Published online Jul 15, 2004. doi: 10.3748/wjg.v10.i14.2147
Revised: March 4, 2004
Accepted: March 12, 2004
Published online: July 15, 2004
AIM: To study the relationship between microvessel density (MVD), telomerase activity and biological characteristics in hepatocellular carcinoma (HCC).
METHODS: S-P immunohistochemical method and telomeric repeat amplification protocol (TRAP) were respectively used to analyze the MVD and telomerase activity in 58 HCC and adjacent normal tissues.
RESULTS: The MVD in HCC with metastasis, lower differentiation or without intact capsule was significantly higher than that in HCC with intact capsule, higher differentiation, or without metastasis. While MVD had no relationship with tumor size, hepatic virus infection and other clinical factors. Telomerase activity was related to differentiation degree, but not to tumor size or histological grade. MVD in HCC with telomerase activity was higher than that in HCC without telomerase activity.
CONCLUSION: MVD and telomerase activity may serve as diagnostic criteria of HCC in earlier stage. Meanwhile, there may be a cooperative effect between MVD and telomerase on the growth and metastasis of HCC.
- Citation: Piao YF, He M, Shi Y, Tang TY. Relationship between microvessel density and telomerase activity in hepatocellular carcinoma. World J Gastroenterol 2004; 10(14): 2147-2149
- URL: https://www.wjgnet.com/1007-9327/full/v10/i14/2147.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i14.2147
Primary hepatocellular carcinoma (HCC) is a common malignant solid tumor, which is rich of blood. It has many characteristics, such as fast infiltrating growth, metastasis in early stage, high grade malignancy, poorly therapeutic efficacy. So it is important to study the angiogenesis of HCC. At present, it has been proved that MVD can serve as a prognostic criterion for relapse, metastasis and survival rate of all kinds of carcinoma[1-6].
Telomerase is a reverse transcriptase. It can make up telomeres that lose 50-200 bp during each DNA replication so as to retain the telomere length and stabilize the cells. The expression of telomerase activity is important to cell proliferation, senescence, immortalization and carcinogenesis[7-9]. It is known that telomerase activity can be detected in many carcinomas, but not in most of normal tissues[10-16].
MVD and telomerase activity can act as specific markers for malignant tumors, and are used to analyze the biologic characteristics, infiltration, metastasis and prognosis of tumors. But the relationship between them has not been reported. In this study, S-P immunohistochemistry method and telomeric repeat amplification protocol (TRAP) were used to respectively detect MVD and telomerase activity, so as to study their relationship and other clinical factors.
Fifty-eight HCC and adjacent normal tissues specimens were obtained from Hepatobiliary Surgery Department of Changchun Infectious Hospital from December 2000 to December 2002. They were all proved as primary HCC. These patients did not receive radiotherapy, chemotherapy, or other therapies. Of them, 35 cases had liver cirrhosis, 52 cases had chronic hepatitis, 6 cases had carcinoma emboli in portal vein, 48 cases had positive marker of HBV, 4 cases had positive anti-HCV antibody, and 4 cases had negative marker of hepatitis virus. Each specimen was divided into two parts. One was stained by immunohistochemical method, and routinely fixed in formalin, embeded in paraffin, and then cut into 4-5 μm thick sections. The other was frozen in liquid nitrogen and used to analyze telomerase activity by TRAP.
UltraSensitiveTM S-P kit, anti-VIII factor related antigen monoantibody (anti-FVIII RAG), and positive control were purchased from Fuzhou Maixin Biotechnology Development Company. The sections were deparaffinized in xylene and rehydrated in a serial gradient of ethanol solutions. Then S-P immunohistochemical method was used according to its manual. At last, they were restained with hematoxylin and observed. The negative control included empty control with normal rabbit IgG instead of primary antibodies or with the second antibody only. MVD was determined in triplicate in the area of the most intense vascularization (hot spot) of each tumor, and the average count was recorded.
About 50-100 mg tissue was split. Then the supernatant was collected for analysis. The telomerase activity was detected using a TRAP kit following instructions of the manufacturer (Beijing Tiangekangning Biotech Institute). The reaction system containing 25 μL TRAP agent, 0.2 μL Taq enzyme and 1 μL cell extract was incubated for 30 min at 25 °C, then 0.5 μL of primer was added and PCR was conducted for 30 cycles with denaturing at 94 °C for 30 s, annealing at 60 °C for 30 s, extending at 72 °C for 30 s. Fifteen microliter PCR product was loaded onto a 90 g/L non-degenerative SDS gel, resolved through the SDS-PAGE, demonstrated by a reaction in 2 g/L silver nitrate for 15 min, and visualized by incubation in 30 g/L anhydrous sodium carbonate containing formaldehydes (1 mL/L). The activity of telomerase was indicated by the presence of a 6 bp-DNA ladder. The cell extract inactivated by incubation at 75 °C for 10 min was used as negative control.
Result were expressed as mean ± SD. Student’s t test and χ2 test were used. P < 0.05 was considered statistically significant.
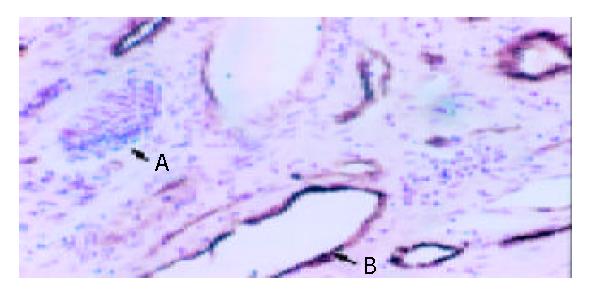
Observed with light microscope, venous endothelium but not lymphatic vessel endothelium was brown (Figure 1).
Table 1 shows that MVD in HCC tissues with metastasis within vein or liver was significantly higher than that in HCC without metastasis (P < 0.05), MVD in HCC without intact capsule was higher than that in HCC with intact capsule (P < 0.05). There was no significant difference in MVD between big (> 5 cm) and small (≤ 5 cm) tumors.
In this study, the distribution of MVD was different in various regions. MVD was higher in HCC with lower differentiation than that in HCC with higher differentiation (Table 2). MVD in adjacent normal tissues (13.2 ± 2.7) was mainly distributed in the area near the carcinoma tissues, especially around the pseudolobules with liver cirrhosis. It was reported that MVD in normal tissues was 11.8 ± 0.2.
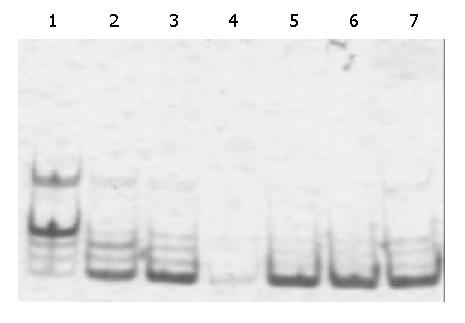
The results are shown in Figure 2. The positive rate of telomerase activity in HCC tissues (89.6%) was significantly higher than that in adjacent normal liver tissues (20.7%). The telomerase activity in HCC was related to differentiation grade of HCC, but was not related to HCC size, capsule integrity, number of liver nods, metastasis, or hepatitis virus infection. While the telomerase activity in adjacent normal liver tissues was related to capsule integrity, number of liver nods and hepatitis virus infection (Table 3).
| Group | HCC | Adjacent normal tissues | ||||||
| Cases | Positive number | Positive rate (%) | P | Positive number | Positive rate (%) | P | ||
| Differentiation | High | 11 | 6 | 54.4 | 0 | 0 | ||
| Middle | 38 | 37 | 97.4 | 5 | 13.2 | |||
| Low | 9 | 9 | 100 | < 0.05 | 7 | 77.8 | < 0.05 | |
| Size | ≤ 5 cm | 16 | 14 | 87.5 | 3 | 18.8 | ||
| > 5 cm | 42 | 38 | 90.9 | > 0.05 | 9 | 21.4 | > 0.05 | |
| Capsule | - | 20 | 16 | 80.0 | 1 | 5.0 | ||
| Integrity | + | 38 | 36 | 94.7 | > 0.05 | 11 | 29.8 | < 0.05 |
| Hepatitis virus | + | 48 | 44 | 91.6 | 1 | 22.9 | ||
| Infection | - | 10 | 8 | 80.0 | > 0.05 | 1 | 10.0 | < 0.05 |
| Nod number | Mono-nod | 15 | 12 | 80.0 | 1 | 6.7 | ||
| Multi-nod | 43 | 40 | 93.0 | > 0.05 | < 0.05 | |||
| Metastasis | + | 36 | 34 | 94.4 | 10 | 27.8 | ||
| - | 22 | 18 | 81.8 | > 0.05 | 2 | 9.1 | < 0.05 | |
Among the 58 HCC and adjacent normal liver tissues, MVD in those with positive telomerase activity was significantly higher than that in those with negative activity (Table 4).
Carcinogenesis of HCC is a multi-factor, multi-step and complex process. Angiogenesis is necessary for solid tumors larger than 1 mm × 1 mm. Or else the tumor would remain at dormancy phase and would not metastasize. As soon as it entered angiogenesis stage, the potency of metastasis exhibited at once[17-20].
MVD is an important marker of angiogenesis in tumor and is valuable in prognosis of various carcinomas. In this study, S-P immunohistochemical staining was used to analyze MVD in 58 HCC and corresponding adjacent normal tissues. F VIII in cytoplasma of various vascular endothelium cells is synthesized in endothelial cells. F VIII-RAg is specific to endothelial cells so that it could be differentiated blood vessel from lymphatic vessel. The role of vascular genesis, development and distribution in occurrence, development, infiltration, metastsis and advancement of tumors has been studied[21]. MVD in poorly differentiated HCC with metastasis in vein or liver, intact capsule was significantly higher than that in corresponding ones. This result was consistent with others, suggesting that HCC with higher MVD may obtain more nutrition from new blood vessels, so that it can grow faster and has the ability to infiltrate and metastasize. MVD might play a key role in development of HCC. There was no close relationship between MVD and tumor size, HBV infection. Maybe HBV infection is merely a carcinogenic factor, and is not related to the growth, infiltration and metastasis of HCC.
We also detected telomerase activity in 58 HCC and adjacent normal tissues by TRAP. Similar to Nouso’s research, the expression of telomerase activity in HCC was negatively related to differentiation, but was not related to tumor size or histologic grade. Telomerase activity in adjacent normal tissues with middle or big size, multi-nods, unintegral capsule, positive HBsAg was higher than that in adjacent normal tissues without them, suggesting that there is some relationship between telomerase activity in adjacent normal tissue and prognosis of HCC. Hepatitis viruses, especially HBV, have been found to be an important factor in causing HCC. HBx, encoded by HBV, could block the function of p53, and make genome unstable. With the cell division, telomerase was activated. The cells with positive telomerase activity became immortalized and further developed to carcinoma cells. Histological examination in combination with detection of telomerase activity could increase the accuracy of diagnosis in early stage and improve the judgment on its prognosis.
In addition, there was some relationship between MVD and telomerase activity. Activation of telomerase could make liver cells immortalized, and MVD could offer nutrition for their growth and stimulate metastasis. These two factors would result in carcinogenesis at last.
In a word, MVD and telomerase activity have a key role in occurrence, development, infiltration and metastasis of HCC and have notable clinical values in diagnosing and treating HCC in early stage.
Edited by Wang XL and Chen WW Proofread by Xu FM
| 1. | Du JR, Jiang Y, Zhang YM, Fu H. Vascular endothelial growth factor and microvascular density in esophageal and gastric carcinomas. World J Gastroenterol. 2003;9:1604-1606. [PubMed] |
| 2. | Ding YB, Chen GY, Xia JG, Zang XW, Yang HY, Yang L. Association of VCAM-1 overexpression with oncogenesis, tumor angiogenesis and metastasis of gastric carcinoma. World J Gastroenterol. 2003;9:1409-1414. [PubMed] |
| 3. | Moon WS, Rhyu KH, Kang MJ, Lee DG, Yu HC, Yeum JH, Koh GY, Tarnawski AS. Overexpression of VEGF and angiopoietin 2: a key to high vascularity of hepatocellular carcinoma. Mod Pathol. 2003;16:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Takahashi R, Tanaka S, Kitadai Y, Sumii M, Yoshihara M, Haruma K, Chayama K. Expression of vascular endothelial growth factor and angiogenesis in gastrointestinal stromal tumor of the stomach. Oncology. 2003;64:266-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Poon RT, Ng IO, Lau C, Yu WC, Yang ZF, Fan ST, Wong J. Tumor microvessel density as a predictor of recurrence after resection of hepatocellular carcinoma: a prospective study. J Clin Oncol. 2002;20:1775-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 227] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 6. | Ng IO, Poon RT, Lee JM, Fan ST, Ng M, Tso WK. Microvessel density, vascular endothelial growth factor and its receptors Flt-1 and Flk-1/KDR in hepatocellular carcinoma. Am J Clin Pathol. 2001;116:838-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 127] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2511] [Cited by in RCA: 2637] [Article Influence: 77.6] [Reference Citation Analysis (1)] |
| 8. | Morin GB. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989;59:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1068] [Cited by in RCA: 1122] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 9. | Shay JW, Wright WE. Ageing and cancer: the telomere and telomerase connection. Novartis Found Symp. 2001;235:116-125; discussion 125-129, 146-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5156] [Cited by in RCA: 5233] [Article Influence: 168.8] [Reference Citation Analysis (0)] |
| 11. | Mu J, Wei LX. Telomere and telomerase in oncology. Cell Res. 2002;12:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Masutomi K, Yu EY, Khurts S, Ben-Porath I, Currier JL, Metz GB, Brooks MW, Kaneko S, Murakami S, DeCaprio JA. Telomerase maintains telomere structure in normal human cells. Cell. 2003;114:241-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 546] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 13. | Testorelli C. Telomerase and cancer. J Exp Clin Cancer Res. 2003;22:165-169. [PubMed] |
| 14. | Yokota T, Suda T, Igarashi M, Kuroiwa T, Waguri N, Kawai H, Mita Y, Aoyagi Y. Telomere length variation and maintenance in hepatocarcinogenesis. Cancer. 2003;98:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Mariani E, Meneghetti A, Formentini I, Neri S, Cattini L, Ravaglia G, Forti P, Facchini A. Telomere length and telomerase activity: effect of ageing on human NK cells. Mech Ageing Dev. 2003;124:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Tomita M, Matsuzaki Y, Onitsuka T. Effect of mast cells on tumor angiogenesis in lung cancer. Ann Thorac Surg. 2000;69:1686-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Lichtenbeld HH, van Dam-Mieras MC, Hillen HF. Tumour angiogenesis: pathophysiology and clinical significance. Neth J Med. 1996;49:42-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Paweletz N, Knierim M. Tumor-related angiogenesis. Crit Rev Oncol Hematol. 1989;9:197-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 131] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Desai SB, Libutti SK. Tumor angiogenesis and endothelial cell modulatory factors. J Immunother. 1999;22:186-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Saaristo A, Karpanen T, Alitalo K. Mechanisms of angiogenesis and their use in the inhibition of tumor growth and metastasis. Oncogene. 2000;19:6122-6129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 181] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 21. | Ruiter DJ, Schlingemann RO, Rietveld FJ, de Waal RM. Monoclonal antibody-defined human endothelial antigens as vascular markers. J Invest Dermatol. 1989;93:25S-32S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |