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 6. | Furuta T, El-Omar EM, Xiao F, Shirai N, Takashima M, Sugimura H. Interleukin 1beta polymorphisms increase risk of hypochlorhydria and atrophic gastritis and reduce risk of duodenal ulcer recurrence in Japan. Gastroenterology. 2002;123:92-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 262] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 7. | Zeng ZR, Hu PJ, Hu S, Pang RP, Chen MH, Ng M, Sung JJ. Association of interleukin 1B gene polymorphism and gastric cancers in high and low prevalence regions in China. Gut. 2003;52:1684-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 141] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | El-Omar EM, Oien K, El-Nujumi A, Gillen D, Wirz A, Dahill S, Williams C, Ardill JE, McColl KE. Helicobacter pylori infection and chronic gastric acid hyposecretion. Gastroenterology. 1997;113:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 399] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 9. | Xu GP, Reed PI. N-nitroso compounds in fresh gastric juice and their relation to intragastric pH and nitrite employing an improved analytical method. Carcinogenesis. 1993;14:2547-2551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Broutet N, Plebani M, Sakarovitch C, Sipponen P, Mégraud F. Pepsinogen A, pepsinogen C, and gastrin as markers of atrophic chronic gastritis in European dyspeptics. Br J Cancer. 2003;88:1239-1247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Stockbruegger RW. Bacterial overgrowth as a consequence of reduced gastric acidity. Scand J Gastroenterol Suppl. 1985;111:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Svendsen JH, Dahl C, Svendsen LB, Christiansen PM. Gastric cancer risk in achlorhydric patients. A long-term follow-up study. Scand J Gastroenterol. 1986;21:16-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Beales IL, Calam J. Inhibition of carbachol stimulated acid secretion by interleukin 1beta in rabbit parietal cells requires protein kinase C. Gut. 2001;48:782-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Loparev VN, Cartas MA, Monken CE, Velpandi A, Srinivasan A. An efficient and simple method of DNA extraction from whole blood and cell lines to identify infectious agents. J Virol Methods. 1991;34:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | McColl KE, el-Omar E, Gillen D. Helicobacter pylori gastritis and gastric physiology. Gastroenterol Clin North Am. 2000;29:687-703, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Schepp W, Dehne K, Herrmuth H, Pfeffer K, Prinz C. Identification and functional importance of IL-1 receptors on rat parietal cells. Am J Physiol. 1998;275:G1094-G1105. [PubMed] |
| 17. | Wolfe MM, Nompleggi DJ. Cytokine inhibition of gastric acid secretion--a little goes a long way. Gastroenterology. 1992;102:2177-2178. [PubMed] |
| 18. | Mahr S, Neumayer N, Gerhard M, Classen M, Prinz C. IL-1beta-induced apoptosis in rat gastric enterochromaffin-like cells is mediated by iNOS, NF-kappaB, and Bax protein. Gastroenterology. 2000;118:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Beales IL, Calam J. Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut. 1998;42:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 221] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Hwang IR, Kodama T, Kikuchi S, Sakai K, Peterson LE, Graham DY, Yamaoka Y. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1beta production in Helicobacter pylori infection. Gastroenterology. 2002;123:1793-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 292] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 21. | Lundell L, Miettinen P, Myrvold HE, Pedersen SA, Thor K, Andersson A, Hattlebakk J, Havu N, Janatuinen E, Levander K. Lack of effect of acid suppression therapy on gastric atrophy. Nordic Gerd Study Group. Gastroenterology. 1999;117:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 122] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11271] [Article Influence: 490.0] [Reference Citation Analysis (2)] |
| 23. | Fox JG, Wang TC. Reply to "The 'African enigma' - another explanation". Nat Med. 2000;6:1297-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |