Published online Jul 14, 2005. doi: 10.3748/wjg.v11.i26.4098
Revised: November 14, 2004
Accepted: November 19, 2004
Published online: July 14, 2005
AIM: To investigate the radiation response of various human tumor cells and normal liver cells.
METHODS: Cell lines of human hepatoma cells (SMMC-7721), liver cells (L02), melanoma cells (A375) and cervical tumor (HeLa) were irradiated with 60Co γ-rays. Cell survive was documented by a colony assay. Chromatid breaks were measured by counting the number of chromatid breaks and isochromatid breaks immediately after prematurely chromosome condensed by Calyculin-A.
RESULTS: Linear quadratic survival curve was observed in all of four cell lines, and dose-dependent increase in radiation-induced chromatid and isochromatid breaks were observed in GB2B phase. Among these four cell lines, A375 was most sensitive to radiation, while, L02 had the lowest radiosensitivity. For normal liver cells, chromatid breaks were easy to be repaired, isochromatid breaks were difficult to be repaired.
CONCLUSION: The results suggest that the γ-rays induced chromatid breaks can be possibly used as a good predictor of radiosensitivity, also, unrejoined isochromatid breaks probably tightly related with cell cancerization.
- Citation: Yang JS, Li WJ, Zhou GM, Jin XD, Xia JG, Wang JF, Wang ZZ, Guo CL, Gao QX. Comparative study on radiosensitivity of various tumor cells and human normal liver cells. World J Gastroenterol 2005; 11(26): 4098-4101
- URL: https://www.wjgnet.com/1007-9327/full/v11/i26/4098.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i26.4098
Liver cancer is one kind of malignancy tumor with the highest incidence and clinical risk, especially popular in China. As till now, radiotherapy is the most acceptable and effective method. Low LET (Linear energy transfer) rays, such as X-ray, γ-ray, were often used. Radiobiologists aim to develop an assay or a combinative assay to predict the radiation response of human cancers. Precise prediction of response to radiation could provide the basis for selecting and designing clinical treatment project.
Colony assay or growth assay has the good correlation with the radiation response[1-5]. But as a routine predictive assay, it will take at least 2 or 3 weeks to form the clone, is unlikely to be used for clinical diagnosis and treatment.
The PCC (premature chromosome condensation) technique is very useful for measuring the radiation-induced chromatin breaks in all of the cell cycles[6-9]. especially in G2 phase. In the present study, we investigated the G2 phase radiosensitivity of human hepatoma cells and normal liver cells, compared with other two type of human malignancy tumors. The results showed that the radiosensitivity of normal liver cells is lowest among these four cell lines, suggest that in the course of liver cancer radiotherapy, liver cells will less injured than hepatoma cells and other two tumor cell lines when absorbed same dose of radiation.
Four cell lines (From CCTCC) were grown in RPMI-1640 medium supplemented with 10% fetal calf serum at 37°C in 50 mL/L CO2, additional 0.25 U/mL insulin was added into the L02 culture medium.
Exponentially growing cells were irradiated with γ-rays generated by 60Co source with a dose rate 0.2 Gy/min at Lanzhou medicine college, dose range was from 0 to 8 Gy.
Calyculin-A (BIOMOL America) was used as the PCC inducer, which was dissolved in 100% ethanol as 1 mmol/L stock solution; 50 nmol/L of Calyculin-A was added to the cell cultures before irradiation to score the initial chromatid breaks. Then, cells were incubated for a further 30 min at 37°C in 50 mL/L CO2. Chromosome spreads were then harvested by swelling cells in 75 mmol/L KCl for 20 min at 37°C and fixed with Carnoy’s fixation. A final wash and fixation in the same fixative was completed before dropping cells onto a glass slide and hot humidity drying. Chromosome was stained with 5% Giemsa for 20 min.
More than 40 G2 phase cells were scored for each dose point according to the standard criteria[10]. Briefly, chromatid discontinuing, misalignment of the distal to the lesion, or a non-stained region longer than the chromatid width was classified as a break. Isochromatid breaks were scored two breaks. The total chromatid breaks were calculated by summing the production of chromatid and isochromatid breaks. The process of kinetic repair of damaged chromosome by PCC technique was same as initial ones.
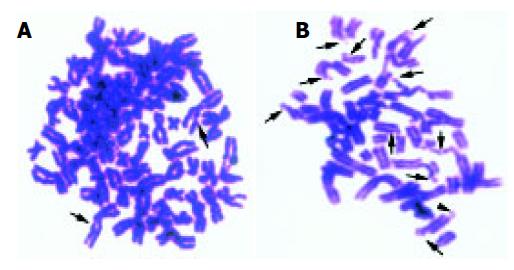
Calyculin A is a phosphatase inhibitor, can effectively induce the cells into G2 phase by very little time. It is easy to detect the chromosome damage using PCC technique. Figure 1 shows the G2 PCC chromosome spreads of L02 cell lines exposed to 8 Gy of γ-rays and SMMC-7721 cell lines of 0.5 Gy.
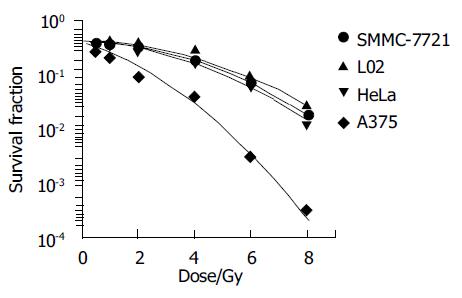
Colony assay is a routine method for analyzing the cell survival fraction. Classical model of cell survival curve after exposed to low LET rays, such as X-ray, γ-ray, was linear quadratic. In present study, four cell lines’ fitted survival curve were well agreement with the linear quadratic model. Figure 2 shows the detail of it.
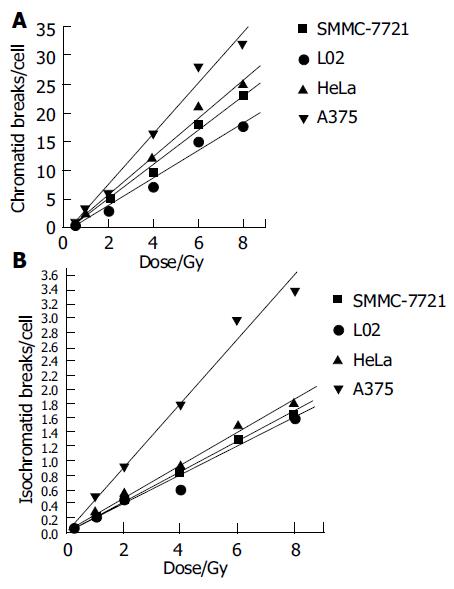
Figures 3A and B express the chromatid and isochromatid breaks of four cell lines after irradiated with various absorbed dose. Though some inter-exchanges or inner-exchanges exist in chromosome spreads, its number was rare. We only observed the main chromosome break type, i.e., chromatid and isochromatid breaks. Chromatid and isochromatid breaks linearly increased with the dose increase of these four cell lines, the number of chromatid breaks was much more than that of isochromatid breaks at each dose point.
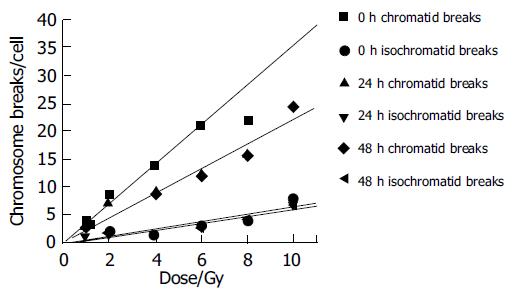
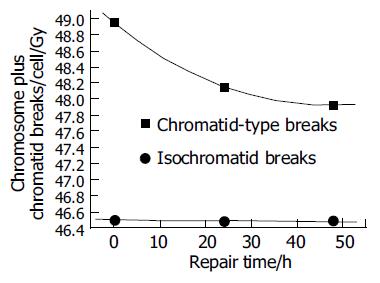
Figures 4 and 5 show the unrejoined chromatid breaks/ cell/Gy at 0, 24, and 48 h. In this kinetic process, almost 50% chromatid breaks got repaired, for isochromatid ones, just 15% got repaired at most. Also we can see, during 24-48 h, both of these two type breaks were nearly the same level, i.e., from 2.16 to 1.93 breaks/cell/Gy for chromatid breaks, from 0.476 to 0.48 breaks/cell/Gy for isochromatid ones. However, from 0 to 24 h these values were 2.95-2.16 breaks/cell/Gy, 0.502-0.476 breaks/cell/Gy, respectively. Compared with chromatid breaks, isochromatid breaks nearly keep the original state within 48 h after exposed to radiation. This is to say, at the early 24 h after exposure, chromosome repair process finished.
Liver cancer is of high risk and mortality. Besides the surgical excision, radiotherapy is the main method after the discovery of X-rays. Before radiotherapy, prompt and exact detection of radiosensitivity of target cells is of great importance.
Many researchers have reported a linear-quadratic cell survival curve in various cell lines irradiated by X-rays, γ-rays, also, linear relationship between chromosome breaks and absorbed dose was discovered[5,8,9,11-15]. With the introduction of the premature chromosome condensation technique[16-18], it is easy to study initial radiation-induced chromosome damage (Figure 1).
In the present study, fitted cell survival curve of four cell lines were linear quadratic (Figure 2), which were consilient with previous studies[8-9]. Among these four cell lines, survival fraction of L02 was highest, A375 was lowest after absorbed same dose of radiation, suggested that human normal liver cells was of much higher resistance to γ-rays than human hepatoma cells, melanoma cells and cervical tumor cells. Radiation injury mainly resulted from oxidative free radicals, reductive glycogens synthesized in liver cells which interact with free radicals reduce the radiation injury.
Linear relationship was found between dose and the chromatid breaks after the cells were exposed to 60Co γ-rays in this study (Figure 3). This relationship has proved by previous studies[5,8,14,19,20]. An increased production of chromatid breaks induced by X-ray irradiation has been reported by Durante[21-23], an increased production of isochromatid breaks produced by exposure to X-ray was also reported by Kawata[8,9]. It suggested that with the higher dose, the electrons which hit the target-chromosome increased, so, the production increased. While, the absolutely production increase of isochomatid breaks was smaller than that of chromatid breaks. Kawata et al[8] has reported that after low LET irradiation, the chromatid breaks dominated, while for high LET rays, such as heavy ions, isochromatid breaks dominated, suggesting that most isochromatid breaks resulted from two separate breaks on sister chromatids induced by independent electron tracks. For low LET rays, it can not deposit enough energy during unit range to penetrate sister chromatids meantime, so , most breaks were chromatid ones.
Since normal liver cells were more radioresistant to γ-rays than other three kind of cancer cells, study the kinetic repair of chromosome damage of L02 cells has the essentiality. Data[4,9,15,16] showed that these irradiation could induce the chromosome damage, the damage will probably result in cancerization. Correct and effective repair of injured chromosome is very important to stop cancerization course. Figures 4 and 5 show the correlation between chromatid breaks and dose at 0, 24 and 48 h after radiation. In the present study, at each time point, chromatid breaks and isochromatid breaks were increased linearly with the dose, and the absolute number of chromatid breaks was much more than that of isochromatid ones, this phenomenon was described by Kawata et al[8]; Suzuki et al[15] reported that this repairing process occurred during the early 10 h after irradiation. This means in the early 24 h, most of any kind of cells end their spontaneous repair process if they are injured. After 24 h, without any artificial factors, injured and normal cells will be stable and continue synthesizing, mitosing, and so on.
Though the radiotherapy by using common irradiation such as γ-ray is very effective, we could not neglect the side-effect which largely reduce the curative effect due to the injury of normal tissue. Heavy ions have the good biophysics characteristics such as Bragg peak of dose distribution which result in the lowest side scatter, higher RBE (relative biological effect) and lower OER (oxygen enhancement ratio). Present study will enrich the essential data for the Heavy-Ion-Radiotherapy project in the Institute of Modern Physics, Chinese academy of Sciences when the national great engineering HIRFL-CSR (heavy ion research facility of Lanzhou-cooling restore cycle) complete at 2005.
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Girinsky T, Bernheim A, Lubin R, Tavakoli-Razavi T, Baker F, Janot F, Wibault P, Cosset JM, Duvillard P, Duverger A. In vitro parameters and treatment outcome in head and neck cancers treated with surgery and/or radiation: cell characterization and correlations with local control and overall survival. Int J Radiat Oncol Biol Phys. 1994;30:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | West CM, Davidson SE, Burt PA, Hunter RD. The intrinsic radiosensitivity of cervical carcinoma: correlations with clinical data. Int J Radiat Oncol Biol Phys. 1995;31:841-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | West CM, Davidson SE, Roberts SA, Hunter RD. The independence of intrinsic radiosensitivity as a prognostic factor for patient response to radiotherapy of carcinoma of the cervix. Br J Cancer. 1997;76:1184-1190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Coco Martin JM, Mooren E, Ottenheim C, Burrill W, Nunez MI, Sprong D, Bartelink H, Begg AC. Potential of radiation-induced chromosome aberrations to predict radiosensitivity in human tumour cells. Int J Radiat Biol. 1999;75:1161-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Kawata T, Ito H, George K, Wu H, Uno T, Isobe K, Cucinotta FA. Radiation-induced chromosome aberrations in ataxia telangiectasia cells: high frequency of deletions and misrejoining detected by fluorescence in situ hybridization. Radiat Res. 2003;159:597-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Gotoh E, Kawata T, Durante M. Chromatid break rejoining and exchange aberration formation following gamma-ray exposure: analysis in G2 human fibroblasts by chemically induced premature chromosome condensation. Int J Radiat Biol. 1999;75:1129-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Durante M, Furusawa Y, George K, Gialanella G, Greco O, Grossi G, Matsufuji N, Pugliese M, Yang TC. Rejoining and misrejoining of radiation-induced chromatin breaks. IV. Charged particles. Radiat Res. 1998;149:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Kawata T, Gotoh E, Durante M, Wu H, George K, Furusawa Y, Cucinotta FA. High-LET radiation-induced aberrations in prematurely condensed G2 chromosomes of human fibroblasts. Int J Radiat Biol. 2000;76:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Kawata T, Durante M, Furusawa Y, George K, Takai N, Wu H, Cucinotta FA. Dose--response of initial G2-chromatid breaks induced in normal human fibroblasts by heavy ions. Int J Radiat Biol. 2001;77:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Savage JR. Classification and relationships of induced chromosomal structual changes. J Med Genet. 1976;13:103-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 370] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Pantelias GE, Maillie HD. Direct analysis of radiation-induced chromosome fragments and rings in unstimulated human peripheral blood lymphocytes by means of the premature chromosome condensation technique. Mutat Res. 1985;149:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Bedford JS, Goodhead DT. Breakage of human interphase chromosomes by alpha particles and X-rays. Int J Radiat Biol. 1989;55:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Goodwin EH, Blakely EA, Tobias CA. Chromosomal damage and repair in G1-phase Chinese hamster ovary cells exposed to charged-particle beams. Radiat Res. 1994;138:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Cornforth MN, Goodwin EH. The dose-dependent fragmentation of chromatin in human fibroblasts by 3.5-MeV alpha particles from 238Pu: experimental and theoretical considerations pertaining to single-track effects. Radiat Res. 1991;127:64-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Suzuki M, Watanabe M, Suzuki K, Nakano K, Matsui K. Heavy ion-induced chromosome breakage studied by premature chromosome condensation (PCC) in Syrian hamster embryo cells. Int J Radiat Biol. 1992;62:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Suzuki M, Watanabe M, Kanai T, Kase Y, Yatagai F, Kato T, Matsubara S. LET dependence of cell death, mutation induction and chromatin damage in human cells irradiated with accelerated carbon ions. Adv Space Res. 1996;18:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Suzuki M, Kase Y, Kanai T, Yatagai F, Watanabe M. LET dependence of cell death and chromatin-break induction in normal human cells irradiated by neon-ion beams. Int J Radiat Biol. 1997;72:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Johnson RT, Rao PN. Mammalian cell fusion: induction of premature chromosome condensation in interphase nuclei. Nature. 1970;226:717-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 455] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Hittelman WN, Rao PN. Premature chromosome condensation. I. Visualization of x-ray-induced chromosome damage in interphase cells. Mutat Res. 1974;23:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 90] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Cornforth MN, Bedford JS. X-ray--induced breakage and rejoining of human interphase chromosomes. Science. 1983;222:1141-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 123] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Pandita TK, Hittelman WN. The contribution of DNA and chromosome repair deficiencies to the radiosensitivity of ataxia-telangiectasia. Radiat Res. 1992;131:214-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Sasai K, Evans JW, Kovacs MS, Brown JM. Prediction of human cell radiosensitivity: comparison of clonogenic assay with chromosome aberrations scored using premature chromosome condensation with fluorescence in situ hybridization. Int J Radiat Oncol Biol Phys. 1994;30:1127-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Durante M, Gialanella G, Grossi GF, Nappo M, Pugliese M, Bettega D, Calzolari P, Chiorda GN, Ottolenghi A, Tallone-Lombardi L. Radiation-induced chromosomal aberrations in mouse 10T1/2 cells: dependence on the cell-cycle stage at the time of irradiation. Int J Radiat Biol. 1994;65:437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |