Published online Sep 8, 2024. doi: 10.35713/aic.v5.i2.97317
Revised: July 11, 2024
Accepted: July 17, 2024
Published online: September 8, 2024
Processing time: 100 Days and 5.2 Hours
Artificial intelligence is rapidly evolving and its application is increasing day-by-day in the medical field. The application of artificial intelligence is also valuable in gastrointestinal diseases, by calculating various scoring systems, evaluating radio
Core Tip: Artificial intelligence (AI), a term coined by John McCarthy in 1955, is the new-kid-on-the-block in the medical arena, with immense potential to revolutionize how patients may be managed in coming years. It is a science of creating machines with capa
- Citation: Shukla A, Chaudhary R, Nayyar N. Role of artificial intelligence in gastrointestinal surgery. Artif Intell Cancer 2024; 5(2): 97317
- URL: https://www.wjgnet.com/2644-3228/full/v5/i2/97317.htm
- DOI: https://dx.doi.org/10.35713/aic.v5.i2.97317
The platform of artificial intelligence is revolutionizing the medical field and changing approaches to reach a definitive diagnosis. Furthermore, artificial intelligence is expanding its horizons not only in clinical diagnoses, but also in the operative management of patients. Leonard et al[1] and John Hopkins University have shown this by developing the Smart Tissue Autonomous Robot (STAR) to perform ex vivo and in vivo anastomosis of the small bowel in porcine models[2]. Artificial intelligence can be vaguely defined as the study of sets of algorithms giving machines the ability to perform reasoning and solve problems, along with image, object, and word identification[3,4]. Another innovation in the surgical field was Gestonurse, which is a robotic scrub nurse used to hand instruments to the operating surgeon[5].
The index application of artificial intelligence in gastrointestinal surgery was documented in 1976, when it was introduced to aid computer analysis for acute abdominal pain[6]. The term artificial intelligence was coined by McCarthy et al[7] in 1955 during his summer research project. However, its origin can be traced back to 1950, when Turing[8], a British mathematician, used a computer to display human-like behavior in the Turing test.
Most of the initial advancements of artificial intelligence are depicted in the field of dermatology, radiology, and pathology, noted by an increase in Food and Drug Administration approvals for artificial intelligence devices in these fields[9]. Also, in pathology, a reduction in error rates from 3.4% to 0.5% was seen with the help of artificial intelligence[10]. Artificial intelligence has also seeped deeply into our lives in the form of daily assistance devices such as Siri, Alexa, chatbots, Google assistant, smart homes, navigation apps, autonomous vehicles, etc. Similarly, machine and deep learning is assisting physicians in diagnosing various gastrointestinal diseases, liver tumors, pancreatic neoplasms, and various infections.
Applying artificial intelligence to large and complex data is also helping to identify new variables and their associations, shaping changes in today’s clinical practices. In the surgical field, the surgeon must associate with data scientists to unfold the role of artificial intelligence. In the future, human roles may be limited to only supervision and authentication of different models of artificial intelligence.
We performed a PubMed search for relevant articles, and then searched the article reference lists for additional appropriate studies. The keywords and combinations included in the search were as follows: “Artificial intelligence” and “radiology”; “artificial intelligence” and “pathology”; “artificial intelligence” and “endoscopic management”; “artificial intelligence” and “gastrointestinal surgery”; “artificial intelligence” and “gastric cancer”; “artificial intelligence” and “colorectal malignancy”; “artificial intelligence” and “pancreatic cancer”; and “artificial intelligence” and “robotic surgery”. The search was limited to publications in English. All the authors agreed that the articles selected for review were relevant.
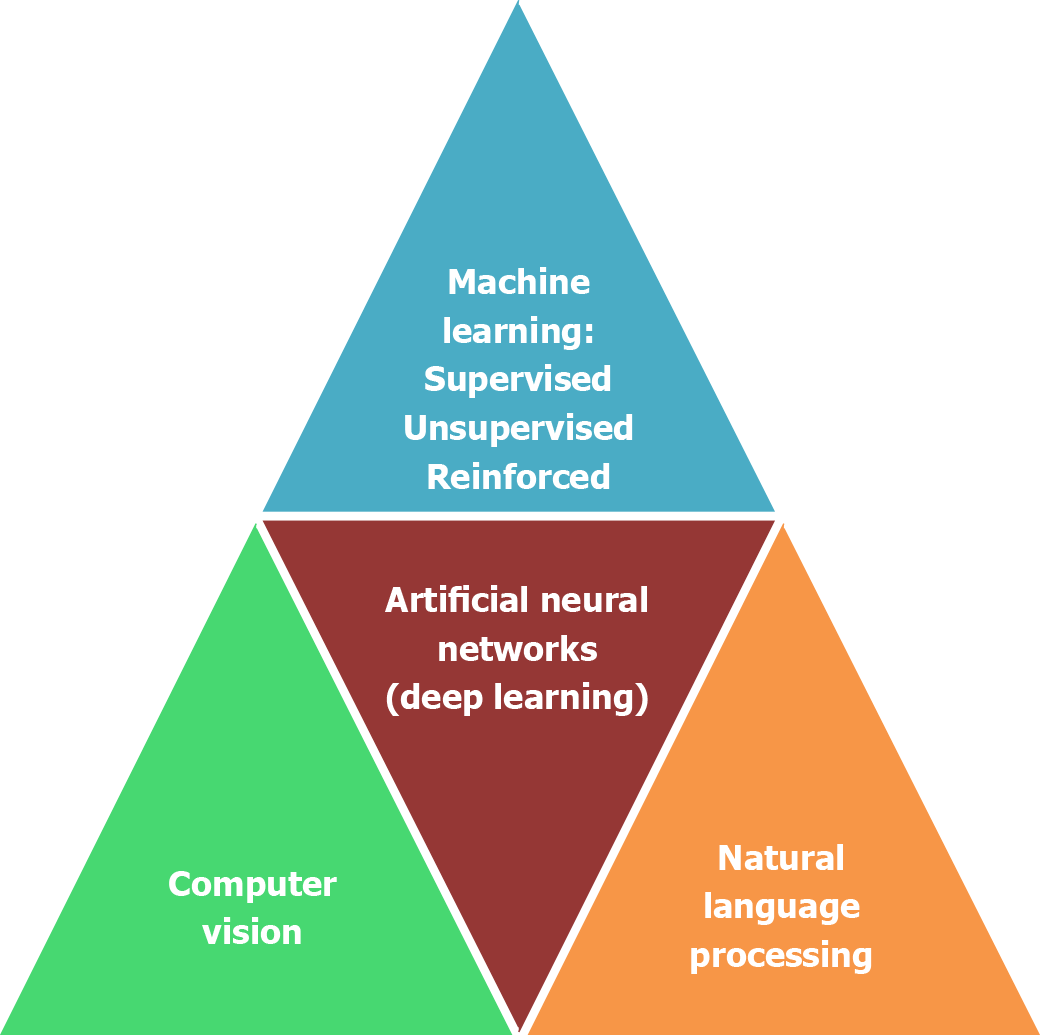
The basis and advances of artificial intelligence relies on machine learning and deep learning. Artificial intelligence has found many uses in surgical diseases, from diagnosis and scoring systems to preoperative and intraoperative planning and assistance, as well as in surgical training and robotics[11-13]. Principal subfields of artificial intelligence are: (1) Machine learning; (2) artificial neural networks; (3) natural language processing; and (4) computer vision (Figure 1).
The purpose of machine learning is to equip machines to learn and predict by identifying similar patterns[14]. Machine learning is based on the ability to do a task with the help of a set of algorithms without programming, and is further sub
Reinforced learning is a type of machine learning wherein the device makes decisions to achieve the most optimal results. It is similar to the trial and error learning process that humans use to fulfill their goals. An example of such a module is an artificial pancreas device used to accurately measure and inject doses of insulin for diabetic patients[17]. Another use of machine learning is precisely predicting mortality, sepsis, and acute kidney injury with the help of an intraoperative database[18,19].
An artificial neural network applying binary threshold functions was conceived in 1943 by McCulloch and Pitts[20]. It is the subdivision of artificial intelligence persuaded by the human nervous system and applied in various medical applications. Deep learning is a new type of artificial intelligence based on artificial neural networks. The deep learning neural network is composed of multiple layers, which enables them to learn minuscule patterns from the given datasets in comparison to simple or two-layered neural networks[21]. In deep learning, every network subgroup of inputs leads to various concealed subgroups in response to distinct characteristics of inputs, which allows for an in-depth understanding of datasets similar to multiple neuronal connections occurring in the human brain[22,23]. In the arena of clinical applications, the artificial neural network has shown high accuracy and sensitivity and specificity in predicting in-hospital mortality following abdominal aortic aneurysms. Similarly, its application in acute pancreatitis to assess severity has surpassed conventional APACHE II scoring (sensitivity and specificity of 80% and 85%, respectively), with a sensitivity and specificity of 89% and 96%, respectively[24]. To analyze images, convolutional neural networks are a type of deep neural network used in the detection of gastrointestinal malignancy within endoscopic images, radiologic images, and pathologic specimen assessments[14,25].
Computer vision, a subtype of artificial intelligence, portrays the ability of a device or machine to obtain and interpret images or videos like humans. It has been widely used in axial imaging for virtual colonoscopy, computer aided dia
Natural language processing is the subfield of artificial intelligence dealing with interpretation of text form and voice data from electronic health records[28]. It has a role in both preoperative and postoperative assessments of surgical patients to predict risks, outcomes, and complication rates. It is used in the surgical field to follow up on patients and monitor complications[29]. One of the most frequent applications of natural language processing is to foretell the risk of surgical site infection in various surgical procedures[30-32].
Artificial intelligence is improving the field of gastrointestinal surgery, and helping in the diagnosis and management in many gastrointestinal diseases where images (endoscopy, radiology, and pathology) are used such as in Barrett’s esophagus, esophageal cancers, gastric cancer, Helicobacter infection, pancreatic diseases, liver tumors, colonic polyps, and malignancies. It also helps in identifying precancerous lesions more efficiently and to reduce missing small lesions. In comparison to other fields, the diagnosis of surgical diseases require more dynamic and complex algorithms, sometimes involving real time decision making, similar to differences in artificial intelligence software used to recognize faces and to drive autonomous vehicles.
In abdominal wall hernia repair, artificial intelligence uses supervised machine learning algorithms and preoperative computed tomography images to anticipate risks and requirements of component separation techniques. In a study, this model was found to be superior to a panel of consultant surgeons[33]. Artificial intelligence has been used in convolutional neural network-based algorithms to find stomach malignancy with endoscopic images since 2018[34]. One multicenter study introduced the Gastrointestinal Artificial Intelligence Diagnostic System model to diagnose upper gastrointestinal cancers in late 2019, comparing its accuracy with a specialist and trainee endoscopist, and noticed it to be better than the trainee and similar to the specialist in diagnosing stomach carcinoma[35]. Convolutional neural networks using narrow band imaging in endoscopy have been used to differentiate stomach cancer and gastritis with an upper limit of sensitivity of 95% and positive predictive value of 91%[36]. In studies of Barrett’s esophagus, deep learning model algorithms have been used to find neoplastic changes based on datasets of images, showing superior accuracy to amateur endoscopists, along with the optimum site for biopsy in 97% cases[37]. Another study using a convolutional neural network-based model was found to be more accurate in detecting early gastric carcinoma than a specialist endoscopist[38]. Other studies have used deep learning modules along with biomarkers, histologic images, and radiologic images to anticipate liver and lymph node spread in stomach carcinoma[39,40]. Various studies have also used evolved models anticipating risk stratification of stomach carcinoma and its survival prediction[41,42]. In esophageal carcinoma, deep learning models have been developed to find squamous cell carcinoma of the esophagus using narrow band imaging as the input; models anticipating depth of esophageal malignancy have also been developed[43-45].
In the large bowel, artificial intelligence has been used to find polyps and differentiate hyperplastic or adenomatous polyps based on deep learning and convolutional neural networks[46,47]. Artificial intelligence is also helpful in segre
Anticipation of resection of peritoneal carcinomatosis necessitates laparotomy to optimally classify the disease burden. Random forest models based on machine learning can determine resectability in this condition with accuracy to the tune of 98%, preventing unwanted laparotomies[52]. Another machine learning based model has been developed to examine intrahepatic cholangiocarcinoma patients preoperatively using various parameters, and to anticipate which patients would benefit from surgery[53,54]. Using artificial neural networks, the uterine artery can be distinguished from the ureter in laparoscopic hysterectomy, as well as other surgeries posing risk of ureteral injury[55]. In hepatic surgery, arti
In various gastrointestinal surgeries, postoperative management can be augmented with the help of artificial inte
Radiology assists in gastrointestinal diseases using images generated through X-ray, computed tomography, and magnetic resonance imaging, which are used by deep learning models as data inputs to identify anatomy and differentiate malignant or benign tumors[64]. Radiomics has been used to assess and differentiate the malignant potential of various tumors by assessing images via deep learning of shapes, texture, and histograms[65,66]. Some studies have developed survival prediction models with the help of radiomics analysis of computed tomography images. Following image segmentation, the analysis was accomplished and risk scores stratified, showing the superiority of radiomics over normal nomograms in stomach cancer patients[67,68].
In colorectal liver metastases using computed tomography and magnetic resonance imaging, radiomics have depicted splendid accuracy in finding early liver spread, which might aid in effective management and better prognosis. It is also helpful in predicting responses to chemotherapy[69]. Studies have tried to predict peritoneal spread with the help of con
As with any new technology, artificial intelligence also has limitations and drawbacks, as it depends heavily on the data provided. The quality, amount, and variation in the data used to train the model is an important aspect. Outcomes depend on the quality of the dataset provided for input, and any shortcomings will affect the results. Biases while accumulating clinical data have an impact on the kind of patterns artificial intelligence perceives and the predictions it makes[79,80]. In gastrointestinal malignancy, IBM Watson Oncology—a question–answer-based computer device—was supposed to help physicians in decision making and to keep updated with recent evidence; however, it has not lived up to the standard[81,82]. In the presence of well-devised models, depending entirely on artificial intelligence is not recommended, as a skilled endoscopist is still needed to accurately capture the data[83].
Radiological models are deficient in automatic segmentation in gastrointestinal malignancies due to large discrepancies in imaging of the gastrointestinal tract. Radiomics appears to be efficient in finding malignant probability with analyses of intratumor heterogeneity; however, it also has limitations in the form of interobserver variability during segmentation, scanning equipment, image acquisition protocols, and algorithm reconstruction[84-86]. In endoscopic imaging, most studies are retrospective in nature and do not represent real clinical scenarios, and it is not clear that artificial intelligence will be cost effective and beneficial in improving clinical results[87]. Similarly, in pathology, there is interobserver discord commonly noticed in various diagnoses among pathologists, which affects the learning or input data and the cost effectiveness of artificial intelligence[88,89].
Traditionally, the responsibility and legal implications onus was on the surgeon; however, with autonomous artificial intelligence, these need to be sorted out in respect of accountability, liability, and culpability. Only accountability can be assessed in artificial intelligence with the help of recording actions, but liability and culpability require further intro
Artificial intelligence is advancing at a rampant pace, and is playing a major role in shaping the way patients will be managed in coming years. The implementation of artificial intelligence in diagnosing gastrointestinal disease seems to be at par with the experts; however, it is important to realize that artificial intelligence cannot totally replace humans, and will need assistance for its use, further development, and enhancement. The aim of artificial intelligence is to enrich the skills of a surgeon and not to replace him. Due to its various limitations, more must be done to use artificial intelligence to improve healthcare.
| 1. | Leonard S, Wu KL, Kim Y, Krieger A, Kim PC. Smart tissue anastomosis robot (STAR): a vision-guided robotics system for laparoscopic suturing. IEEE Trans Biomed Eng. 2014;61:1305-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Shademan A, Decker RS, Opfermann JD, Leonard S, Krieger A, Kim PC. Supervised autonomous robotic soft tissue surgery. Sci Transl Med. 2016;8:337ra64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 233] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 3. | Bellman R. An introduction to artificial intelligence: Can computers think? 1978. [cited 11 July 2024]. Available from: https://searchworks.stanford.edu/view/2762753. |
| 4. | Hashimoto DA, Rosman G, Rus D, Meireles OR. Artificial Intelligence in Surgery: Promises and Perils. Ann Surg. 2018;268:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 595] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 5. | Jacob M, Li YT, Akingba G, Wachs JP. Gestonurse: a robotic surgical nurse for handling surgical instruments in the operating room. J Robot Surg. 2012;6:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Gunn AA. The diagnosis of acute abdominal pain with computer analysis. J R Coll Surg Edinb. 1976;21:170-172. [PubMed] |
| 7. | McCarthy J, Minsky ML, Rochester N, Shannon CE. A proposal for the dartmouth summer research project on artificial intelligence. AI Mag. 2006;27:12-14. [DOI] [Full Text] |
| 8. | Turing AM. Computing machinery and intelligence. In: Epstein R, Roberts G, Beber G, editors. Dordrecht: Springer, 2009. |
| 9. | Rajpurkar P, Irvin J, Ball RL, Zhu K, Yang B, Mehta H, Duan T, Ding D, Bagul A, Langlotz CP, Patel BN, Yeom KW, Shpanskaya K, Blankenberg FG, Seekins J, Amrhein TJ, Mong DA, Halabi SS, Zucker EJ, Ng AY, Lungren MP. Deep learning for chest radiograph diagnosis: A retrospective comparison of the CheXNeXt algorithm to practicing radiologists. PLoS Med. 2018;15:e1002686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 743] [Cited by in RCA: 587] [Article Influence: 83.9] [Reference Citation Analysis (0)] |
| 10. | Wang D, Khosla A, Gargeya R, Irshad H, Beck AH. Deep Learning for Identifying Metastatic Breast Cancer. Jun18, 2016. [cited 11 July 2022]. Available from: https://arxiv.org/abs/1606.05718. |
| 11. | LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36149] [Cited by in RCA: 20025] [Article Influence: 2002.5] [Reference Citation Analysis (0)] |
| 12. | Erickson BJ, Korfiatis P, Akkus Z, Kline TL. Machine Learning for Medical Imaging. Radiographics. 2017;37:505-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 770] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 13. | McBee MP, Awan OA, Colucci AT, Ghobadi CW, Kadom N, Kansagra AP, Tridandapani S, Auffermann WF. Deep Learning in Radiology. Acad Radiol. 2018;25:1472-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 257] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 14. | Deo RC. Machine Learning in Medicine. Circulation. 2015;132:1920-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1155] [Cited by in RCA: 1955] [Article Influence: 217.2] [Reference Citation Analysis (6)] |
| 15. | Cruz JA, Wishart DS. Applications of machine learning in cancer prediction and prognosis. Cancer Inform. 2007;2:59-77. [PubMed] |
| 16. | Soguero-Ruiz C, Fei WM, Jenssen R, Augestad KM, Álvarez JL, Jiménez IM, Lindsetmo RO, Skrøvseth SO. Data-driven Temporal Prediction of Surgical Site Infection. AMIA Annu Symp Proc. 2015;2015:1164-1173. [PubMed] |
| 17. | Bothe MK, Dickens L, Reichel K, Tellmann A, Ellger B, Westphal M, Faisal AA. The use of reinforcement learning algorithms to meet the challenges of an artificial pancreas. Expert Rev Med Devices. 2013;10:661-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Koyner JL, Carey KA, Edelson DP, Churpek MM. The Development of a Machine Learning Inpatient Acute Kidney Injury Prediction Model. Crit Care Med. 2018;46:1070-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 205] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 19. | Bertsimas D, Dunn J, Velmahos GC, Kaafarani HMA. Surgical Risk Is Not Linear: Derivation and Validation of a Novel, User-friendly, and Machine-learning-based Predictive OpTimal Trees in Emergency Surgery Risk (POTTER) Calculator. Ann Surg. 2018;268:574-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 205] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 20. | McCulloch WS, Pitts W. A logical calculus of the ideas immanent in nervous activity. Bull Math Biol. 1943;5:115-133. |
| 21. | Hinton GE, Osindero S, Teh YW. A fast learning algorithm for deep belief nets. Neural Comput. 2006;18:1527-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9547] [Cited by in RCA: 3189] [Article Influence: 167.8] [Reference Citation Analysis (0)] |
| 22. | Bini SA. Artificial Intelligence, Machine Learning, Deep Learning, and Cognitive Computing: What Do These Terms Mean and How Will They Impact Health Care? J Arthroplasty. 2018;33:2358-2361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 255] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 23. | Haeberle HS, Helm JM, Navarro SM, Karnuta JM, Schaffer JL, Callaghan JJ, Mont MA, Kamath AF, Krebs VE, Ramkumar PN. Artificial Intelligence and Machine Learning in Lower Extremity Arthroplasty: A Review. J Arthroplasty. 2019;34:2201-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 24. | Mofidi R, Duff MD, Madhavan KK, Garden OJ, Parks RW. Identification of severe acute pancreatitis using an artificial neural network. Surgery. 2007;141:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Yang YJ, Bang CS. Application of artificial intelligence in gastroenterology. World J Gastroenterol. 2019;25:1666-1683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 211] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (5)] |
| 26. | Kenngott HG, Wagner M, Nickel F, Wekerle AL, Preukschas A, Apitz M, Schulte T, Rempel R, Mietkowski P, Wagner F, Termer A, Müller-Stich BP. Computer-assisted abdominal surgery: new technologies. Langenbecks Arch Surg. 2015;400:273-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Volkov M, Hashimoto DA, Rosman G, Meireles OR, Rus D. Machine Learning and Coresets for Automated Real-Time Video Segmentation of Laparoscopic and Robot-Assisted Surgery. IEEE International Conference on Robotics and Automation. Singapore, 2017: 754-759. |
| 28. | Hashimoto DA, Ward TM, Meireles OR. The Role of Artificial Intelligence in Surgery. Adv Surg. 2020;54:89-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Mellia JA, Basta MN, Toyoda Y, Othman S, Elfanagely O, Morris MP, Torre-Healy L, Ungar LH, Fischer JP. Natural Language Processing in Surgery: A Systematic Review and Meta-analysis. Ann Surg. 2021;273:900-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 30. | Shi J, Liu S, Pruitt LCC, Luppens CL, Ferraro JP, Gundlapalli AV, Chapman WW, Bucher BT. Using Natural Language Processing to improve EHR Structured Data-based Surgical Site Infection Surveillance. AMIA Annu Symp Proc. 2019;2019:794-803. [PubMed] |
| 31. | Chapman AB, Mowery DL, Swords DS, Chapman WW, Bucher BT. Detecting Evidence of Intra-abdominal Surgical Site Infections from Radiology Reports Using Natural Language Processing. AMIA Annu Symp Proc. 2017;2017:515-524. [PubMed] |
| 32. | Sohn S, Larson DW, Habermann EB, Naessens JM, Alabbad JY, Liu H. Detection of clinically important colorectal surgical site infection using Bayesian network. J Surg Res. 2017;209:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Elhage SA, Deerenberg EB, Ayuso SA, Murphy KJ, Shao JM, Kercher KW, Smart NJ, Fischer JP, Augenstein VA, Colavita PD, Heniford BT. Development and Validation of Image-Based Deep Learning Models to Predict Surgical Complexity and Complications in Abdominal Wall Reconstruction. JAMA Surg. 2021;156:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Hirasawa T, Aoyama K, Tanimoto T, Ishihara S, Shichijo S, Ozawa T, Ohnishi T, Fujishiro M, Matsuo K, Fujisaki J, Tada T. Application of artificial intelligence using a convolutional neural network for detecting gastric cancer in endoscopic images. Gastric Cancer. 2018;21:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 426] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 35. | Luo H, Xu G, Li C, He L, Luo L, Wang Z, Jing B, Deng Y, Jin Y, Li Y, Li B, Tan W, He C, Seeruttun SR, Wu Q, Huang J, Huang DW, Chen B, Lin SB, Chen QM, Yuan CM, Chen HX, Pu HY, Zhou F, He Y, Xu RH. Real-time artificial intelligence for detection of upper gastrointestinal cancer by endoscopy: a multicentre, case-control, diagnostic study. Lancet Oncol. 2019;20:1645-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 253] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 36. | Horiuchi Y, Aoyama K, Tokai Y, Hirasawa T, Yoshimizu S, Ishiyama A, Yoshio T, Tsuchida T, Fujisaki J, Tada T. Convolutional Neural Network for Differentiating Gastric Cancer from Gastritis Using Magnified Endoscopy with Narrow Band Imaging. Dig Dis Sci. 2020;65:1355-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 37. | de Groof AJ, Struyvenberg MR, van der Putten J, van der Sommen F, Fockens KN, Curvers WL, Zinger S, Pouw RE, Coron E, Baldaque-Silva F, Pech O, Weusten B, Meining A, Neuhaus H, Bisschops R, Dent J, Schoon EJ, de With PH, Bergman JJ. Deep-Learning System Detects Neoplasia in Patients With Barrett's Esophagus With Higher Accuracy Than Endoscopists in a Multistep Training and Validation Study With Benchmarking. Gastroenterology. 2020;158:915-929.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 38. | Wu L, Zhou W, Wan X, Zhang J, Shen L, Hu S, Ding Q, Mu G, Yin A, Huang X, Liu J, Jiang X, Wang Z, Deng Y, Liu M, Lin R, Ling T, Li P, Wu Q, Jin P, Chen J, Yu H. A deep neural network improves endoscopic detection of early gastric cancer without blind spots. Endoscopy. 2019;51:522-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 156] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 39. | Gao Y, Zhang ZD, Li S, Guo YT, Wu QY, Liu SH, Yang SJ, Ding L, Zhao BC, Li S, Lu Y. Deep neural network-assisted computed tomography diagnosis of metastatic lymph nodes from gastric cancer. Chin Med J (Engl). 2019;132:2804-2811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 40. | Jagric T, Potrc S, Jagric T. Prediction of liver metastases after gastric cancer resection with the use of learning vector quantization neural networks. Dig Dis Sci. 2010;55:3252-3261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Chen T, Zhang C, Liu Y, Zhao Y, Lin D, Hu Y, Yu J, Li G. A gastric cancer LncRNAs model for MSI and survival prediction based on support vector machine. BMC Genomics. 2019;20:846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Nakahira H, Ishihara R, Aoyama K, Kono M, Fukuda H, Shimamoto Y, Nakagawa K, Ohmori M, Iwatsubo T, Iwagami H, Matsuno K, Inoue S, Matsuura N, Shichijo S, Maekawa A, Kanesaka T, Yamamoto S, Takeuchi Y, Higashino K, Uedo N, Matsunaga T, Tada T. Stratification of gastric cancer risk using a deep neural network. JGH Open. 2020;4:466-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Fukuda H, Ishihara R, Kato Y, Matsunaga T, Nishida T, Yamada T, Ogiyama H, Horie M, Kinoshita K, Tada T. Comparison of performances of artificial intelligence versus expert endoscopists for real-time assisted diagnosis of esophageal squamous cell carcinoma (with video). Gastrointest Endosc. 2020;92:848-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 44. | Nakagawa K, Ishihara R, Aoyama K, Ohmori M, Nakahira H, Matsuura N, Shichijo S, Nishida T, Yamada T, Yamaguchi S, Ogiyama H, Egawa S, Kishida O, Tada T. Classification for invasion depth of esophageal squamous cell carcinoma using a deep neural network compared with experienced endoscopists. Gastrointest Endosc. 2019;90:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 45. | Shimamoto Y, Ishihara R, Kato Y, Shoji A, Inoue T, Matsueda K, Miyake M, Waki K, Kono M, Fukuda H, Matsuura N, Nagaike K, Aoi K, Yamamoto K, Inoue T, Nakahara M, Nishihara A, Tada T. Real-time assessment of video images for esophageal squamous cell carcinoma invasion depth using artificial intelligence. J Gastroenterol. 2020;55:1037-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 46. | Byrne MF, Chapados N, Soudan F, Oertel C, Linares Pérez M, Kelly R, Iqbal N, Chandelier F, Rex DK. Real-time differentiation of adenomatous and hyperplastic diminutive colorectal polyps during analysis of unaltered videos of standard colonoscopy using a deep learning model. Gut. 2019;68:94-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 411] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 47. | Urban G, Tripathi P, Alkayali T, Mittal M, Jalali F, Karnes W, Baldi P. Deep Learning Localizes and Identifies Polyps in Real Time With 96% Accuracy in Screening Colonoscopy. Gastroenterology. 2018;155:1069-1078.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 432] [Article Influence: 61.7] [Reference Citation Analysis (1)] |
| 48. | Tokai Y, Yoshio T, Aoyama K, Horie Y, Yoshimizu S, Horiuchi Y, Ishiyama A, Tsuchida T, Hirasawa T, Sakakibara Y, Yamada T, Yamaguchi S, Fujisaki J, Tada T. Application of artificial intelligence using convolutional neural networks in determining the invasion depth of esophageal squamous cell carcinoma. Esophagus. 2020;17:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 49. | Ichimasa K, Kudo SE, Mori Y, Misawa M, Matsudaira S, Kouyama Y, Baba T, Hidaka E, Wakamura K, Hayashi T, Kudo T, Ishigaki T, Yagawa Y, Nakamura H, Takeda K, Haji A, Hamatani S, Mori K, Ishida F, Miyachi H. Artificial intelligence may help in predicting the need for additional surgery after endoscopic resection of T1 colorectal cancer. Endoscopy. 2018;50:230-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 50. | Kitaguchi D, Takeshita N, Matsuzaki H, Takano H, Owada Y, Enomoto T, Oda T, Miura H, Yamanashi T, Watanabe M, Sato D, Sugomori Y, Hara S, Ito M. Real-time automatic surgical phase recognition in laparoscopic sigmoidectomy using the convolutional neural network-based deep learning approach. Surg Endosc. 2020;34:4924-4931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 51. | Brennan M, Puri S, Ozrazgat-Baslanti T, Feng Z, Ruppert M, Hashemighouchani H, Momcilovic P, Li X, Wang DZ, Bihorac A. Comparing clinical judgment with the MySurgeryRisk algorithm for preoperative risk assessment: A pilot usability study. Surgery. 2019;165:1035-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 52. | Maubert A, Birtwisle L, Bernard JL, Benizri E, Bereder JM. Can machine learning predict resecability of a peritoneal carcinomatosis? Surg Oncol. 2019;29:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Tsilimigras DI, Mehta R, Moris D, Sahara K, Bagante F, Paredes AZ, Moro A, Guglielmi A, Aldrighetti L, Weiss M, Bauer TW, Alexandrescu S, Poultsides GA, Maithel SK, Marques HP, Martel G, Pulitano C, Shen F, Soubrane O, Koerkamp BG, Endo I, Pawlik TM. A Machine-Based Approach to Preoperatively Identify Patients with the Most and Least Benefit Associated with Resection for Intrahepatic Cholangiocarcinoma: An International Multi-institutional Analysis of 1146 Patients. Ann Surg Oncol. 2020;27:1110-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 54. | Müller L, Mähringer-Kunz A, Gairing SJ, Foerster F, Weinmann A, Bartsch F, Heuft LK, Baumgart J, Düber C, Hahn F, Kloeckner R. Survival Prediction in Intrahepatic Cholangiocarcinoma: A Proof of Concept Study Using Artificial Intelligence for Risk Assessment. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 55. | Serban N, Kupas D, Hajdu A, Török P, Harangi B. Distinguishing the Uterine Artery, the Ureter, and Nerves in Laparoscopic Surgical Images Using Ensembles of Binary Semantic Segmentation Networks. Sensors (Basel). 2024;24. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 56. | Veerankutty FH, Jayan G, Yadav MK, Manoj KS, Yadav A, Nair SRS, Shabeerali TU, Yeldho V, Sasidharan M, Rather SA. Artificial Intelligence in hepatology, liver surgery and transplantation: Emerging applications and frontiers of research. World J Hepatol. 2021;13:1977-1990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 57. | Han IW, Cho K, Ryu Y, Shin SH, Heo JS, Choi DW, Chung MJ, Kwon OC, Cho BH. Risk prediction platform for pancreatic fistula after pancreatoduodenectomy using artificial intelligence. World J Gastroenterol. 2020;26:4453-4464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 58. | Wang S, Liu X, Zhao J, Liu Y, Liu S, Liu Y, Zhao J. Computer auxiliary diagnosis technique of detecting cholangiocarcinoma based on medical imaging: A review. Comput Methods Programs Biomed. 2021;208:106265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 59. | Merath K, Hyer JM, Mehta R, Farooq A, Bagante F, Sahara K, Tsilimigras DI, Beal E, Paredes AZ, Wu L, Ejaz A, Pawlik TM. Use of Machine Learning for Prediction of Patient Risk of Postoperative Complications After Liver, Pancreatic, and Colorectal Surgery. J Gastrointest Surg. 2020;24:1843-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 60. | Nudel J, Bishara AM, de Geus SWL, Patil P, Srinivasan J, Hess DT, Woodson J. Development and validation of machine learning models to predict gastrointestinal leak and venous thromboembolism after weight loss surgery: an analysis of the MBSAQIP database. Surg Endosc. 2021;35:182-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 61. | Hashimoto DA, Rosman G, Witkowski ER, Stafford C, Navarette-Welton AJ, Rattner DW, Lillemoe KD, Rus DL, Meireles OR. Computer Vision Analysis of Intraoperative Video: Automated Recognition of Operative Steps in Laparoscopic Sleeve Gastrectomy. Ann Surg. 2019;270:414-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 62. | Wingfield LR, Ceresa C, Thorogood S, Fleuriot J, Knight S. Using Artificial Intelligence for Predicting Survival of Individual Grafts in Liver Transplantation: A Systematic Review. Liver Transpl. 2020;26:922-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 63. | Khorsandi SE, Hardgrave HJ, Osborn T, Klutts G, Nigh J, Spencer-Cole RT, Kakos CD, Anastasiou I, Mavros MN, Giorgakis E. Artificial Intelligence in Liver Transplantation. Transplant Proc. 2021;53:2939-2944. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 64. | Bi WL, Hosny A, Schabath MB, Giger ML, Birkbak NJ, Mehrtash A, Allison T, Arnaout O, Abbosh C, Dunn IF, Mak RH, Tamimi RM, Tempany CM, Swanton C, Hoffmann U, Schwartz LH, Gillies RJ, Huang RY, Aerts HJWL. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J Clin. 2019;69:127-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 848] [Cited by in RCA: 818] [Article Influence: 136.3] [Reference Citation Analysis (3)] |
| 65. | Mayerhoefer ME, Materka A, Langs G, Häggström I, Szczypiński P, Gibbs P, Cook G. Introduction to Radiomics. J Nucl Med. 2020;61:488-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 1007] [Article Influence: 201.4] [Reference Citation Analysis (0)] |
| 66. | Forghani R, Savadjiev P, Chatterjee A, Muthukrishnan N, Reinhold C, Forghani B. Radiomics and Artificial Intelligence for Biomarker and Prediction Model Development in Oncology. Comput Struct Biotechnol J. 2019;17:995-1008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 67. | Li W, Zhang L, Tian C, Song H, Fang M, Hu C, Zang Y, Cao Y, Dai S, Wang F, Dong D, Wang R, Tian J. Prognostic value of computed tomography radiomics features in patients with gastric cancer following curative resection. Eur Radiol. 2019;29:3079-3089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 68. | Zhang W, Fang M, Dong D, Wang X, Ke X, Zhang L, Hu C, Guo L, Guan X, Zhou J, Shan X, Tian J. Development and validation of a CT-based radiomic nomogram for preoperative prediction of early recurrence in advanced gastric cancer. Radiother Oncol. 2020;145:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 69. | Rompianesi G, Pegoraro F, Ceresa CD, Montalti R, Troisi RI. Artificial intelligence in the diagnosis and management of colorectal cancer liver metastases. World J Gastroenterol. 2022;28:108-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (3)] |
| 70. | Huang Z, Liu D, Chen X, Yu P, Wu J, Song B, Hu J, Wu B. Retrospective imaging studies of gastric cancer: Study protocol clinical trial (SPIRIT Compliant). Medicine (Baltimore). 2020;99:e19157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Xie A, Fang C, Huang Y, Fan Y, Pan J, Peng F. Application of three-dimensional reconstruction and visible simulation technique in reoperation of hepatolithiasis. J Gastroenterol Hepatol. 2013;28:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Fang CH, Kong D, Wang X, Wang H, Xiang N, Fan Y, Yang J, Zhong SZ. Three-dimensional reconstruction of the peripancreatic vascular system based on computed tomographic angiography images and its clinical application in the surgical management of pancreatic tumors. Pancreas. 2014;43:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 73. | Miyamoto R, Oshiro Y, Nakayama K, Kohno K, Hashimoto S, Fukunaga K, Oda T, Ohkohchi N. Three-dimensional simulation of pancreatic surgery showing the size and location of the main pancreatic duct. Surg Today. 2017;47:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Ieiri S, Uemura M, Konishi K, Souzaki R, Nagao Y, Tsutsumi N, Akahoshi T, Ohuchida K, Ohdaira T, Tomikawa M, Tanoue K, Hashizume M, Taguchi T. Augmented reality navigation system for laparoscopic splenectomy in children based on preoperative CT image using optical tracking device. Pediatr Surg Int. 2012;28:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 75. | Onda S, Okamoto T, Kanehira M, Suzuki F, Ito R, Fujioka S, Suzuki N, Hattori A, Yanaga K. Identification of inferior pancreaticoduodenal artery during pancreaticoduodenectomy using augmented reality-based navigation system. J Hepatobiliary Pancreat Sci. 2014;21:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 76. | Kenngott HG, Neuhaus J, Müller-Stich BP, Wolf I, Vetter M, Meinzer HP, Köninger J, Büchler MW, Gutt CN. Development of a navigation system for minimally invasive esophagectomy. Surg Endosc. 2008;22:1858-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 77. | Gupta A, Singla T, Chennatt JJ, David LE, Ahmed SS, Rajput D. Artificial intelligence: A new tool in surgeon's hand. J Educ Health Promot. 2022;11:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 78. | Hou F, Yang Z, Gu W, Yu Y, Liang Y. Automatic identification of metastatic lymph nodes in OCT images. Optical Coherence Tomography and Coherence Domain Optical Methods in Biomedicine XXIII, 108673G; 2019 Feb 22. [DOI] [Full Text] |
| 79. | Jüni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001. 323:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2379] [Cited by in RCA: 2160] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 80. | Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database Syst Rev. 2009;2009:MR000006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 393] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 81. | Somashekhar SP, Sepúlveda MJ, Norden AD, Rauthan A, Arun K, Patil P, Ethadka RY, Kumar RC. Early experience with IBM Watson for Oncology (WFO) cognitive computing system for lung and colorectal cancer treatment. J ClinOnco. 2017;35:8527. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 82. | Tian Y, Liu X, Wang Z, Cao S, Liu Z, Ji Q, Li Z, Sun Y, Zhou X, Wang D, Zhou Y. Concordance Between Watson for Oncology and a Multidisciplinary Clinical Decision-Making Team for Gastric Cancer and the Prognostic Implications: Retrospective Study. J Med Internet Res. 2020;22:e14122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 83. | Nawab K, Athwani R, Naeem A, Hamayun M, Wazir M. A Review of Applications of Artificial Intelligence in Gastroenterology. Cureus. 2021;13:e19235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 84. | Fave X, Mackin D, Yang J, Zhang J, Fried D, Balter P, Followill D, Gomez D, Jones AK, Stingo F, Fontenot J, Court L. Can radiomics features be reproducibly measured from CBCT images for patients with non-small cell lung cancer? Med Phys. 2015;42:6784-6797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 85. | Berenguer R, Pastor-Juan MDR, Canales-Vázquez J, Castro-García M, Villas MV, Mansilla Legorburo F, Sabater S. Radiomics of CT Features May Be Nonreproducible and Redundant: Influence of CT Acquisition Parameters. Radiology. 2018;288:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 432] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 86. | Shiri I, Rahmim A, Ghaffarian P, Geramifar P, Abdollahi H, Bitarafan-Rajabi A. The impact of image reconstruction settings on 18F-FDG PET radiomic features: multi-scanner phantom and patient studies. Eur Radiol. 2017;27:4498-4509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 147] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 87. | Kudou M, Kosuga T, Otsuji E. Artificial intelligence in gastrointestinal cancer: Recent advances and future perspectives. Artif Intell Gastroenterol. 2020;1:71-85. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 88. | Chassagnon G, Vakalopoulou M, Paragios N, Revel MP. Artificial intelligence applications for thoracic imaging. Eur J Radiol. 2020;123:108774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 89. | Rudie JD, Rauschecker AM, Bryan RN, Davatzikos C, Mohan S. Emerging Applications of Artificial Intelligence in Neuro-Oncology. Radiology. 2019;290:607-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 90. | O'Sullivan S, Nevejans N, Allen C, Blyth A, Leonard S, Pagallo U, Holzinger K, Holzinger A, Sajid MI, Ashrafian H. Legal, regulatory, and ethical frameworks for development of standards in artificial intelligence (AI) and autonomous robotic surgery. Int J Med Robot. 2019;15:e1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 91. | DeCamp M, Tilburt JC. Why we cannot trust artificial intelligence in medicine. Lancet Digit Health. 2019;1:e390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |