Published online Aug 15, 2023. doi: 10.4239/wjd.v14.i8.1280
Peer-review started: April 19, 2023
First decision: April 28, 2023
Revised: May 16, 2023
Accepted: June 21, 2023
Article in press: June 21, 2023
Published online: August 15, 2023
Currently, the lack of comparative studies between weekly and daily formulations of glucagon-like peptide-1 receptor agonists (GLP-1RAs) for glucose excursion is worth investigation.
To investigate the effects of weekly and daily formulations of GLP-1RA on glucose excursion and inflammation in overweight and obese patients with type 2 diabetes.
Seventy patients with type 2 diabetes mellitus who were treated at our hospital between January 2019 and January 2022 were enrolled in this retrospective analysis. All patients were treated with metformin. We evaluated changes in blood glucose levels and a series of important indicators in patients before and after treatment with either a weekly or daily preparation of GLP-1RA (group A; n = 33 and group B; n = 37).
The degree of decrease in the levels of fasting blood glucose, mean blood glucose, mean amplitude of glycemic excursions, total cholesterol, triglycerides, tumor necrosis factor-α, interleukin-6, and high-sensitivity C-reactive protein after treatment in group A was higher than that in group B (P < 0.05), whereas the 2-h postprandial blood glucose levels decreased more so in group B than in group A (P < 0.001). However, there were no statistically significant differences in the levels of glycated hemoglobin, standard deviation of blood glucose, coefficient of variation, absolute mean of daily differences, percentage of time with 3.9 mmol/L < glucose < 10 mmol/L, and high- and low-density lipoproteins between the two groups (P > 0.05). The incidence of adverse reactions was significantly lower in group A than in group B (P < 0.05).
The effect of the weekly preparation of GLP-1RA in controlling blood glucose levels in the patients, suppressing inflammation, and reducing adverse reactions was significantly higher than that of the daily preparations, which is worthy of clinical promotion.
Core Tip: Weekly formulation of glucagon-like peptide-1 receptor agonists (GLP-1RAs) exhibited superior efficacy in treating obese patients with type 2 diabetes mellitus compared to the daily formulation. It effectively controls blood glucose levels, better regulates blood lipids, inhibits inflammatory reactions, and reduces adverse reactions. Therefore, the weekly formulation of GLP-1RA is a promising treatment option worthy of clinical promotion.
- Citation: Huang XM, Zhong X, Du YJ, Guo YY, Pan TR. Effects of glucagon-like peptide-1 receptor agonists on glucose excursion and inflammation in overweight or obese type 2 diabetic patients. World J Diabetes 2023; 14(8): 1280-1288
- URL: https://www.wjgnet.com/1948-9358/full/v14/i8/1280.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i8.1280
The prevalence of diabetes is increasing worldwide, with nearly 500 million people living with diabetes and is expected to increase by 25% by 2030 and 51% by 2045[1]. Currently, the prevalence of diabetes in China is as high as 11.2% and patients with type 2 diabetes mellitus (T2DM) account for > 90% of the population. However, the awareness rate (36.5%), treatment rate (32.2%), and control rate (49.2%) of diabetes are low, and the prevalence of diabetes in obese and over
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) have received much attention because of their unique glucose-lowering mechanisms, which mainly include a glucose concentration-dependent approach to promote insulin release, inhibit glucagon secretion, protect β-cells to increase their numbers, reduce hepatic glucose output, suppress appetite to increase satiety, and delay gastric emptying and gastrointestinal motility to lower blood glucose levels and reduce body weight[5-7]. In addition to these mechanisms, the role of GLP-1RAs in suppressing inflammation is currently receiving widespread attention. Diabetes mellitus is a complex chronic metabolic disease that requires continuous medical management with glycemic control along with multifactorial risk reduction strategies (e.g., blood pressure, lipid, and weight control)[8,9].
GLP-1RA is another injectable agent other than insulin in the treatment of T2DM, which is divided into two categories: daily and long-acting weekly agents according to the duration of action, among which the clinical use of weekly agents greatly reduces the number of injections and increases patient compliance, which is one of the important factors for long-term glycemic control in diabetic patients[10]. The weekly formulation has good prospects for clinical application as a once-weekly injection regimen. The current lack of comparative studies between weekly and daily formulations of GLP-1RAs on glucose excursion is worth in-depth exploration, especially for providing a new avenue for improving patient compliance and glycemic control in clinical treatment.
Seventy patients with T2DM who were treated at our hospital between January 2019 and January 2022 were enrolled in this retrospective analysis. All patients were treated with metformin. Specifically, patients were treated with either a weekly or daily preparation of GLP-1RA (group A; n = 33 and group B; n = 37). This study was approved by our Medical Ethics Committee.
The inclusion criteria were as follows: (1) Patients with symptoms who met the latest World Health Organization diagnostic criteria for diabetes mellitus[11]; (2) 18-60-years-old; (3) metformin monotherapy of up to 1500 mg in the last 3 mo for substandard glucose control; (4) 7.5%-10.0% glycated hemoglobin (HbA1c); and (5) 24 kg/m2 ≤ body mass index (BMI) ≤ 35 kg/m2.
Exclusion criteria: (1) Type 1 diabetes or other specific types of diabetes; (2) T2DM combined with acute complications of diabetes, infection, or stress; (3) severe liver, kidney, and gastrointestinal diseases (alanine aminotransferase and aspartate aminotransferase 2.5 times higher than the upper limit of normal, bilirubin 1.5 times higher than the upper limit of normal, and blood creatinine > 106 μmol/L); (4) myocardial infarction and chronic cardiac insufficiency (New York Heart Association [NYHA] classes III-IV); (5) history of acute or chronic pancreatitis; (6) patients with thyroid disease or serum calcitonin levels > 20 pg/mL; and (7) pregnancy, lactation, or planning a pregnancy in the near future.
All patients received an education on diabetes knowledge from dedicated staff, followed by the diet and appropriate amount of post-meal exercise as specified by a physician; they could skillfully apply the blood glucose meter for self-measurement, and all blood glucose meters were calibrated before use. This was combined with 500 mg metformin three times daily. Patients in group A were started with dulaglutide (S20190021; Eli Lilly Nederland B.V., The Netherlands) 0.75 mg subcutaneously once a week and increased to 1.5 mg once per week after 1 wk if formamidopyrimidine DNA glycosylase (FPG) > 7.0 mmol/L or 2-h plasma glucose (2hPG) > 11.0 mmol/L was measured. Patients in group B started with 0.6 mg liraglutide subcutaneously once daily and increased to 1.2 mg twice daily if FPG > 7.0 mmol/L or 2hPG > 11.0 mmol/L was measured after 1 wk and increased to 1.8 mg once daily if FPG > 7.0 mmol/L or 2hPG > 11.0 mmol/L was measured after 1 wk. Patients who were originally taking oral antihypertensive and lipid-regulating drugs continued the original regimen.
Patients wore a 72-h ambulatory continuous glucose monitor (CGM; MMT-7745; Medtronic, Minneapolis, MN, United States) before and after 12 wk of treatment, and monitored three times a day before meals and before bedtime as well as fasting fingertip glucose to calibrate ambulatory glucose values. The software analysis system was used to process the blood glucose data to derive the following parameters: 24-h mean blood glucose (MBG), 24-h standard deviation of blood glucose (SDBG), coefficient of variation (CV%), mean amplitude of glycemic excursions (MAGEs), absolute mean of daily differences (MODDs), and percentage of time with 3.9 mmol/L < glucose < 10 mmol/L (TIR).
The main outcome measures were as follows: blood glucose levels (plasma fasting blood glucose [FBG], 2-h postprandial blood glucose [PBG], and HbA1c) were compared before and after treatment between the two groups. The changes in ambulatory glucose monitoring indices, including MBG, SDBG, CV%, MAGE, MODD, and TIR, were compared between the two groups.
The secondary outcome measures were as follows: the baseline clinical data of the two groups were compared. The incidence of adverse reactions and changes in lipid indices (total cholesterol and triglyceride levels) were compared between the two groups. The levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and high-sensitivity C-reactive protein (hs-CRP) were compared between the two groups before and after treatment using an enzyme-linked immunosorbent assay, and the changes in BMI were compared before and after treatment.
Before and after 12 wk of treatment, the patient was equipped with a 72-h ambulatory CGM (Medtronic), which monitored fasting fingertip blood glucose three times a day before meals and before bedtime. A software analysis system was used to process blood glucose data to obtain the following parameters: MBG, SDBG, CV%, MAGE, MODD, and TIR.
The counting data are expressed as rates and counted by the χ2 test. The measurement data are expressed as the mean ± standard deviation, and the paired samples t-test was used for intra-group comparison before and after treatment, and the independent samples t-test was used for intergroup comparison. P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 20.0 software.
The clinical data of the two groups were compared. There was no statistical difference in age, sex, course of disease, BMI, and history of hypertension and smoking between patients in group A and group B (P > 0.05; Table 1).
| Factor | Group A, n = 33 | Group B, n = 37 | P value |
| Age in yr | |||
| ≥ 60 | 13 | 17 | 0.580 |
| < 60 | 20 | 20 | |
| Sex | |||
| Male | 22 | 35 | 0.447 |
| Female | 11 | 12 | |
| Course of disease in yr | |||
| ≥ 5 | 19 | 20 | 0.767 |
| < 5 | 14 | 17 | |
| BMI in kg/m2 | |||
| ≥ 30 | 25 | 30 | 0.587 |
| < 30 | 8 | 7 | |
| History of hypertension | |||
| Yes | 8 | 10 | 0.790 |
| No | 25 | 27 | |
| History of smoking | |||
| Yes | 22 | 35 | 0.447 |
| No | 11 | 12 |
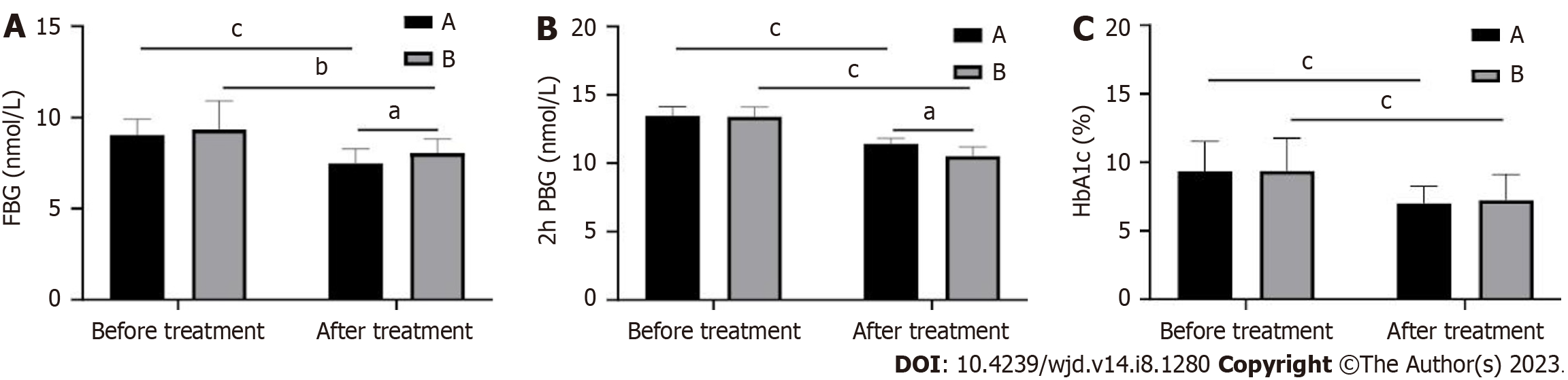
Comparison of the changes in blood glucose levels between the two groups revealed that FBG, 2h PBG, and HbA1c were significantly decreased in both groups after treatment (P < 0.05). Among them, FBG decreased more in group A than in group B after treatment, and 2h PBG decreased more in group B than in group A (P < 0.001; Figure 1). There was no difference in HbA1c between the two groups after treatment (P > 0.05).
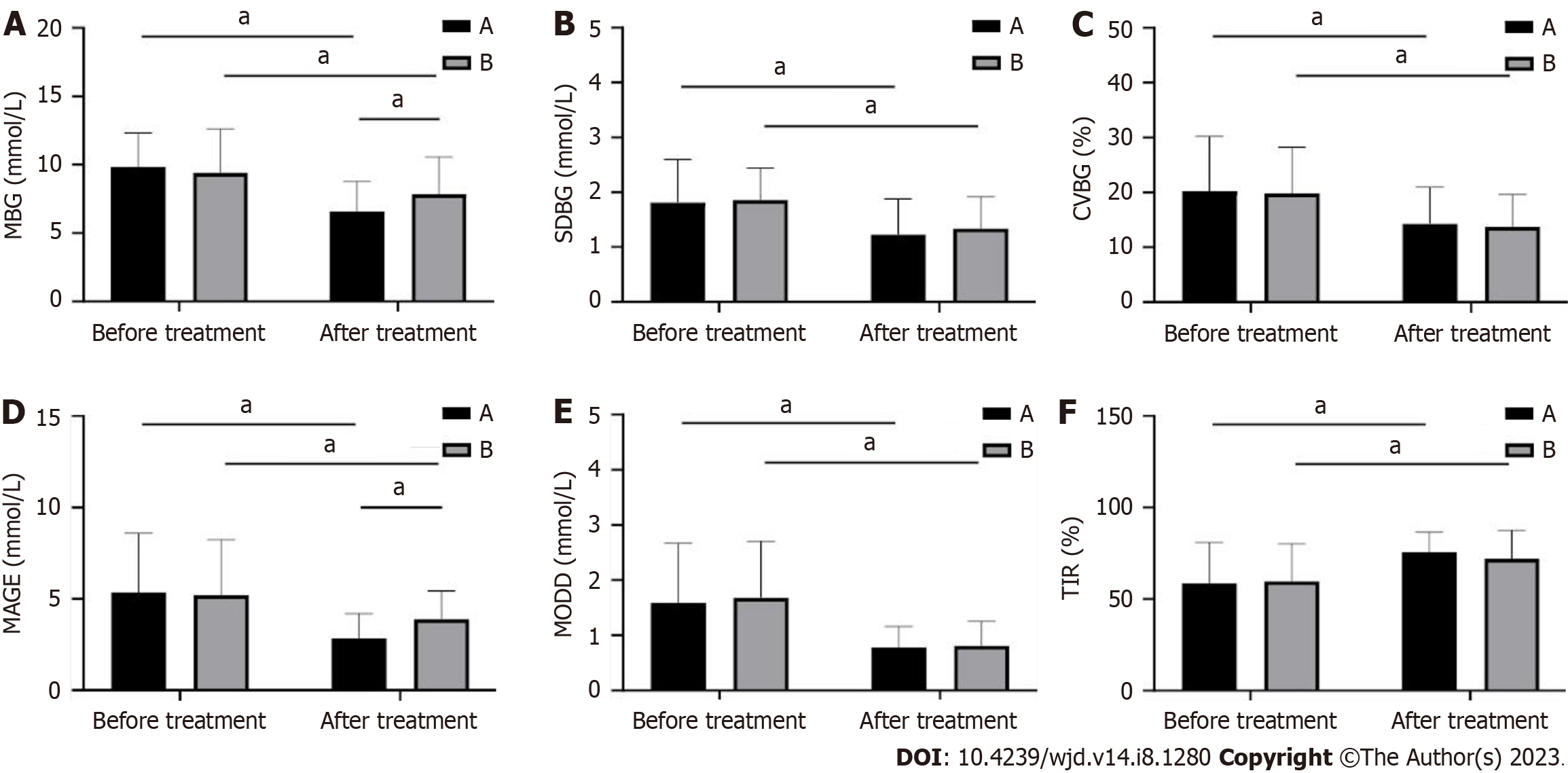
The dynamic indicators of blood glucose before and after treatment in the two groups were found to be statistically non-different in MBG, SDBG, CV%, MAGE, MODD, and TIR before treatment in the two groups (P > 0.05; Figure 2). Patients were treated with a significant decrease in MBG, SDBG, CV%, MAGE, and MODD in both groups compared to pre-treatment, whereas TIR increased dramatically (P < 0.001; Figure 2). Further comparison revealed that MBG and MAGE were significantly lower in group A than in group B (P < 0.01; Figure 2), but there was no statistical difference in SDBG, CV%, MODD, and TIR between both groups (P > 0.05; Figure 2).
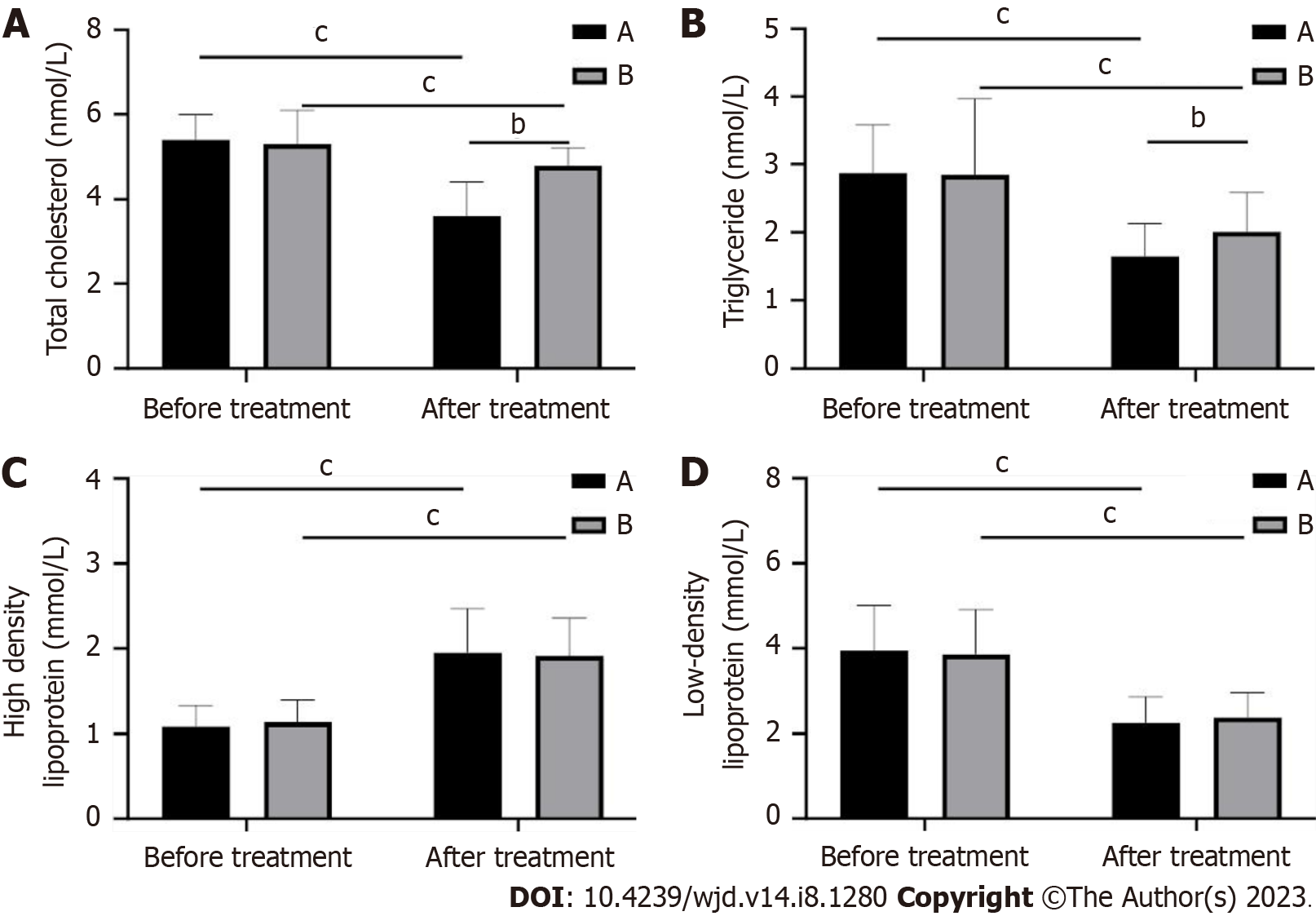
In this study, we also examined the changes in the lipid indexes of patients. Total cholesterol, triglyceride and low-density lipoprotein (LDL) levels were significantly lower and high-density lipoprotein (HDL) levels were significantly higher (P < 0.01) in both groups after treatment compared to those before treatment. Among them, total cholesterol and triglyceride levels decreased more in group A than in group B after treatment (P < 0.05; Figure 3), but there was no difference between HDL and LDL (P > 0.05).
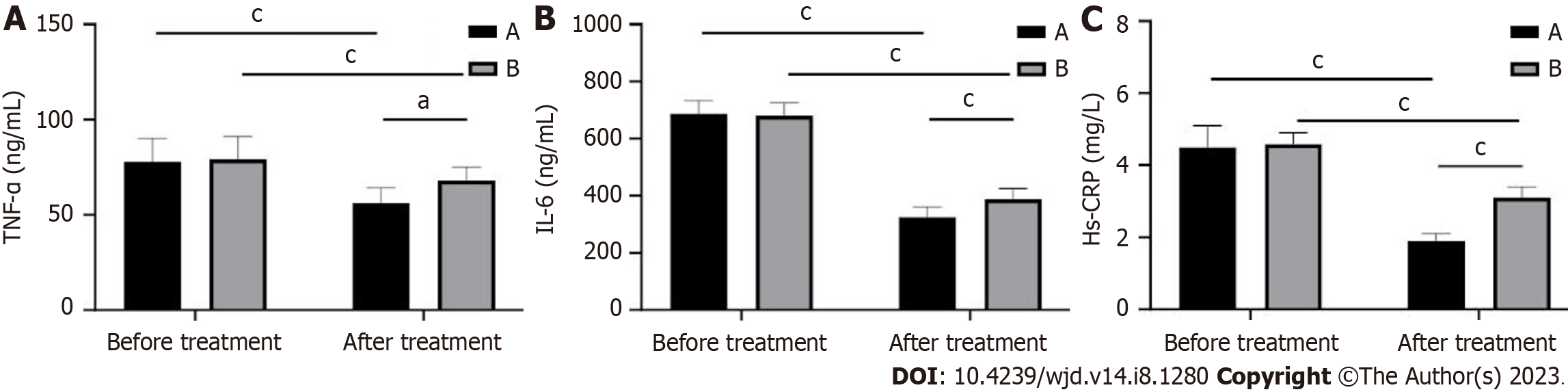
In this study, we also detected changes in inflammatory factors in patients. The levels of TNF-α, IL-6, and hs-CRP were significantly lower in both groups after treatment compared with those before treatment (P < 0.01). Among them, the level of TNF-α, IL-6, and hs-CRP decreased to a greater extent in group A than in group B after treatment (P < 0.05; Figure 4).
Comparison of the adverse reactions between the two groups revealed that the incidence of adverse reactions was significantly lower in group A than in group B. There was a statistical difference (P < 0.05; Table 2).
| Group | Nausea | Vomiting | Diarrhea | Total incidence |
| Group A, n = 33 | 1 (3.03%) | 1 (3.03%) | 0 (0.00%) | 2 (6.06) |
| Group B, n = 37 | 4 (10.80%) | 3 (8.10%) | 2 (5.40) | 9 (24.30) |
| χ2 value | 4.393 | |||
| P value | 0.036 |
In this study, we also examined the changes in BMI before and after treatment in both groups. Patients in both groups showed a significant decrease in BMI through treatment (P < 0.05), with patients in group A showing a higher decrease in BMI after treatment than those in group B (P < 0.001, Table 3).
| Group | BMI in kg/m2 | t value | P value | |
| Before treatment | After treatment | |||
| Group A, n = 33 | 33.29 ± 4.05 | 26.89 ± 1.59 | 8.096 | < 0.001 |
| Group B, n = 37 | 33.35 ± 3.29 | 30.22 ± 1.48 | 5.099 | < 0.001 |
| t value | 0.125 | 8.999 | ||
| P value | 0.900 | < 0.001 | ||
Studying the causes of T2DM has expanded from the well-known deficiency of islet β-cell secretion, increased hepatic glycogen output, and decreased muscle glucose uptake to lipid metabolic disorder, weakening of the effect of intestinal glucagon, inappropriate secretion of glucagon in islet α-cells, increase of glucose reabsorption by renal tubules, and hypothalamic regulatory disorder of blood glucose[12-14].
Numerous types of drugs are available for the treatment of T2DM including insulin promoters, insulin sensitizers, α-glucosidase inhibitors, insulin or insulin analogs, dipeptidyl peptidase 4 inhibitors, and sodium-glucose co-transporter 2 inhibitors[15-17]. However, most of these drugs are not only ineffective in maintaining blood glucose in the long term but also have side effects such as weight gain, progressive pancreatic β-cell failure, liver and renal impairment, gastro
In the present study, we compared the blood glucose control effects of weekly GLP-1RA administration with that of daily GLP-1RA administration and analyzed glucose excursion and inflammation in overweight or obese patients with T2DM. After treatment, FBG, MBG, MAGE, total cholesterol, triglyceride, and TNF-α levels were measured in patients in group A. The decrease in IL-6 and hs-CRP levels was greater in group A than in group B. We also found through glucose dynamic tests that the MBG, SDBG, CV%, MAGE, and MODD of patients in the two groups were decreased significantly after treatment compared to those before treatment, whereas TIR increased in both groups. This indicates that both treatment schemes can improve blood glucose drift in patients. However, we found that the MBG and MAGE levels in group A were significantly lower than those in group B. Previously, in a meta-analysis performed by Yang et al[21], it was found that the use of a GLP-1RA weekly formula was superior to the daily formula in improving HbA1c and FBG levels in patients with T2DM, which is consistent with our findings. This is because weekly preparations can effectively control FBG levels by stimulating insulin secretion and inhibiting glucagon secretion, whereas daily preparations mainly rely on delaying gastric emptying and slowing glucose absorption in the duodenum, mainly to lower PBG levels. In addition, weekly preparations have a half-life of several days and can continuously agitate GLP-1R to produce hypoglycemic effects; therefore, the hypoglycemic efficacy of weekly preparations, especially for FBG control, is better than that of daily preparations[22]. Most of the initially diagnosed obese patients with T2DM have inflammation, and inflammatory factors such as TNF-α, IL-6, and hs-CRP are abnormally elevated, and this inflammation leads to insulin resistance and increases the risk of cardiovascular disease, so it is significant to control inflammation in the body[23]. The results of the current study showed that after treatment, the levels of TNF-α and IL-6 in group A were lower than those in group B, indicating that the use of weekly preparations of GLP-1RA for treating obese patients with T2DM is more effective, facilitates the reduction of inflammatory factors, and has a higher safety for clinical application.
GLP-1 receptors are expressed on cardiomyocytes, vascular smooth muscle cells, and vascular endothelial cells. GLP-1RA can inhibit smooth muscle cell proliferation and high glucose-induced apoptosis in endothelial cells, promote endothelial cell proliferation, stabilize the endothelial environment, and reduce injury, which directly affects the cardiovascular system and ultimately the outcome of patients with T2DM[24]. GLP-1RA acts on the hypothalamic feeding center to delay the emptying of food into the stomach, suppressing the appetite of patients with a significant weight loss effect, and thus reducing the risk of cardiovascular disease[25]. In addition, GLP-1RA inhibitors directly participate in lipid metabolism and accelerate fat mobilization. The present study showed that the levels of total cholesterol and triglycerides post-treatment were lower in patients treated with the weekly formulation of GLP-1RA than in those treated with the daily formulation, suggesting that both drugs are suitable for obese patients with high lipid levels, but that the weekly formulation is the most effective.
This study had some limitations. First, we did not conduct patient follow-up sessions. Second, this study was retrospective, which may have introduced a recall bias in the analysis of the results. Finally, only the short-term treatment effects of the two drugs were analyzed, and it remains unclear whether there are differences in the long-term. Future studies should address these limitations through extensive clinical trials to refine our conclusions.
In conclusion, the effect of weekly preparations of GLP-1RA in controlling the blood glucose levels in obese patients with T2DM, inhibiting inflammation, and reducing adverse reactions was significantly higher than that of daily preparations, which is worthy of clinical promotion.
The background of the research is the increasing prevalence and challenges associated with type 2 diabetes mellitus (T2DM). The study explores the causes of T2DM and the limitations of current treatment options.
The motivation behind the research is to address the limitations of existing drugs for T2DM treatment and explore the potential of glucagon-like peptide-1 receptor agonists (GLP-1RAs) as a more effective therapeutic option.
The objectives of the research are to compare the blood glucose control effects of weekly and daily formulations of GLP-1RAs, analyze glucose excursion and inflammation in overweight or obese patients with T2DM, and evaluate the safety and clinical application prospects of the weekly formulation.
The study involved administering weekly and daily formulations of GLP-1RA to the participants and measuring various parameters such as fasting blood glucose, mean blood glucose, glucose excursion, lipid levels, and inflammation markers. Glucose dynamic tests were conducted to assess blood glucose fluctuations.
The results indicated that the weekly formulation of GLP-1RA had superior blood glucose control effects compared to the daily formulation. It resulted in lower mean blood glucose levels, reduced glucose excursion, and improved lipid profiles. Additionally, the weekly formulation showed a greater decrease in inflammation markers.
Based on the findings, the research concludes that the weekly formulation of GLP-1RA is more effective in controlling blood glucose levels, inhibiting inflammation, and reducing adverse reactions in obese patients with T2DM. It suggests that the weekly formulation has promising clinical applications and should be considered for wider implementation.
To investigate the effects of weekly and daily formulations of GLP-1RA on glucose excursion and inflammation in overweight and obese patients with type 2 diabetes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gregg EW, United Kingdom; Schlesinger S, Germany S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Xu ZH
| 1. | Valenti G, Tamma G. History of Diabetes Insipidus. G Ital Nefrol. 2016;33 Suppl 66:33.S66.1. [PubMed] [Cited in This Article: ] |
| 2. | Palumbo C, Nicolaci N, La Manna AA, Branek N, Pissano MN. [Association between central diabetes insipidus and type 2 diabetes mellitus]. Medicina (B Aires). 2018;78:127-130. [PubMed] [Cited in This Article: ] |
| 3. | Scherbaum WA. Autoimmune diabetes insipidus. Handb Clin Neurol. 2021;181:193-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Refardt J, Winzeler B, Christ-Crain M. Diabetes Insipidus: An Update. Endocrinol Metab Clin North Am. 2020;49:517-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Rossing P, Agarwal R, Anker SD, Filippatos G, Pitt B, Ruilope LM, Amod A, Marre M, Joseph A, Lage A, Scott C, Bakris GL; FIDELIO-DKD Investigators. Efficacy and safety of finerenone in patients with chronic kidney disease and type 2 diabetes by GLP-1RA treatment: A subgroup analysis from the FIDELIO-DKD trial. Diabetes Obes Metab. 2022;24:125-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 6. | Giugliano D, Scappaticcio L, Longo M, Caruso P, Maiorino MI, Bellastella G, Ceriello A, Chiodini P, Esposito K. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Cardiovasc Diabetol. 2021;20:189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 7. | Andreasen CR, Andersen A, Knop FK, Vilsbøll T. Understanding the place for GLP-1RA therapy: Translating guidelines for treatment of type 2 diabetes into everyday clinical practice and patient selection. Diabetes Obes Metab. 2021;23 Suppl 3:40-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Brunton SA, Wysham CH. GLP-1 receptor agonists in the treatment of type 2 diabetes: role and clinical experience to date. Postgrad Med. 2020;132:3-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 9. | Dave CV, Kim SC, Goldfine AB, Glynn RJ, Tong A, Patorno E. Risk of Cardiovascular Outcomes in Patients With Type 2 Diabetes After Addition of SGLT2 Inhibitors Versus Sulfonylureas to Baseline GLP-1RA Therapy. Circulation. 2021;143:770-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Nelson AJ, Pagidipati NJ, Aroda VR, Cavender MA, Green JB, Lopes RD, Al-Khalidi H, Gaynor T, Kaltenbach LA, Kirk JK, Lingvay I, Magwire ML, O'Brien EC, Pak J, Pop-Busui R, Richardson CR, Reed M, Senyucel C, Webb L, McGuire DK, Granger CB. Incorporating SGLT2i and GLP-1RA for Cardiovascular and Kidney Disease Risk Reduction: Call for Action to the Cardiology Community. Circulation. 2021;144:74-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract. 2014;103:341-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 407] [Cited by in F6Publishing: 466] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 12. | Javeed N, Matveyenko AV. Circadian Etiology of Type 2 Diabetes Mellitus. Physiology (Bethesda). 2018;33:138-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 13. | Brunton S. Pathophysiology of Type 2 Diabetes: The Evolution of Our Understanding. J Fam Pract. 2016;65. [PubMed] [Cited in This Article: ] |
| 14. | Wu Y, Ding Y, Tanaka Y, Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int J Med Sci. 2014;11:1185-1200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 512] [Cited by in F6Publishing: 540] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 15. | Lingvay I, Sumithran P, Cohen RV, le Roux CW. Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet. 2022;399:394-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 178] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 16. | Ferguson D, Finck BN. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17:484-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 212] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 17. | Dashti HM, Mathew TC, Al-Zaid NS. Efficacy of Low-Carbohydrate Ketogenic Diet in the Treatment of Type 2 Diabetes. Med Princ Pract. 2021;30:223-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Padhi S, Nayak AK, Behera A. Type II diabetes mellitus: a review on recent drug based therapeutics. Biomed Pharmacother. 2020;131:110708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 19. | Xu L, Li Y, Dai Y, Peng J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol Res. 2018;130:451-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 241] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 20. | Lisco G, De Tullio A, Guastamacchia E, Triggiani V. Fixed-Ratio Combinations of Basal Insulin and GLP-1RA in the Management of Type 2 Diabetes Mellitus: Highlights from the Literature. Endocr Metab Immune Disord Drug Targets. 2021;21:626-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Yang B, Wang Z, Dong J. The Specific Magnetic Resonance Imaging Indicators in Predicting Clear-Cell Renal Cell Carcinoma Metastatic to the Sinonasal Region. J Comput Assist Tomogr. 2020;44:70-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Nauck MA, D'Alessio DA. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovasc Diabetol. 2022;21:169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 68] [Reference Citation Analysis (1)] |
| 23. | Scheithauer TPM; Rampanelli E; Nieuwdorp M, Vallance BA, Verchere CB, van Raalte DH, Herrema H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front Immunol. 2020;11:571731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 237] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 24. | Andersen A, Knop FK, Vilsbøll T. A Pharmacological and Clinical Overview of Oral Semaglutide for the Treatment of Type 2 Diabetes. Drugs. 2021;81:1003-1030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Castellana M, Cignarelli A, Brescia F, Laviola L, Giorgino F. GLP-1 receptor agonist added to insulin versus basal-plus or basal-bolus insulin therapy in type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab Res Rev. 2019;35:e3082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |