Copyright
©The Author(s) 2004.
World J Gastroenterol. May 15, 2004; 10(10): 1387-1391
Published online May 15, 2004. doi: 10.3748/wjg.v10.i10.1387
Published online May 15, 2004. doi: 10.3748/wjg.v10.i10.1387
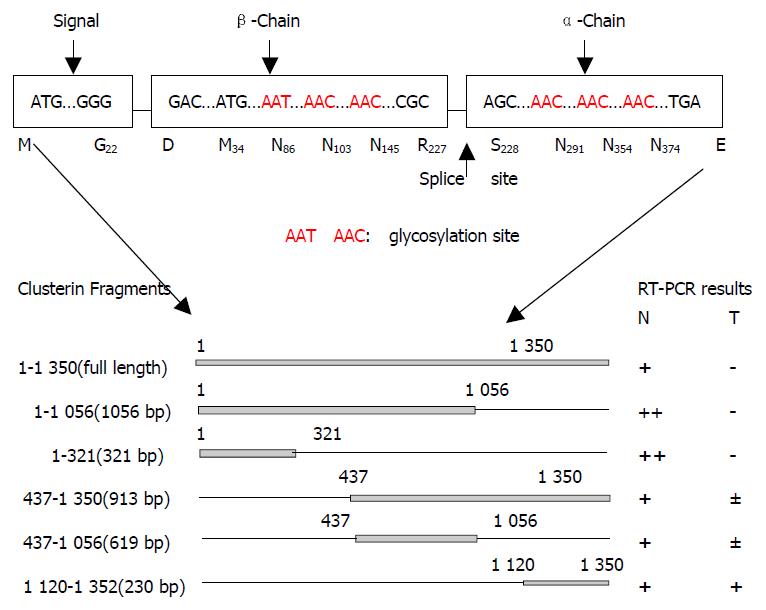
Figure 1 Schematic diagram of analyzed fragments of clusterin.
A: structural map of the human clusterin protein. Letters and boxes signify the following: ER-targeting signal, endoplasmic reticulum-targeting hydrophobic leader peptide; splice site, α/β-cleavage site (amino acid 205 and 206) of cytoplasmic - 60 ku pre-secreted clusterin which was glycosylated and cleavaged into α and β chains to form an 80 ku matured secre-tory clusterin protein; rectangles, hydrophobic leader peptide (N-terminal 66 bp encode aa M1 to G22, 22 aa), β-chain (5’ end) and α-chain (3’ end); Red letters, the glycosylation sites. B: Schematic diagram of analyzed fragments of clusterin and their RT-PCR results.
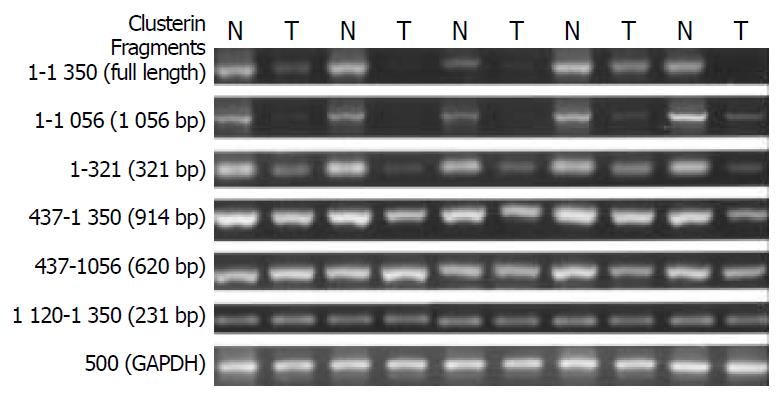
Figure 2 N-terminal truncated clusterin was analyzed by RT-PCR.
GAPDH was used as internal control. N: tumor matched far edge normal esophageal mucosa. T: ESCC specimens. The expression of full-length and different of clusterin fragments were analyzed comparing with the tumor matched normal esophageal mucosa. The truncated region was narrowed down at the 5’ end of clusterin (1-437 bp).
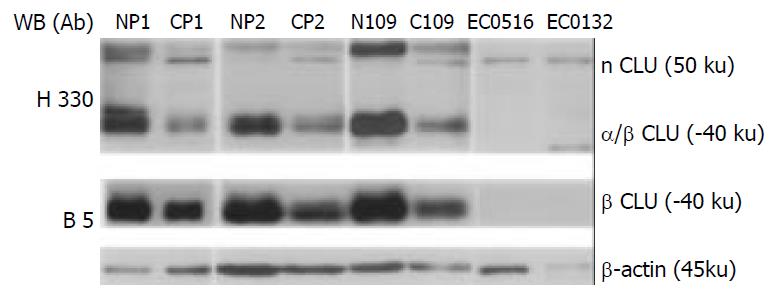
Figure 3 Western blot analysis of expression of multiple forms of clusterin in ESCC.
nCLU: the nonglycosylated uncleaved nuclear clusterin; α/β CLU: cleaved and glycosylated secreted clusterin protein; β CLU: cleaved and glycosylated clusterin β chain; B-5: monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) reacted with the C-terminus of clusterin-β of human origin and did not react with the nuclear clusterin; H-330: polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) recognized the major part of clusterin from 120 to 449 aa at the C-terminus of clusterin-α/β chains including unleaved nuclear clusterin (-50 ku and the matured secreted clusterin (-37-40 ku); Np: pooled normal esophageal mucosa from 10 cases; Cp: pooled ESCC tumor tissues from 10 cases; N109: normal esophageal mucosa from a single individual; C109: ESCC tumor tissue from a single individual. β-actin was used as loading control.
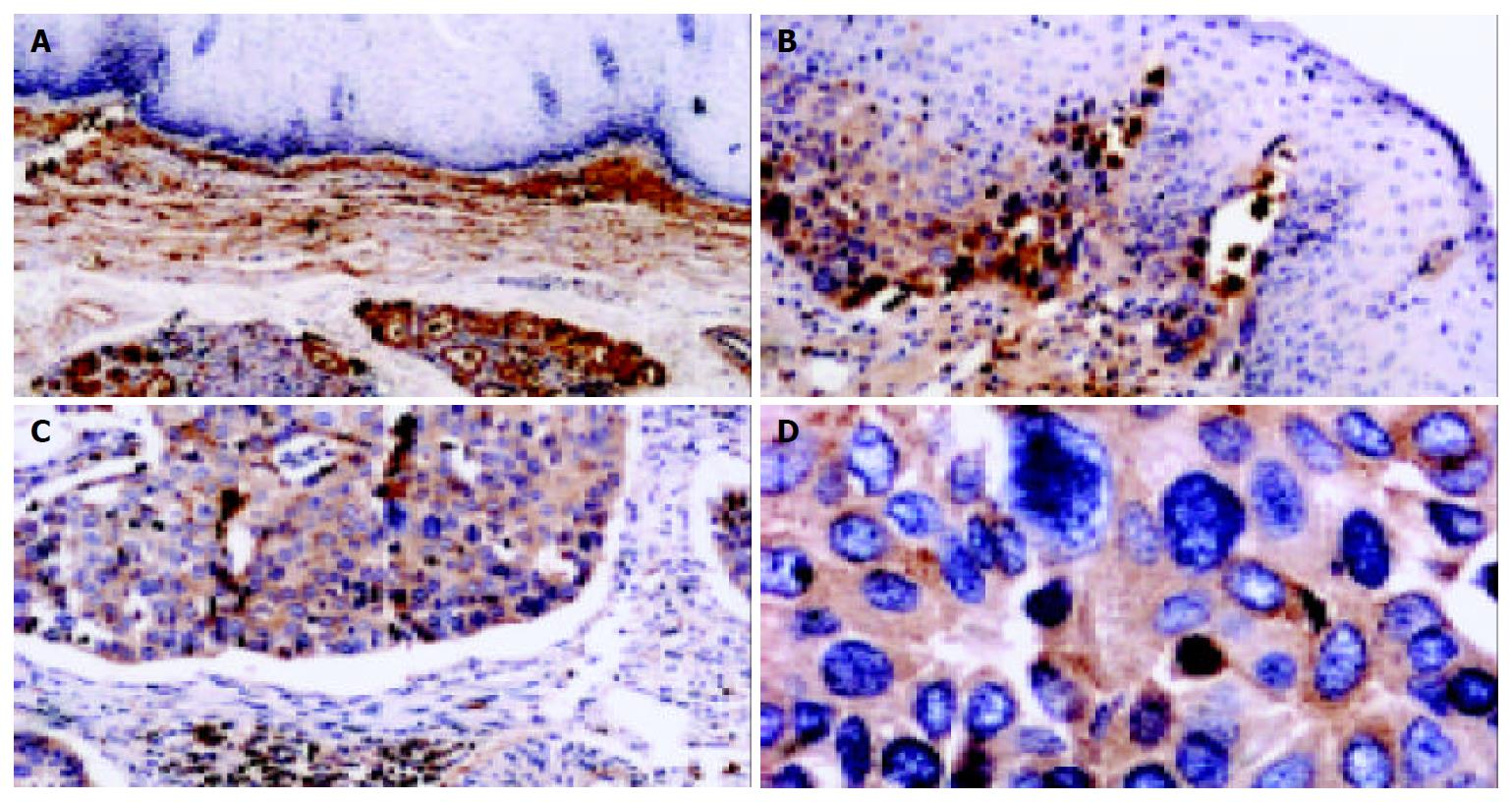
Figure 4 Detection of clusterin protein in tissue sections of human ESCC by immunohistochemistry.
In normal esophageal mucosa, the expression of clusterin in squamous epithelial cells and basal lamina was negative, while clusterin immunostaining was visualized obviously in the stroma of epithelial mucosa (A, 100 ×); clusterin was positive in middle dysplasia, while the vast majority of stromal cells were negative in normal squamous epithelia of esophagus (B, 100 ×); clusterin was strong positive in the remnants of stromal extracellular matrix invaded by tumor cells in well-differentiated ESCC (C, 100 ×). D show that stromal and basal membrane are almost completely disrupted and invaded by tumor cells. (D, same section, different field of that of C, 400 ×). Counterstaining was performed with hematoxylin.
- Citation: He HZ, Song ZM, Wang K, Teng LH, Liu F, Mao YS, Lu N, Zhang SZ, Wu M, Zhao XH. Alterations in expression, proteolysis and intracellular localizations of clusterin in esophageal squamous cell carcinoma. World J Gastroenterol 2004; 10(10): 1387-1391
- URL: https://www.wjgnet.com/1007-9327/full/v10/i10/1387.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i10.1387