Published online Dec 15, 2004. doi: 10.3748/wjg.v10.i24.3670
Revised: February 26, 2004
Accepted: March 4, 2004
Published online: December 15, 2004
AIM: To study the inhibitory effect of transfected PTEN on LoVo cells.
METHODS: Human PTEN cDNA was transferred into LoVo cells via lipofectin and PTEN mRNA levels and its expression were analyzed by Western blot and flow cytometry. Before or after transfection, the effects of 5-Fu on inhibiting cell proliferation and inducing apoptosis were measured by flow cytometry, DNA bands and MTT.
RESULTS: PTEN transfection significantly up-regulated PTEN expression in LoVo cells. 5-Fu inhibited cell proliferation and induced apoptosis in transfected LoVo cells.
CONCLUSION: Transfected PTEN can remarkably up-regulate PTEN expression in LoVo cells and promote the apoptosis. PTEN transfection is associated with 5-Fu treatment effect and has a cooperatively cytotoxic effect.
- Citation: Xu SS, Shen WL, Ouyang SY. Inhibition of transfected PTEN on human colon cancer. World J Gastroenterol 2004; 10(24): 3670-3673
- URL: https://www.wjgnet.com/1007-9327/full/v10/i24/3670.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i24.3670
Apoptosis and abnormal proliferation play a critical role in the development of carcinoma[1-8]. 5-Fu is one of the most popular drugs in colon cancer treatment. In vivo 5-Fu converts into fluorouracil deoxynucleotide and inhibits thymidylic acid synthetase and consequently, hinders deoxyuridylic acid into deoxythymidylic acid catalyzed by the enzyme thus blocking DNA synthesis and inducing apoptosis in cancer cells. The effect of phosphatase and tensin homologue deleted on chromosome 10 (PTEN)[9-21] transfection associated with 5-Fu on LoVo cells in human colon cancer was studied.
Human LoVo cells and PTEN plasmid pcDNA-PTEN-WT,wild type-WT, plasmid DNA extraction kit were purchased from Hangzhou Weitejie BioTech Co. The samples of normal human tissue and highly malignant colon cancer tissues were obtained from 461 Hospital of PLA and immediately stored in liquid nitrogen. Plasmid pcDNA3.1 (+) of eukaryonal expression vectors was from Invitrogen. E.coli DH5α, LoVo were stored in our laboratory. Diethyl pyrocarbonate (DEPC) was from Sigma. Total RNA was extracted by Trizol (Gibco). Oligo dT, TaKaRa RNA PCR Kit (AMV) pMD 18-T, Ex TaqTM enzyme (including dNTPs and Mg2+), DNA Ligase Kit Ver.2, DNA marker were from TaKaRa. LipofectamineTM 2000 transfection reagent was from Invitrogen. DNA sequence kit was ABI BigDye terminator kit. DNA fragment extraction and purification kit was from Beijing Dingguo Bio Corp. Rabbit-anti-human PTEN polyclonal antibody (rabbit anti-PTEN) was from Beijing Zhongshan Bio Corp. Horseradish enzyme labeled goat-anti-rabbit IgG (H + L) was obtained from Beijing Zhongshan Biotechnology Co., LTD.
Reagents and enzymes Restriction enzymes Bam HI and Hind III, basic proteolytic enzyme, DNA kit, Lipofectin, G418were osed.
Primers The primers were designed according to human PTEN sequence published in GenBank using Primer Premier 5.0 software. The upper stream primer was 5’AAGCTTATGACAGCCATCATCAAAGAGAT3’ with Hind III digestion site. The down stream primer was 5’-GGATCCGGAATAAAACGGGAAAGTGCC£-3’ with BamH I digestion site. The primers were synthesized by Beijing Baisheng Bio Corp.
RNA extraction One hundred milligrams of the samples in liquid nitrogen were taken. Total RNA was extracted by the protocol of Trizol.
RT-PCR amplification of PTEN gene It was carried out by TaKaRa RNA PCR kit. The reaction mixture was 20 µL. Reaction condition was at 30 °C for 10 min, 45 °C for 40 min, 95 °C for 2 min, 5 °C for 5 min. The primer was designed as above. Amplification condition was at 94 °C for 5 min, 94 °C for 30 s, 53 °C for 30 s, 72 °C for 1 min, 72 °C for 10 min. After the reaction 5 µL was taken for agarose gel electrophoresis.
PTEN clone and identification Fifty microliters of PCR product were used for 1% agarose gel electrophoresis to extract cDNA fragment and after extraction it was identified by agarose gel electrophoresis. cDNA fragment was inserted into pMD18-T vectors and ligated at 16 °C overnight to construct the recombinant plasmids pMD-PTEN-WT (wild type) and pMD-PTEN-MT (mutant type). The recombinant plasmids transfected competent DH5α cells by CaCl2. The transfected cells were cultivated in 50 mg/L ampicillin (Amp) LB agar plates at 37 °C for 14-16 h. Single colonies were inoculated in LB liquid medium with Amp at 37 °C overnight. The plasmids were extracted from cell culture. The positive clones were selected and doubly digested by Hind III and BamH at 37 °C for 1 h. After digestion it was identified by electrophoresis and the sequences were verified by ABI 3100 and the results were analysed by DNAsis software.
Construction and identification of eukaryonal expression vectors Recombinant plasmids with correct sequences were doubly digested by Hind III and BamH I. The product was used for agarose gel electrophoresis to extract 1.4 kb PTEN fragments. pcDNA3.1 (+) was also doubly digested by Hind III and BamH I and extracted. The linear vector fragments and extracted PTEN fragments were ligated by T4 DNA ligase at 16 °C overnight and the product was transferred into competent DH5α cells. Single colonies were randomly selected and cultivated at 37 °C overnight. Plasmids were extracted and amplified by PCR and identified by digestion.
Transfection According to LipofectamineTM 2000 transfection kit, pcDNA3.1 (+) vacant vector and none-transfected LoVo cells served as controls. After transfection the cells were grown at 37 °C with 50 mL/L CO2 for 4-6 h and 2 mL of medium supplemented with 10% calf serum was changed and cultivated for 48 h. Four to ten cells were transferred and incubated. When the cells were 80% in confluence, 600 µg/mL G418 was added to select the positive clones.
Cell growth The cells were digested by 0.25% trypsin to get monocyte suspension and inoculated into 6 well plates at 1 × 104/well. Seven wells were for each kind of cells. The medium was changed after 2 d. Cells were counted every day. The cell growth was observed for 7 d and cell growth curve was obtained.
Detection of PTEN gene expression Total RNA extracted was used as template for reverse transcription. The reaction system and condition were described as above. Western assay was performed according to the reports[22-24], horseradish peroxidase-labeled rabbit-anti-human IgG was used as the second antibody.
Gene preparation Total RNA was extracted from tumor and normal tissues. PCR product was collected and ligated into pMD 18-T vectors and then transformed. The constructs were verified by DNA sequencing.
Cell culture Frozen cells were taken out of liquid nitrogen and rapidly melted at 37-40 °C for 1 min in water bath. The tube of cells stored was opened and the cell suspension was transferred into the culture plate. After the medium was added the cells were grown at 37 °C. The medium was changed after cell growing on the wall (around 4 h). After the cells were cultivated into a single layer, the next generation was grown. The restored cells were transferred onto the 35 mm plate and cultivated at 37 °C with 50 mL/L CO2 for 18 h. The cells were digested by 0.25% pancreatin and cultured in 2 mL of DMEM supplemented with 10% fetal calf serum to stop digestion. The cells were cultured for 12 h. When the cells were in 30%-50% confluence, they were transfected by plasmid DNA.
Western blot Total RNA was extracted with Trizol from transfected LoVo cells or non-transfected cells. Five microliters of samples were separated by electrophoresis, transblotted, prohybridized, hybridized and stained.
PTEN protein expression Before or after transfection PTEN protein expression was assayed by flow cytometry. The cells were resuspended in PBS, PTEN monoclonal IgG1 antibody was labeled and incubated at room temperature for 30 min and analyzed by flow cytometry.
DNA ladder bands assay 5-Fu at 20 µL/mL was added into PTEN cells transfected or non-transfected and incubated for 48 h and centrifuged. DNA was extracted from the cells and electrophoresis was performed in 2% agar gel in 0.5 × TBS for 2 h. Photos were taken under UV light.
Inhibitory rates of 5-Fu on LoVo cells before or after transfection by MTT assay One hundred microliters of LoVo cells at 1 × 106/mL was inoculated into 96 well plates and 100 µL of 5-Fu was added so the concentrations were 10, 20, 40, 100, 200, 400 µg/mL. After incubation for 48 h, 100 µL of dimethylsulfoxide was added. After 20 min, the absorbance in each well was measured by enzyme-labelled meter and the inhibition rate was calculated.
The inhibition rate = [1-(the value of Abs in experiment/the value of Abs in the control)] × 100%.
Flow cytometry The apoptosis of LoVo cells induced by 5-Fu was analyzed by flow cytometry. 5-Fu 10 µg/mL was added into LoVo cells transfected or non-transfected and incubated for 48 h, then centrifuged, and 20 µL of propidium iodide (PI) was added for half an hour in the dark and analyzed by flow cytometry.
The differences between groups were analyzed by t test.
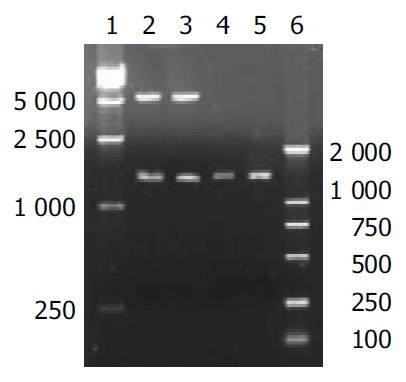
The digested plasmid PTEN-pMD18-T is shown in Figure 1. After plasmid PTEN-pMD18-T was digested, two fragments were 5.3 kb and 1.4 kb, which were consistent with dl 2000 marker.
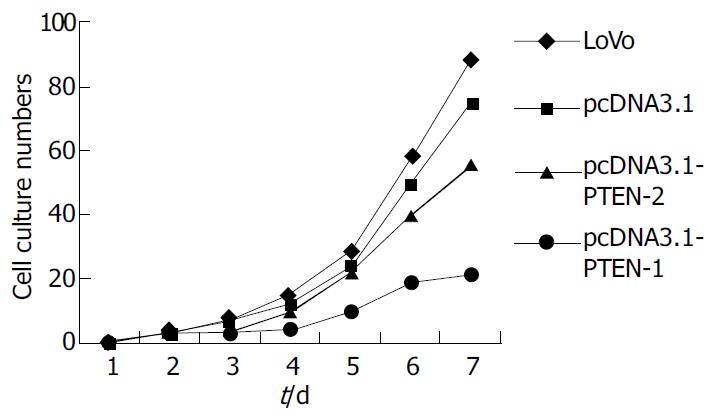
Four cell growth curves are shown pcDNA-PTEN-WT cell growth was slower than other cells (Figure 2).
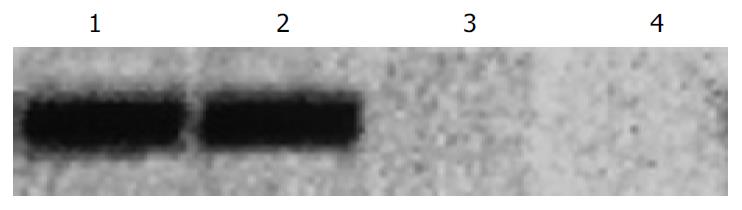
PTEN mRNA expression in LoVo cells was assayed by Western blot. The PTEN mRNA expression level was higher after transfection (Figure 3).
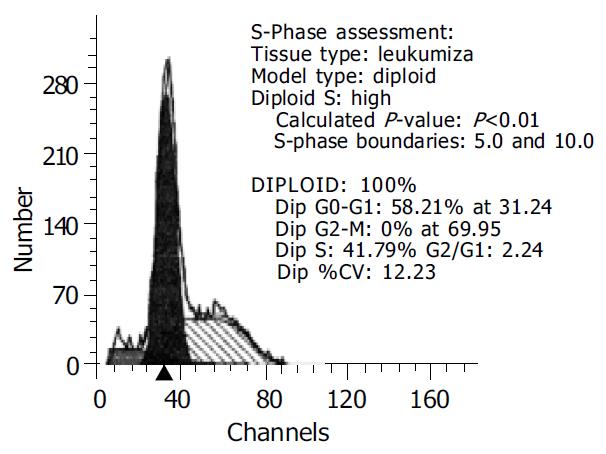
LoVo cells transfected with PTEN-WT gene were assayed by flow cytometry and it was mainly found in cell cycle blockage period G0-G1 (58.21%) (Figure 4).
The results of DNA-ladder bands showed that in transfected LoVo cells treated by 5-Fu there were typical DNA ladder bands, but in non-transfected LoVo cells there was no DNA ladder band.
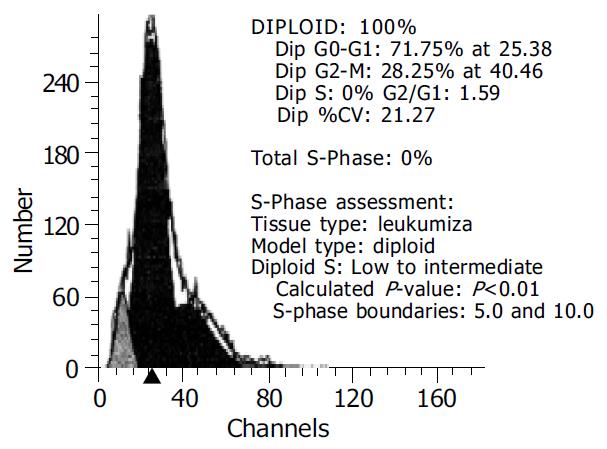
After 5-Fu 10 µL/mL was added for 48 h, the apoptotic LoVo cells reached 71.75% and only 18.84% in the control (Figure 5).
The effect of same concentration of 5-Fu on PTEN transfected and non-transfected LoVo cells was detected using MTT. The inhibition rate on cell proliferation was higher in the former than that in the later, and there was a significant difference between them (P < 0.01).
Tumor formation is a process of multi-factors and multi-stages. The activation of cancer genes and inactivation and abnormality of anticancer genes are considered as important molecular mechanisms. Recently PTEN was found to be an anticancer gene[25-28]. PTEN deletion and mutation were found in many malignant tumors including glioma, squamous carcinoma, prostate cancer, kidney cancer, endometrium cancer, breast cancer, colon cancer, etc[29-35]. The gene is on 10q23 chromosome in human and there are 9 extrons at 5’ terminal end and 804 nucleotides. The open reading frame is 1209 bp encoding 403 amino acid residues of PTEN protein with a molecular weight 47.1 kD. Chromosome allele gene deletions mainly occurred in highly malignant tumors such as glioblastoma multiform (GBM) and malignant meningioma. PTEN mutation also occurred in GBM and was seldom found in low degree malignant glioma. Davies[36] analyzed the DNA sequences in 46 glioma samples and found that PTEN mutation rate in an atlastic astrocytoma was 23% (3/13) and 36% (5/14) in GBM, but it was not found in 19 subjects with low degree malignant glioma. Such results indicated that PTEN might be related to the progress of glioma. Steck et al[37] discovered that 10q23 PTEN gene domain was frequently lost in GBM but was conserved in most low malignant tumors. The gene deletion was in 12q25-q26 domain and then PTEN gene loss appeared in the process of GBM. Dicuonzo et al[23] found that in colon cancer PTEN mutation, deletion and loss or replacement were all on chromosome 10 and the more malignant it was, the more evident the mutation and deletion were.
PTEN mutation rates in glioma cell lines and GBM were 41%-63% and 17%-44%, respectively. The literatures in Medline were reviewed for 674 malignant gliomas, the mutation rate of PTEN was 24%, including frame shift mutation, missense mutation, nonsense mutation and PTEN deletion. The point mutation rate was 41% and most of mutations were in core phosphorylase domain of 5 extrons. Davies et al[38] used adenovirus as vector and PTEN gene was transfected into glioma cell lines U251, pkb/akt, phosphorylation level was decreased and its activities were inhibited. The apoptosis rate was significantly higher than that in the control. It was reported that tumor cell growth was inhibited (60%-70%) by transfected wild type PTEN with reverse transcription and PTEN mutation with U87MG and U178MG of glioma cell lines and cell growth stopped at G1 stage by flow cytometry.
In our study, after transfection PTEN mRNA expression level in LoVo cells was higher than that before PTEN was transfected. After transfection PTEN protein expression level was higher by flow cytometry and the positive rate was increased from 21% to 47%, which indicated that PTEN gene was transferred into LoVo via lipofectin. Typical DNA ladder bands were found by 5-Fu in transfected LoVo cells and DNA ladder bands were not remarkable in non-transfected LoVo cells. The apoptosis rate was 71.75% after incubation of 5-Fu 10 µL/mL in LoVo cells for 48 h and in the control it was only 18.84%. The effect of same concentration of 5-Fu on PTEN transfected LoVo cells and non-transfected LoVo cells was measured. The inhibition rate of cell proliferation was higher in the former than in the later and there was a significant difference between them, which demonstrated that PTEN gene expression promoted signaling in DNA damage by 5-Fu and induced apoptosis and inhibited cell proliferation in tumor.
PTEN protein is in cytoplasm of cells and homologous to tensin which is important in cell framework and in local adhesion. PTEN not only acts on the nuclei but also on membranes. PTEN protein could down-regulate the interaction of tumor cells and extracellular matrix (ECM) by inhibiting activities of focal adhesion kinase. (fak)[39]. Malignant cells proliferate without local adhesion. Fak is an important signal mediated by integrin and its inhibition could induce apoptosis and its activation could induce non-dependent growth and tumor formation[40]. Fak is over-expressed in many high degree malignant tumors. PTEN inhibits non-dependent cell growth by reducing Fak activities.
It was shown that apoptosis reached 71.75% when transfected LoVo cells were incubated with 5-Fu 10 µL/mL for 48 h and it was 18.84% in the control. Maybe 5-Fu up-regulated the new protein synthesis on the surface of cells and promoted signaling in PTEN system in the cells or 5-Fu blocked some protective protein synthesis. The mechanism of cooperative effect of 5-Fu and PTEN gene transfection may be due to PTEN protein expression on the surface of cells and 5-Fu transmembrance transportation. 5-Fu can accumulate in cells and produce cytotoxin and induce cell apoptosis.
Edited by Wang XL and Zhu LH Proofread by Xu FM
| 1. | Mayo LD, Dixon JE, Durden DL, Tonks NK, Donner DB. PTEN protects p53 from Mdm2 and sensitizes cancer cells to chemotherapy. J Biol Chem. 2002;277:5484-5489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 250] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Wu RC, Li X, Schönthal AH. Transcriptional activation of p21WAF1 by PTEN/MMAC1 tumor suppressor. Mol Cell Biochem. 2000;203:59-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Tolkacheva T, Chan AM. Inhibition of H-Ras transformation by the PTEN/MMAC1/TEP1 tumor suppressor gene. Oncogene. 2000;19:680-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Gu J, Tamura M, Yamada KM. Tumor suppressor PTEN inhibits integrin- and growth factor-mediated mitogen-activated protein (MAP) kinase signaling pathways. J Cell Biol. 1998;143:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 257] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | Xu B, Yao Q, Dai SZ. [Detection of mutation and protein expression of PTEN gene in endometrial carcinoma]. Aizheng. 2004;23:69-73. [PubMed] |
| 6. | Mao JH, Wu D, Perez-Losada J, Nagase H, DelRosario R, Balmain A. Genetic interactions between Pten and p53 in radiation-induced lymphoma development. Oncogene. 2003;22:8379-8385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Seminario MC, Precht P, Wersto RP, Gorospe M, Wange RL. PTEN expression in PTEN-null leukaemic T cell lines leads to reduced proliferation via slowed cell cycle progression. Oncogene. 2003;22:8195-8204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Shin Lee J, Seok Kim H, Bok Kim Y, Cheol Lee M, Soo Park C. Expression of PTEN in renal cell carcinoma and its relation to tumor behavior and growth. J Surg Oncol. 2003;84:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Su JD, Mayo LD, Donner DB, Durden DL. PTEN and phosphatidylinositol 3'-kinase inhibitors up-regulate p53 and block tumor-induced angiogenesis: evidence for an effect on the tumor and endothelial compartment. Cancer Res. 2003;63:3585-3592. [PubMed] |
| 10. | Choi Y, Zhang J, Murga C, Yu H, Koller E, Monia BP, Gutkind JS, Li W. PTEN, but not SHIP and SHIP2, suppresses the PI3K/Akt pathway and induces growth inhibition and apoptosis of myeloma cells. Oncogene. 2002;21:5289-5300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Malmer B, Grönberg H, Andersson U, Jonsson BA, Henriksson R. Microsatellite instability, PTEN and p53 germline mutations in glioma families. Acta Oncol. 2001;40:633-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 827] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 13. | Li J, Simpson L, Takahashi M, Miliaresis C, Myers MP, Tonks N, Parsons R. The PTEN/MMAC1 tumor suppressor induces cell death that is rescued by the AKT/protein kinase B oncogene. Cancer Res. 1998;58:5667-5672. [PubMed] |
| 14. | Nassif NT, Lobo GP, Wu X, Henderson CJ, Morrison CD, Eng C, Jalaludin B, Segelov E. PTEN mutations are common in sporadic microsatellite stable colorectal cancer. Oncogene. 2004;23:617-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Saito Y, Swanson X, Mhashilkar AM, Oida Y, Schrock R, Branch CD, Chada S, Zumstein L, Ramesh R. Adenovirus-mediated transfer of the PTEN gene inhibits human colorectal cancer growth in vitro and in vivo. Gene Ther. 2003;10:1961-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Tanaka M, Grossman HB. In vivo gene therapy of human bladder cancer with PTEN suppresses tumor growth, downregulates phosphorylated Akt, and increases sensitivity to doxorubicin. Gene Ther. 2003;10:1636-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Han B, Dong Z, Liu Y, Chen Q, Hashimoto K, Zhang JT. Regulation of constitutive expression of mouse PTEN by the 5'-untranslated region. Oncogene. 2003;22:5325-5337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Radu A, Neubauer V, Akagi T, Hanafusa H, Georgescu MM. PTEN induces cell cycle arrest by decreasing the level and nuclear localization of cyclin D1. Mol Cell Biol. 2003;23:6139-6149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 175] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Ito K, Kubokawa M, Harada N, Mibu R, Nawata H. p53 and PTEN/MMAC1 mutational analysis of the small-intestinal cancer. Dig Liver Dis. 2003;35:347-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Lin Q, Zhuang YZ, Xu DP, Ye JX, Chen PQ. [Expression of PTEN protein and its correlation with p27kip1 and cyclin D1 expression in primary breast cancer]. Zhonghua Zhongliu Zazhi. 2003;25:246-249. [PubMed] |
| 21. | Marino M, Acconcia F, Trentalance A. Biphasic estradiol-induced AKT phosphorylation is modulated by PTEN via MAP kinase in HepG2 cells. Mol Biol Cell. 2003;14:2583-2591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Taniyama K, Goodison S, Ito R, Bookstein R, Miyoshi N, Tahara E, Tarin D, Urquidi V. PTEN expression is maintained in sporadic colorectal tumours. J Pathol. 2001;194:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Dicuonzo G, Angeletti S, Garcia-Foncillas J, Brugarolas A, Okrouzhnov Y, Santini D, Tonini G, Lorino G, De Cesaris M, Baldi A. Colorectal carcinomas and PTEN/MMAC1 gene mutations. Clin Cancer Res. 2001;7:4049-4053. [PubMed] |
| 24. | Lilja JF, Wu D, Reynolds RK, Lin J. Growth suppression activity of the PTEN tumor suppressor gene in human endometrial cancer cells. Anticancer Res. 2001;21:1969-1974. [PubMed] |
| 25. | Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3525] [Cited by in RCA: 3583] [Article Influence: 128.0] [Reference Citation Analysis (0)] |
| 26. | Tashiro H, Blazes MS, Wu R, Cho KR, Bose S, Wang SI, Li J, Parsons R, Ellenson LH. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res. 1997;57:3935-3940. [PubMed] |
| 27. | Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2004] [Cited by in RCA: 2036] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 28. | Davies MA, Koul D, Dhesi H, Berman R, McDonnell TJ, McConkey D, Yung WK, Steck PA. Regulation of Akt/PKB activity, cellular growth, and apoptosis in prostate carcinoma cells by MMAC/PTEN. Cancer Res. 1999;59:2551-2556. [PubMed] |
| 29. | Li DM, Sun H. PTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cells. Proc Natl Acad Sci USA. 1998;95:15406-15411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 354] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 30. | Furnari FB, Huang HJ, Cavenee WK. The phosphoinositol phosphatase activity of PTEN mediates a serum-sensitive G1 growth arrest in glioma cells. Cancer Res. 1998;58:5002-5008. [PubMed] |
| 31. | Zhao H, Dupont J, Yakar S, Karas M, LeRoith D. PTEN inhibits cell proliferation and induces apoptosis by downregulating cell surface IGF-IR expression in prostate cancer cells. Oncogene. 2004;23:786-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Kato H, Fujimura M, Kumabe T, Ishioka C, Kanamaru R, Yoshimoto T. PTEN gene mutation and high MIB-1 labeling index may contribute to dissemination in patients with glioblastoma. J Clin Neurosci. 2004;11:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Saito Y, Gopalan B, Mhashilkar AM, Roth JA, Chada S, Zumstein L, Ramesh R. Adenovirus-mediated PTEN treatment combined with caffeine produces a synergistic therapeutic effect in colorectal cancer cells. Cancer Gene Ther. 2003;10:803-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Saga Y, Mizukami H, Takei Y, Ozawa K, Suzuki M. Suppression of cell migration in ovarian cancer cells mediated by PTEN overexpression. Int J Oncol. 2003;23:1109-1113. [PubMed] |
| 35. | Nassif NT, Lobo GP, Wu X, Henderson CJ, Morrison CD, Eng C, Jalaludin B, Segelov E. PTEN mutations are common in sporadic microsatellite stable colorectal cancer. Oncogene. 2004;23:617-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Davies MP, Gibbs FE, Halliwell N, Joyce KA, Roebuck MM, Rossi ML, Salisbury J, Sibson DR, Tacconi L, Walker C. Mutation in the PTEN/MMAC1 gene in archival low grade and high grade gliomas. Br J Cancer. 1999;79:1542-1548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Steck PA, Lin H, Langford LA, Jasser SA, Koul D, Yung WK, Pershouse MA. Functional and molecular analyses of 10q deletions in human gliomas. Genes Chromosomes Cancer. 1999;24:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Davies MA, Lu Y, Sano T, Fang X, Tang P, LaPushin R, Koul D, Bookstein R, Stokoe D, Yung WK. Adenoviral transgene expression of MMAC/PTEN in human glioma cells inhibits Akt activation and induces anoikis. Cancer Res. 1998;58:5285-5290. [PubMed] |
| 39. | Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 924] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 40. | Tamura M, Gu J, Danen EH, Takino T, Miyamoto S, Yamada KM. PTEN interactions with focal adhesion kinase and suppression of the extracellular matrix-dependent phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1999;274:20693-20703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 281] [Article Influence: 10.8] [Reference Citation Analysis (0)] |