Copyright
©The Author(s) 2016.
World J Gastroenterol. Jun 21, 2016; 22(23): 5415-5421
Published online Jun 21, 2016. doi: 10.3748/wjg.v22.i23.5415
Published online Jun 21, 2016. doi: 10.3748/wjg.v22.i23.5415
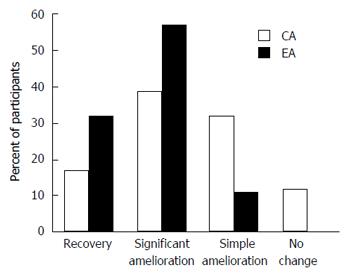
Figure 1 Improvement of dermatological features in both groups after 1 mo.
Illustrated are the percentages of patients with facial dermatoses in the control arm (CA) and experimental arm (EA) over the time of the trial. They are categorized according to the degree of change in clinical manifestation.
Figure 2 Female patient; EA group before and one month after treatment.
Clinical example of a patient treated with E. coli Nissle. The facial popular-pustular exanthema was significantly improved over the 1 mo treatment period.
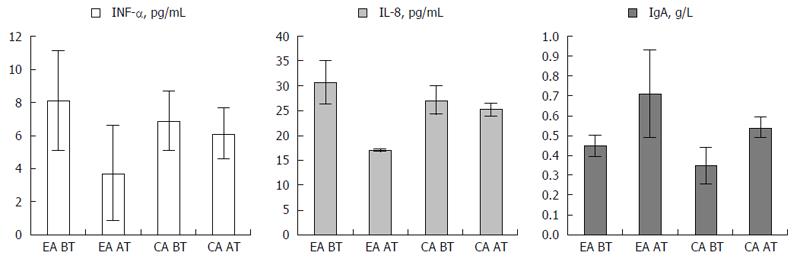
Figure 3 Improvement of serum levels of INF-α, IL-8 and IgA in experimental arm before (EA BT) and after treatment (EA AT), and control arm before (CA BT) and after treatment (CA AT).
Serum INF-α, IL-8 and IgA levels over the trial course. Illustrated are the values of INF-α , IL-8 and IgA in the experimental arm before (EA BT) and after treatment (EA AT) and the control arm before (CA BT) and after treatment (CA AT).
- Citation: Manzhalii E, Hornuss D, Stremmel W. Intestinal-borne dermatoses significantly improved by oral application of Escherichia coli Nissle 1917. World J Gastroenterol 2016; 22(23): 5415-5421
- URL: https://www.wjgnet.com/1007-9327/full/v22/i23/5415.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i23.5415