Copyright
©The Author(s) 2021.
World J Gastroenterol. Oct 7, 2021; 27(37): 6277-6289
Published online Oct 7, 2021. doi: 10.3748/wjg.v27.i37.6277
Published online Oct 7, 2021. doi: 10.3748/wjg.v27.i37.6277
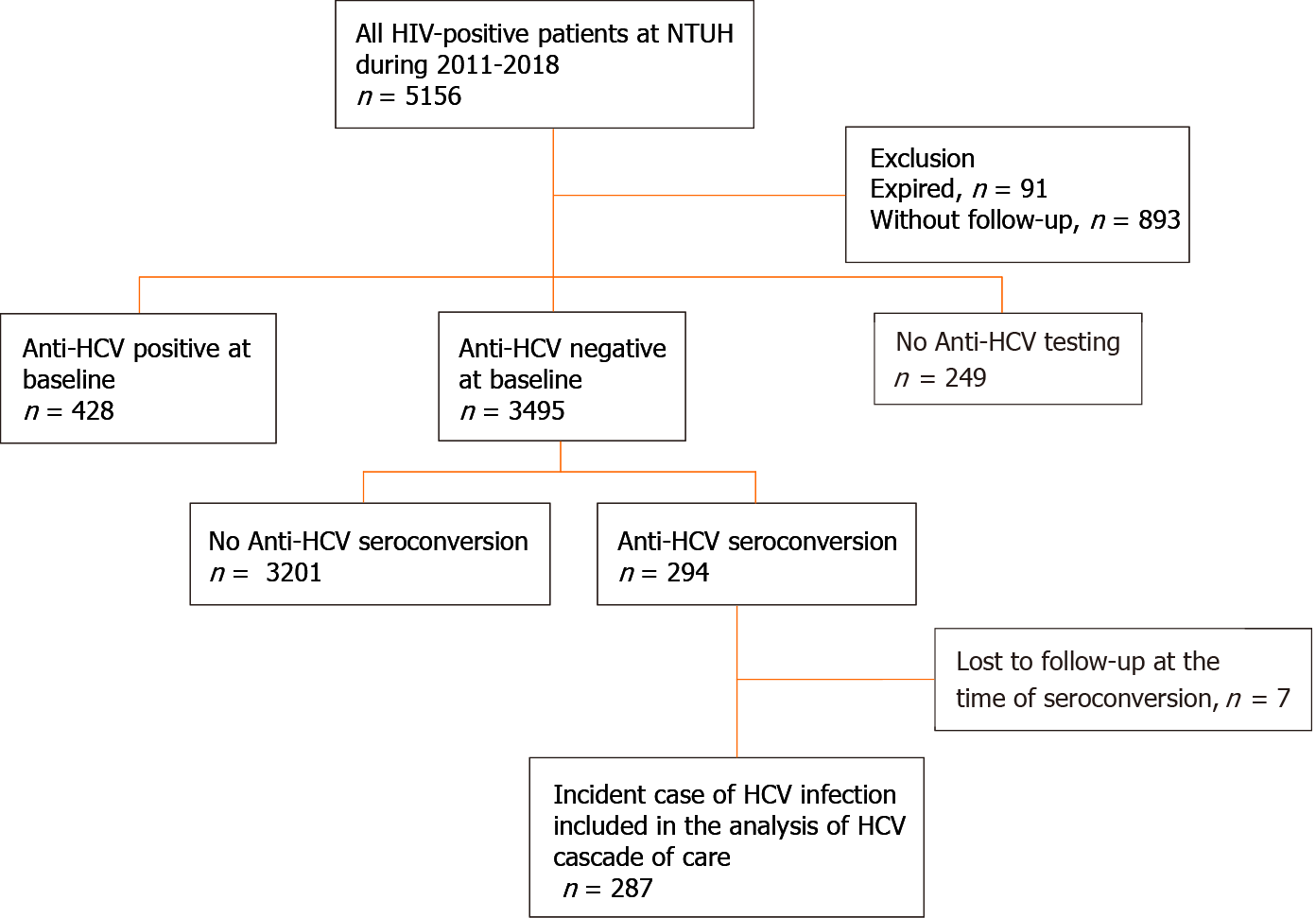
Figure 1 Patient flow.
Between 2011 and 2018, 3495 people living with human immunodeficiency virus (HIV) (PLWH) had negative anti-hepatitis C virus (HCV) antibody tests at baseline and HCV seroconversion was detected in 294 (8.4%) PLWH. After excluding 7 PLWH who were lost to follow-up when HCV seroconversion occurred, a total of 287 PLWH were included in the analysis of the care cascade of incident HCV infections. HIV: Human immunodeficiency virus; NTUH: National Taiwan University Hospital; HCV: Hepatitis C virus.
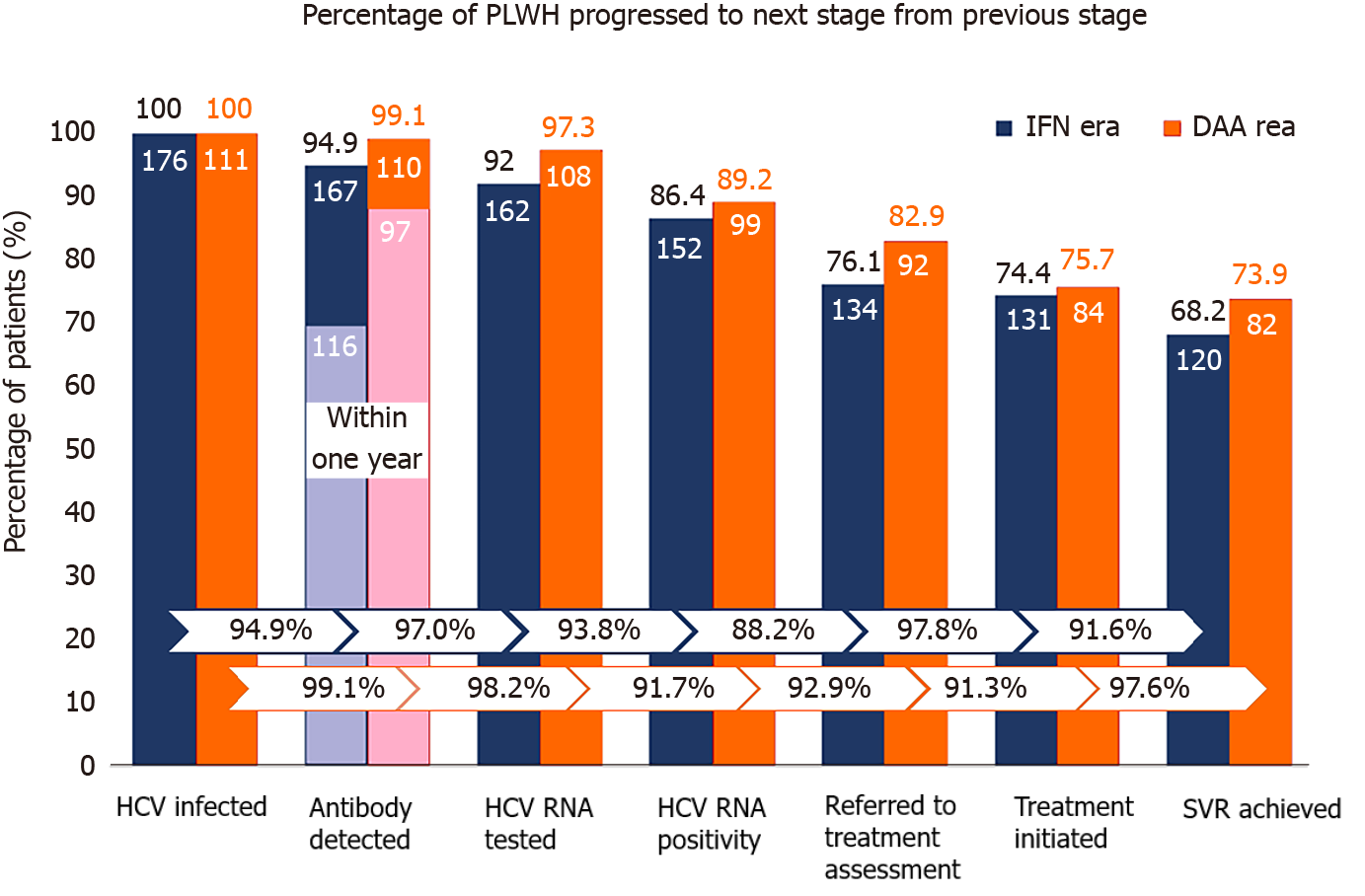
Figure 2 Care cascade of incident hepatitis C virus infections in the interferon and direct-acting antiviral eras.
In the interferon era (blue columns), 176 people living with human immunodeficiency virus (HIV) (PLWH) had incident hepatitis C virus (HCV) infections by retrospective testing of all archive blood samples; 167 (94.9%) were found to have HCV seroconversion by HIV-treating physicians; and the diagnostic rate within one year after seroconversion was 69.4% (116 out of 167). Plasma HCV RNA was tested in 162 (97.0%) PLWH after seroconversion, of which 152 (93.8%) were viremic. A total of 134 (88.2%) viremic PLWH were referred to hepatology clinics; anti-HCV treatments were initiated in 131 (97.8%), and 120 (91.6% of all treated PLWH) achieved sustained virologic response (SVR). In the direct-acting antiviral era (orange columns), 111 PLWH had incident HCV infections; 110 (99.1%) were found to have HCV seroconversion by HIV-treating physicians; and the diagnostic rate within one year after seroconversion was 88.2% (97 out of 110). Plasma HCV RNA was detected in 108 (98.2%) PLWH after seroconversion and 99 (91.7%) were viremic. A total of 92 (92.9%) viremic PLWH received treatment assessment, anti-HCV treatments were initiated in 84 (91.3%), and 82 (97.6% of all treated patients) achieved SVR. IFN: Interferon; DAA: Direct-acting antiviral; HCV: Hepatitis C virus; PLWH: People living with human immunodeficiency virus.
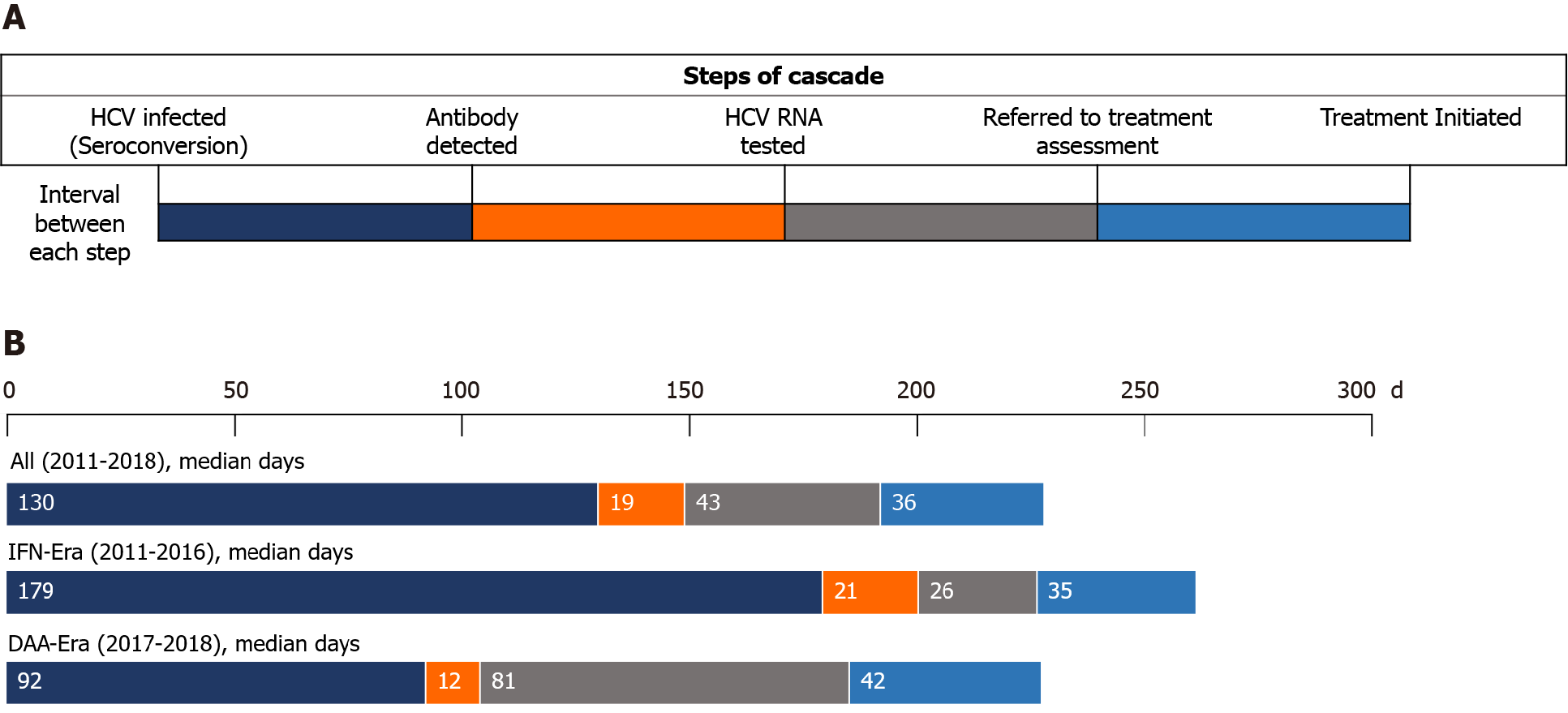
Figure 3 Intervals between steps of the care cascade of incident hepatitis C virus infections.
A: Demonstration of the intervals between each step of the care cascade; B: In all included people living with human immunodeficiency virus (HIV) (PLWH), the median interval from seroconversion to detection of hepatitis C virus (HCV) seropositivity by HIV-treating physicians was 130 days, which was significantly shorter in the direct-acting antiviral (DAA) era (median 92 d) than that in the interferon (IFN) era (median 179 d) (P < 0.001). In the IFN era, the median interval from detection of HCV seropositivity to HCV RNA testing was 21 d, that from RNA testing to referral to treatment assessment was 26 d, and that from assessment to treatment initiation was 35 d. In the DAA era, the median interval from detection of HCV seropositivity to HCV RNA testing was 12 d, that from RNA testing to referral to treatment assessment was 81 d, and that from assessment to treatment initiation was 42 d. The differences in the intervals after antibody diagnosis were not statistically significant between PLWH included in the IFN era and those in the DAA era. HCV: Hepatitis C virus; IFN: Interferon; DAA: Direct-acting antiviral.
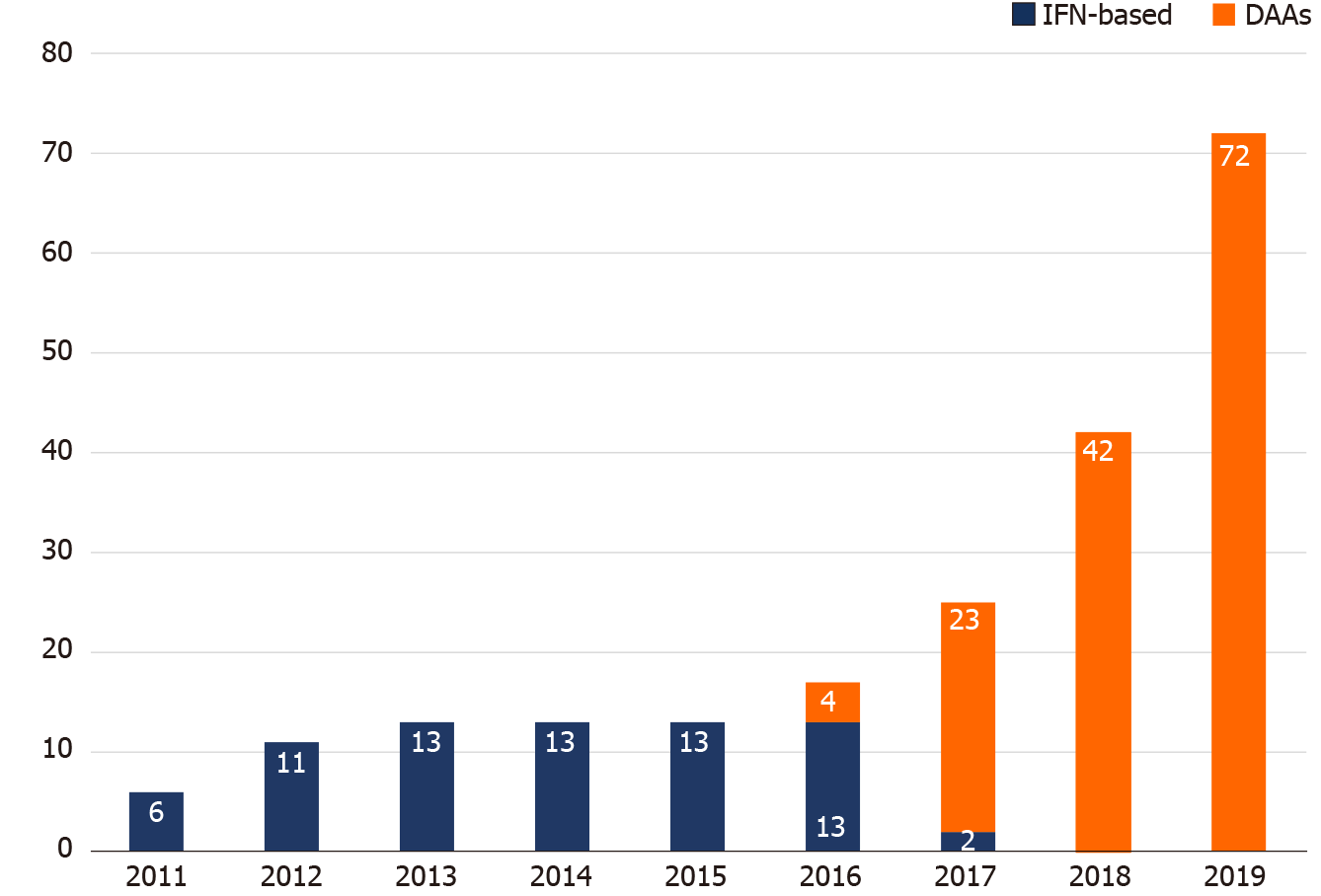
Figure 4 Hepatitis C virus treatment uptake by year from 2011 to 2019.
The annual numbers of people living with human immunodeficiency virus (PLWH) who received interferon-based therapy were unchanged from 2012 to 2016. With the introduction of direct-acting antivirals (DAAs) since 2016, the number of PLWH receiving DAAs increased annually, especially in 2019, when the restrictions on the DAA reimbursement were lifted and all hepatitis C virus viremic patients could be treated with DAAs. DAA: Direct-acting antiviral; IFN: Interferon.
- Citation: Huang MH, Sun HY, Ho SY, Chang SY, Hsieh SM, Sheng WH, Chuang YC, Huang YS, Su LH, Liu WC, Su YC, Hung CC. Recently acquired hepatitis C virus infection among people living with human immunodeficiency virus at a university hospital in Taiwan. World J Gastroenterol 2021; 27(37): 6277-6289
- URL: https://www.wjgnet.com/1007-9327/full/v27/i37/6277.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i37.6277