Copyright
©The Author(s) 2016.
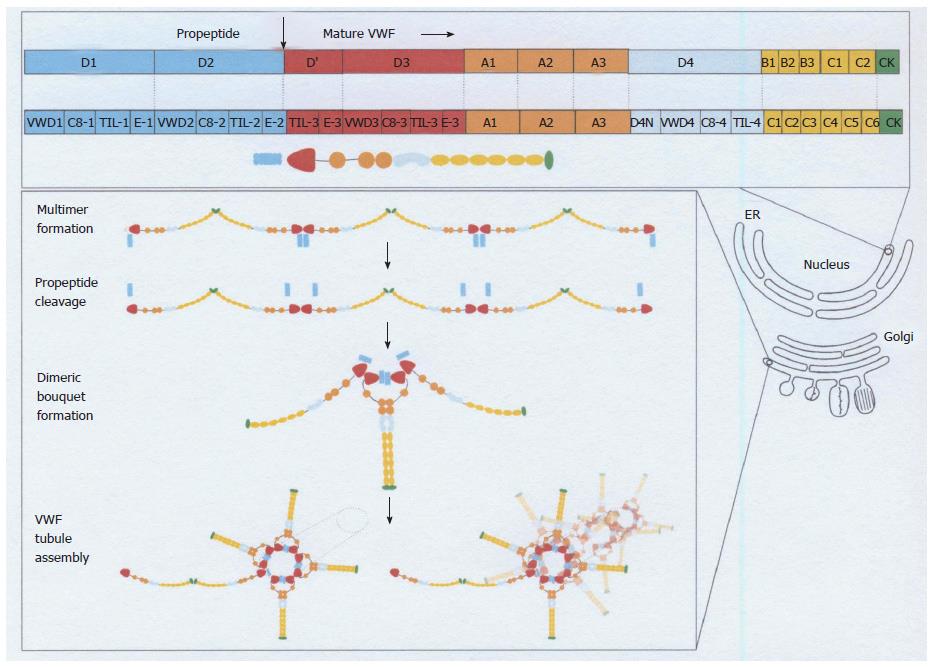
Figure 2 Von Willebrand factor domain structure and assembly throughout the biosynthetic pathways in endothelial cells[3-5].
The top panel shows the different domains of VWF as it is synthesized in the ER[4]. The arrow between the D2 domain and the D’ domain indicates the furin cleavage site at 764 leading to the production of the VWF propeptide (VWFpp) D1-D2 (blue) and the mature VWD protein with the domains D’, D3, A1, A2, D4, C1-6 and the cysteine knot (CK). The lower panel shows the assembly of VWF into multimers in the Golgi compartment, the cleavage VWFpp (blue), and the assembly of VWF into the dimeric bouquet at the trans-Golgi network (TNG). During the translocation of proVWF to the ER the signal peptide is cleaved off, and the proVWF forms dimers in a tail-to-tail fashion through cysteines in its carboxyterminal cysteine knot domain. ProVWF dimers transit to the Golgi apparatus to assemble into multimers in a “head-to-head” fashion through the formation of intermolecular disulphide bonds between cysteine residues in the D3 (multimerization) domain[4]. This is followed by the assembly of VWF in the Golgi network. ER: Endoplasmatic reticulum; VWD: Von Willebrand disease; VWF: Von Willebrand factor. Source: Valentijn and Eikenboom 2013[4].
- Citation: Michiels JJ, Batorova A, Prigancova T, Smejkal P, Penka M, Vangenechten I, Gadisseur A. Changing insights in the diagnosis and classification of autosomal recessive and dominant von Willebrand diseases 1980-2015. World J Hematol 2016; 5(3): 61-74
- URL: https://www.wjgnet.com/2218-6204/full/v5/i3/61.htm
- DOI: https://dx.doi.org/10.5315/wjh.v5.i3.61