Published online May 21, 2005. doi: 10.3748/wjg.v11.i19.3005
Revised: October 10, 2002
Accepted: November 13, 2002
Published online: May 21, 2005
AIM: To determine whether mild hypothermia could protect liver against ischemia and reperfusion injury in pigs.
METHODS: Twenty-four healthy pigs were randomly divided into normothermia, mild hypothermia and normal control groups. The experimental procedure consisted of temporary interruption of blood flow to total hepatic lobe for different lengths of time and subsequent reperfusion. Hepatic tissue oxygen pressure (PtiO2) and aspartate aminotransferase (AST) values were evaluated, and ultrastructural analysis was carried out for all samples.
RESULTS: Serum AST was significantly lower, and hepatic PtiO2 values were significantly higher in the mild hypothermia group than in the normothermia group during liver ischemia-reperfusion periods (P = 0.032, P = 0.028). Meanwhile, the histopathologic injury of liver induced by ischemia-reperfusion was significantly improved in the mild hypothermia group, compared with that in the normothermia group.
CONCLUSION: Mild hypothermia can protect the liver from ischemia-reperfusion injury in pigs.
- Citation: Wang CY, Ni Y, Liu Y, Huang ZH, Zhang MJ, Zhan YQ, Gao HB. Mild hypothermia protects liver against ischemia and reperfusion injury. World J Gastroenterol 2005; 11(19): 3005-3007
- URL: https://www.wjgnet.com/1007-9327/full/v11/i19/3005.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i19.3005
Hepatic vascular exclusion is a major performance of hepatic resection with minimal intraoperative blood loss. Interruption of hepatic inflow is required during extension liver resection. Temporary vascular inflow occlusion by clamping of the hepatic pedicle (Pringle maneuver) is an effective measure to control blood loss during the operative procedure because it is associated with the least hemodynamic consequences and side effects. However, liver dysfunction is still a relatively frequent complication of clinical surgeries as a result of liver ischemic reperfusion[1,2]. Therefore, the ability to improve liver tolerance to ischemia is clinically important.
Several pathways for ischemic reperfusion injury have been postulated, such as oxygen-derived free radicals[3], calcium influx[4], adenosine triphosphate depletion, mitochondrion dysfunction, activation of lysosomal enzymes and disturbance of microcirculation[5,6]. However, the precise mechanisms of hepatic injury remain obscure. It has been shown that ischemic reperfusion injury in the liver first becomes evident in microvascular system particularly in sinusoidal endothelial cells of the liver. Because intrahepatic tissue oxygenation depends on delivery of oxygen by the liver microvasculature, microcirculatory disturbances due to ischemia-reperfusion injury will result in tissue oxygen changes in the liver parenchyma[7]. The partial pressure of oxygen (the PtiO2), which is physically dissolved in interstitial fluid, corresponds to the availability of oxygen at cellular level. The PtiO2 value not only follows metabolic rate, perfusion and microcirculation, but also reflects the functional number of capillaries per tissue volume, the highly variable O2 diffusion in capillary wall, parenchyma and intercellular substance.
The experimental and clinical findings suggest that hypothermic perfusion technique and topical cooling of liver in hepatic resection may be very useful in preserving hepatocytes and sinusoidal endothelial cells and in maintaining stability of the systemic or hepatic circulation after reperfusion[8,9]. In recent years, mild hypothermia has been proved to be of protective role in brain injury with few systemic side-effects. In this study, we evaluated the role of mild hypothermia in liver ischemia-reperfusion injury.
Twenty-four healthy pigs weighing 30-35 kg with male/female ratio of 1:1 (kindly provided by Experimental Animal Center, Sun Yat-Sen University of Medical Sciences, Guang-zhou, China) were included in the study. All pigs were acclim-atized for at least 7 d before the experiment and allowed free access to food and water. The animals were randomly divided into three experimental groups: normothermia, mild hypothermia, and normal control groups.
All surgical procedures were carried out under intravenous anesthesia. The pigs underwent laparotomy with a median incision, and the inflow of liver blood was occluded by the Pringle’s method, i.e., the liver was exposed after supra-median incision into the abdomen, and the hepatic artery, portal vein and common bile duct in the hepato-duodenal ligament were clamped off with non-injury clamps. When the clamps were taken away, the inflow of liver blood was resumed, which is termed as reperfusion. In group A (normothermia group), the inflow of liver blood was occluded for 30 min at normothermia (normal body tempe-rature), and reperfusion was performed for 60 min. In group B (mild hypothermia group), the liver and rectal temperatures were lowered down to 33-35 °C with cooling blanket before liver ischemia, while other treatments were the same as for group A. In group C (controlled group), the liver and hepato-duodenal ligament was exposed at normothermia without interruption of the inflow of liver blood.
The pigs were cooled with cooling blankets (Kangnuo Tech Co. Ltd, Beijing, China) for 1-2 h to reach a temperature in rectal and liver of 34-35 °C (±0.2 °C) with subsequent use of light flash to maintain the temperature.
PtiO2 was measured in each animal by LICOX CMP monitors (Kiel-Mielkendorf Co. Ltd, Germany) with a polarographic microcatheter PtiO2 probe and a micromputer[10]. After calibration in a saline bath, the PtiO2 probe was inserted into the hepatic parenchyma of the middle lobe using an 18-gauge venous cannula. PtiO2 was measured serially before and during the ischemia period (at 10, 20, and 30 min) and after reperfusion at two different points (30 and 60 min).
The serum levels of aspartate aminotransferase (AST) were determined by an autonomic-physiotechnic monitor. Venous blood samples for AST were drawn before ischemia, 30 min after ischemia and 60 min after reperfusion.
Liver tissues were collected before ischemia, 30 min after ischemia and 60 min after reperfusion from the left and right of the liver, and fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin. They were observed under a light microscope to examine histopathological changes.
To study the ultrastructural changes of hepatocytes, liver tissues from all groups were fixed for observation under transmission electron microscope (TEM). The cut samples for TEM underwent technical procedures as previously described[11]. Thin sections were observed under HITACH-H600 electron microscope.
The data were presented as mean±SE, the differences of these groups were compared by a t-test. P<0.05 was considered statistically significant.
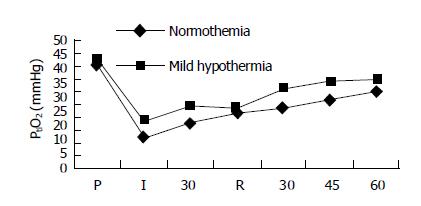
The hepatic PtiO2 values of three groups before ischemia were 43.3±6.9, 42.7±9.5, and 41.9±8.2 mmHg (1 mmHg = 0.133 kPa) respectively. There were no significant differences among these groups (P>0.05). The liver PtiO2 value declined to 18.6±2.8 mmHg at 30 min after ischemia in group A, but increased slowly to 30.9±2.6 mmHg 60 min after reperfusion. There were significant differences between the ischemic and non-ischemic periods (P<0.05). In group B, the hepatic PtiO2 value was 28.7±1.9 mmHg 30 min after ischemia and 37.9±5. mmHg 60 min after reperfusion. There were significant differences between the groups in pre-ischemia and reperfusion periods (P<0.05). Meanwhile, there were significant differences in each stage of ischemia-reperfusion of pig livers between groups A and B (P<0.05, Figure 1).
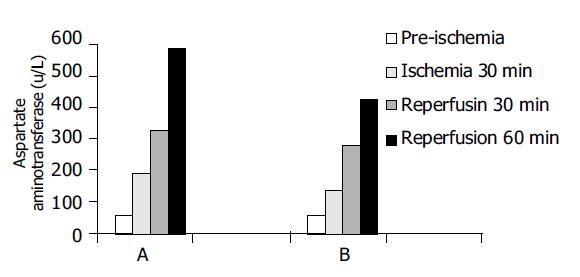
Serum AST values are shown in Figure 2. AST values were 42.6±5.7, 46.2±3.9, and 49.0±6.8 U/L, respectively, before ischemia in groups A, B, and C. There were no significant differences between the groups (P>0.05). However, AST values at 30 min after ischemia, 30 and 60 min after reperfusion were significantly different between groups A and B (P<0.05).
Before ischemia, the histopathological structures were normal in three groups. In the ischemic group of normothermia, light microscopy showed swelling of hepatocytes, stenosis of hepatic sinus, infiltration of inflammatory cells in the interstitial tissue and dotted necrosis of hepatocytes after 30 min of ischemia. The swelling of hepatocytes was even worse and local necrosis of hepatocytes could be observed 60 min after reperfusion. In the mild hypothermia group, the pathological changes of liver were significantly lighter compared to the normothermia group during the periods of ischemia and reperfusion.
Under electromicroscope, swelling of mitochondria and enlargement of endoplasma were observed 30 min after ischemia, even worse swelling, destruction, bubble-degeneration of mitochondria, and degranulation of rough endoplasm were found 60 min after reperfusion. However, the mild hypothermia group only showed widening and swelling of mitochondria with complete membrane and few changes in nuclear membrane and nucleoli.
The induction of hepatic hypothermia began with whole-body cooling in experimental models in 1953 and clinically in 1961. It was designed to minimize the ischemia-reperfusion injury associated with hepatic inflow occlusion. Body surface cooling and cooling via an extracorporeal circuit, however, are not widely accepted for hepatic surgery because of the adverse effects on extrahepatic organs[12-15]. In this study, we found that hypothermia had a protective effect against hepatic ischemia-reperfusion injury in pigs. Our study showed that the PtiO2 values during ischemia and reperfusion in group B were significantly higher than those in group A. It has been previously shown that intrahepatic PtiO2 as an indicator of microvascular perfusion is a parameter of early ischemia-reperfusion injury[6,16-18]. Microcirculatory disturbances during ischemia and reperfusion period might induce local hypoxia in the liver, thereby causing liver damage. We postulate that mild hypothermia might decrease hepatic oxygen consumption during ischemic and attenuate endothelial injury and microcirculatory dysfunction in reperfusion period, and then increase hepatic tissue oxygenation and reduce liver damage.
Liver damage associated with different models of ischemia-reperfusion injury is most commonly characterized by the release of hepatocellular enzymes into extracellular fluids. AST is a comparatively sensible parameter reflecting the injury of liver cells, and serum AST values reflect the extent of injury in liver cells. Mild hypothermia is associated with reduction in serum AST after reperfusion. This means that a relatively well maintained state of hepatic cells by mild hypothermia during ischemia leads to a lesser degree of liver cell injury following reperfusion. The data indicate that the decrease in mitochondria destruction, hepatocyte degeneration and necrosis due to mild hypothermia also presumably contributes to an increase in hepatic tissue PtiO2, resulting in attenuation of tissue damage following hepatic injury.
In conclusion, mild hypothermia prevents hepatic injury after ischemia-reperfusion in pigs, and may play a crucial role in clinical situations associated with liver dysfunction after ischemia-reperfusion.
| 1. | St Peter SD, Post DJ, Rodriguez-Davalos MI, Douglas DD, Moss AA, Mulligan DC. Tacrolimus as a liver flush solution to ameliorate the effects of ischemia/reperfusion injury following liver transplantation. Liver Transpl. 2003;9:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Takahashi Y, Ganster RW, Gambotto A, Shao L, Kaizu T, Wu T, Yagnik GP, Nakao A, Tsoulfas G, Ishikawa T. Role of NF-kappaB on liver cold ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1175-G1184. [PubMed] |
| 3. | Jin MB, Todo S. The role of endothelin-1 in hepatic ischemia and reperfusion injury. J Gastroenterol. 2002;37:763-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Khandoga A, Biberthaler P, Enders G, Axmann S, Hutter J, Messmer K, Krombach F. Platelet adhesion mediated by fibrinogen-intercelllular adhesion molecule-1 binding induces tissue injury in the postischemic liver in vivo. Transplantation. 2002;74:681-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Khandoga A, Enders G, Biberthaler P, Krombach F. Poly(ADP-ribose) polymerase triggers the microvascular mechanisms of hepatic ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2002;283:G553-G560. [PubMed] |
| 6. | Pannen BH. New insights into the regulation of hepatic blood flow after ischemia and reperfusion. Anesth Analg. 2002;94:1448-1457. [PubMed] |
| 7. | Serafín A, Roselló-Catafau J, Prats N, Xaus C, Gelpí E, Peralta C. Ischemic preconditioning increases the tolerance of Fatty liver to hepatic ischemia-reperfusion injury in the rat. Am J Pathol. 2002;161:587-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 168] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Heijnen BH, Elkhaloufi Y, Straatsburg IH, Van Gulik TM. Influence of acidosis and hypoxia on liver ischemia and reperfusion injury in an in vivo rat model. J Appl Physiol (1985). 2002;93:319-323. [PubMed] |
| 9. | Biberthaler P, Luchting B, Massberg S, Teupser D, Langer S, Leiderer R, Messmer K, Krombach F. The influence of organ temperature on hepatic ischemia-reperfusion injury: a systematic analysis. Transplantation. 2001;72:1486-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | van Wagensveld BA, van Gulik TM, Gelderblom HC, Scheepers JJ, Bosma A, Endert E, Gouma DJ. Prolonged continuous or intermittent vascular inflow occlusion during hemihepatectomy in pigs. Ann Surg. 1999;229:376-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Atila K, Coker A, Sagol O, Coker I, Topalak O, Astarcioglu H, Karademir S, Astarcioglu I. Protective effects of carnitine in an experimental ischemia-reperfusion injury. Clin Nutr. 2002;21:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Imber CJ, St Peter SD, Lopez de Cenarruzabeitia I, Pigott D, James T, Taylor R, McGuire J, Hughes D, Butler A, Rees M. Advantages of normothermic perfusion over cold storage in liver preservation. Transplantation. 2002;73:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 175] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Kato A, Singh S, McLeish KR, Edwards MJ, Lentsch AB. Mechanisms of hypothermic protection against ischemic liver injury in mice. Am J Physiol Gastrointest Liver Physiol. 2002;282:G608-G616. [PubMed] |
| 14. | Zhang Y, Zhang B, Pan R. Protective effect of ischemic preconditioning on liver. Chin J Traumatol. 2001;4:123-125. [PubMed] |
| 15. | Ricciardi R, Veal TM, Anwaruddin S, Wheeler SM, Foley DP, Donohue SE, Quarfordt SH, Meyers WC. Porcine hepatic phospholipid efflux during reperfusion after cold ischemia. J Surg Res. 2002;103:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 16. | Poggetti RS, Moore EE, Moore FA, Koike K, Banerjee A. Gut ischemia/reperfusion-induced liver dysfunction occurs despite sustained oxygen consumption. J Surg Res. 1992;52:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Vollmar B, Glasz J, Post S, Menger MD. Role of microcirculatory derangements in manifestation of portal triad cross-clamping-induced hepatic reperfusion injury. J Surg Res. 1996;60:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Vollmar B, Glasz J, Leiderer R, Post S, Menger MD. Hepatic microcirculatory perfusion failure is a determinant of liver dysfunction in warm ischemia-reperfusion. Am J Pathol. 1994;145:1421-1431. [PubMed] |