Published online Dec 18, 2024. doi: 10.13105/wjma.v12.i4.100726
Revised: November 11, 2024
Accepted: November 22, 2024
Published online: December 18, 2024
Processing time: 109 Days and 19.8 Hours
Colon capsule endoscopy (CCE) is a modern, non-invasive method for large bowel visualization, offering a less invasive alternative to traditional colonoscopy (TC). While TC remains the gold standard for comprehensive large bowel assess
To systematically review and compare the polyp detection rates (PDR) of CCE and TC.
A systematic literature search was conducted using four scientific databases: CINAHL, MEDLINE via EBSCO, Cochrane Library, and MEDLINE/PubMed. A standardized search command was utilized to ensure consistency. Full papers were retrieved if they compared PDR between CCE and TC and involved patients over 18 years old. A meta-analysis was then conducted using the meta package in R software.
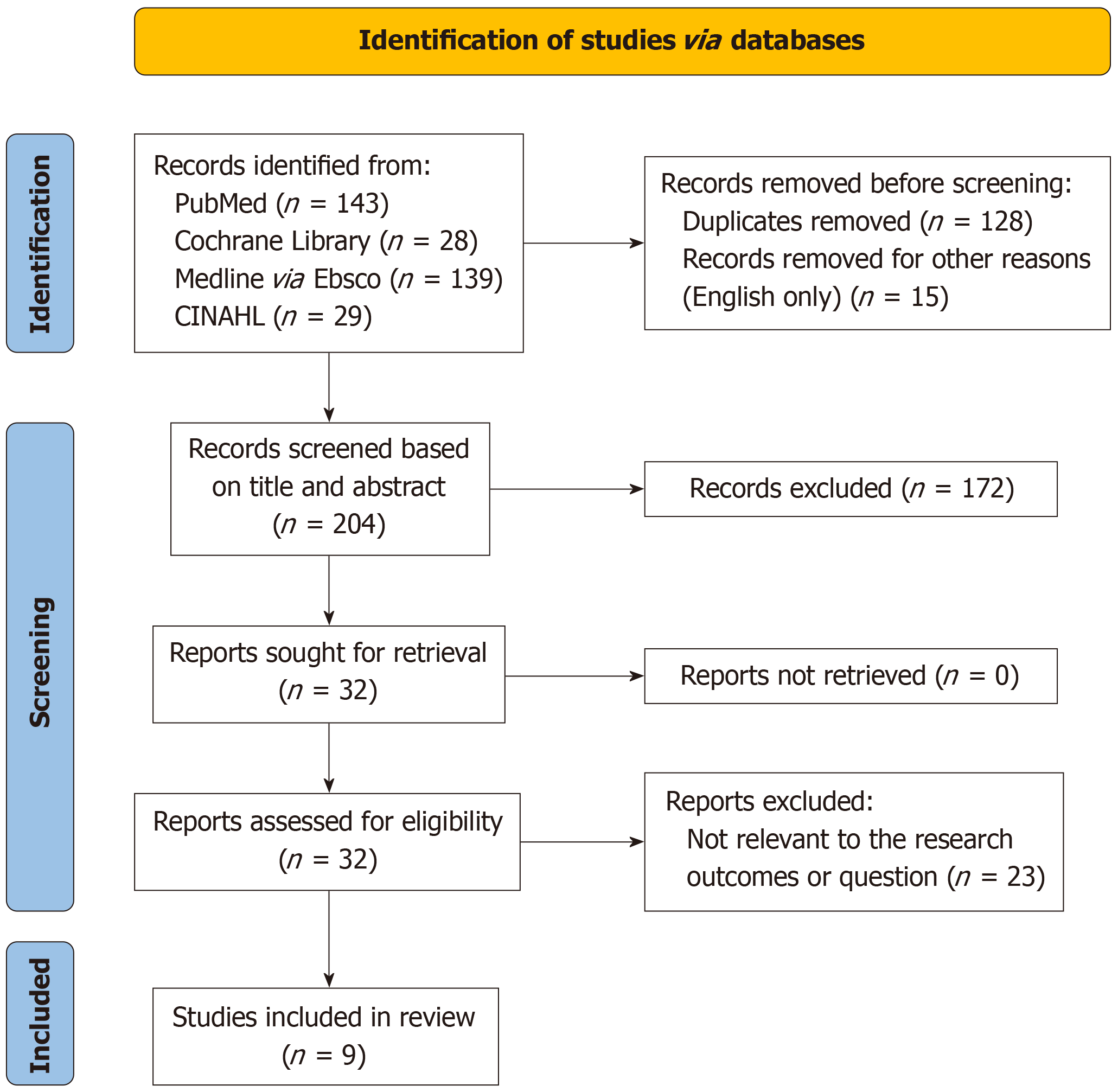
Initially, 339 articles were identified, with 128 duplicates and 15 non-English articles excluded, leaving 196 for screening. After further exclusions, 9 articles were included in the review. The meta-analysis revealed minimal differences in PDR between CCE and TC. The pooled PDR for TC was 0.61 (95%CI: 0.48–0.72), and for CCE, it was 0.61 (95%CI: 0.48–0.73). The overall comparison of the pooled PDR of both methods was 0.96 (95%CI: 0.90–1.02), indicating that CCE is non-inferior to TC.
CCE has emerged as a modern and safe diagnostic alternative to TC for polyp detection, demonstrating non-inferiority when compared to the conventional method.
Core Tip: Colonic polyps are a common finding during colonoscopy. This study is motivated by the necessity for procedural alternatives that ensure both effectiveness and patient acceptance. This is particularly crucial for reaching individuals who may not undergo conventional colonoscopies when recommended. Given that polyps are often asymptomatic but carry malignant potential if not detected and removed. Colon capsule endoscopy is a modern safe alternative to colonoscopy, identifying an optimal diagnostic approach is paramount to maintaining diagnostic quality.
- Citation: Woods M, Soldera J. Colon capsule endoscopy polyp detection rate vs colonoscopy polyp detection rate: Systematic review and meta-analysis. World J Meta-Anal 2024; 12(4): 100726
- URL: https://www.wjgnet.com/2308-3840/full/v12/i4/100726.htm
- DOI: https://dx.doi.org/10.13105/wjma.v12.i4.100726
Colonic polyps typically manifest without symptoms and are often discovered during investigations for bowel-related concerns like rectal bleeding, positive fecal immunochemical testing (FIT), changes in bowel habits, and iron deficiency anemia[1]. These polyps vary in morphology, including sessile, pedunculated, flat, depressed, or excavated forms, and are categorized as hyperplastic, sessile serrated lesions, tubular adenomas, tubular villous adenomas, or polypoidal cancers. Adenomas, which carry a notable risk of malignancy, are generally removed upon discovery[1-3]. The progression from polyp to invasive colorectal cancer (CRC) can take 5-15 years, with adenomas ≥ 10 mm increasing cancer risk by 2.5% after 5 years, 8% after 10 years, and 24% after 20 years[4].
Risk factors like a high-fat diet, excessive alcohol consumption, low-fiber intake, and a family history of CRC or polyps increase the likelihood of developing large bowel polyps. Polyps are more common in males and people over 50, leading many international screening programs to start at this age[1-6]. In the United States, the American Cancer Society recom
Less common causes include Peutz-Jeghers syndrome, characterized by hamartomatous polyps and mucocutaneous pigmentation, and Juvenile polyposis syndrome, marked by juvenile polyps and a high CRC risk, reaching 68% by age 60. Cowden syndrome, caused by phosphatase and tensin homolog gene mutations, increases the lifetime cancer risk to 9%-16%[4].
Understanding risk factors and genetic predispositions is crucial for early detection and prevention strategies. Effective screening and awareness can mitigate these risks, leading to better patient outcomes since CRC is the third most common malignant disease worldwide, accounting for 10.7% of all cancers globally[8]. Asadzadeh et al[9] and Rutter et al[10] emphasize that nearly all CRCs develop from polyps over many years. In the United Kingdom, more than 40000 people are diagnosed, and over 16000 die from CRC annually.
Colon capsule endoscopy (CCE), introduced in 2001, is a modern, safe method for large bowel visualization[11]. However, it has complications like capsule retention, inadequate bowel preparation, aspiration, and technical issues. There are no established standards for capsule readers. Despite these challenges, CCE offers a less invasive alternative to traditional colonoscopy (TC), the gold standard for large bowel assessment.
Given the recent introduction of this technology, there are currently no established standards for "capsuleers" (capsule readers) to assess its performance. Despite these challenges, CCE offers a less invasive alternative to TC, which is regarded as the gold standard for comprehensive large bowel assessment. This less invasive option is important for patients who have previously had failed colonoscopies or been unable to tolerate the traditional method. In addition, as CCE became more established and widely used, the technology could be offered to those in remote locations who may struggle to access a hospital setting.
The RAPID software, developed for capsule endoscopy, interprets data captured by two color-coded cameras (yellow and green) at each end of the capsule. The 'Top 100 Mode' uses artificial intelligence to present images with potential abnormalities. The capsule's speed through the gastrointestinal tract is regulated by medications like Prucalopride, Gastrografin, Phospha-soda, and Bisacodyl suppositories[12,13]. Viewing speeds can be adjusted, and image enhancements like Fujinon Intelligent Color Enhancement System and a blue light filter can help differentiate between adenomatous and hyperplastic polyps[14].
TC is considered the gold standard for comprehensive large bowel assessment, enabling the detection and treatment of neoplastic and non-neoplastic conditions. This procedure can reduce the need for additional procedures but can be uncomfortable and carries risks such as bleeding, perforation, and reactions to medications[15].
Polyp detection rates (PDR) for colonoscopy and colon capsule are the percentage of procedures detecting one or more polyps[16]. PDR is calculated by dividing the number of procedures with polyps by the total number of procedures, then multiplying by 100.
(TC or CCE with polyps / total number of TC or CCE’s performed) × 100 = PDR
Presently, the aspirational target for the PDR in the general United Kingdom population (non-screening) is 20% for colonoscopies, with a minimum standard of 15%. The standard withdrawal time from the caecum is > 6 minutes, with a target of > 10 minutes. This key performance indicator is crucial for enhancing PDRs[16]. Achieving the highest possible PDR is essential to patient outcomes and quality of screening: A higher frequency of polyps being detected and removed means that those polyps cannot develop into possible cancers in the future, reducing the number of patients entering the cancer pathway and ultimately reducing mortality rates. United Kingdom guidelines aim to protect individuals post-polypectomy and post-cancer resection to reduce CRC mortality. High-risk findings (more than two premalignant polyps or one advanced colorectal polyp) necessitate a one-off surveillance colonoscopy after 3 years, unless the patient is over 75 or has a life expectancy of less than 10 years. CRCs require a colonoscopy 1 year post-resection for polyp clearance[10].
Achieving effective bowel preparation is fundamental to conducting a high-quality assessment of the large bowel for polyp detection. For colonoscopy procedures, a scale categorizing bowel preparation as excellent, good, fair, or inadequate is employed[16]. In contrast, colon capsule procedures utilize the Colon Capsule CLEansing Assessment and Report scale, which employs a point system for assessing the right, transverse, and left colon. Each section has a maximum score of 3, resulting in an overall score categorized as excellent (8-9 points), good (6-7 points), or inadequate (0-5 points)[17].
Recent data from August 2023 shows a gradual increase in participation in the Bowel Cancer Screening Program (BCSP) in the United Kingdom from 2021 to 2022, though it remains the lowest among all screening programs[18]. Barriers to participation include fears about procedure outcomes, misconceptions about asymptomatic pathology, concerns about FIT cleanliness, and perceived risks of follow-up colonoscopy[19]. Addressing these issues is crucial for improving participation rates and reducing CRC burden. Having CCE available as an additional, less invasive option for patients could help to increase uptake in the BCSP.
This study seeks procedural alternatives that are both effective and accepted by patients, especially for those who avoid conventional colonoscopies[20]. Since polyps are often asymptomatic but have malignant potential if undetected[21], finding an optimal diagnostic approach is essential.
The aim is to systematically review and compare the PDR of CCE and TC. This will impact the clinical practice of endoscopists, gastroenterologists and surgeons as it will give them the evidence they need to choose the most suitable investigation for their patients. This could increase options and flexibility for treatment, ultimately reducing costs and increasing the detection of abnormalities.
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines were followed in this systematic review[22]. This systematic review has been registered in the PROSPERO database under registration number CRD42024521083[23].
A systematic literature search was conducted on the 4 March 2024 using four scientific databases (CINAHL, MEDLINE via EBSCO, Cochrane Library and PubMed) and was updated on the 6 April 2024.
Articles found comparing PDR between CCE and TC were included in this systematic review and meta-analysis. The PICO criteria (Table 1) were used to formulate a study question and to enable the literature review: Population (adult patients requiring lower gastrointestinal investigation); Intervention (diagnostic testing); Comparison (colonoscopy or colon capsule PDR); Outcome (clear research data, on which an informed decision could be reached).
| Parameter | Inclusion criteria |
| Population | Adult patients over the age of 18 requiring lower gastrointestinal investigation |
| Intervention | Diagnostic testing |
| Comparison | Colonoscopy vs colon capsule endoscopy polyp detection rates |
| Outcomes | Data providing evidence of the superior test in relation to polyp detection |
Results were filtered to English only, with no date restrictions. The search command used in this systematic review was: ("polyp" OR "adenoma" OR "sessile serrated lesion" OR "hyperplastic" OR "adenoma") AND ("Colon capsule endoscopy" OR "capsule endoscopy") AND ("colonoscopy"). The grey literature was not reviewed.
The titles and abstracts were screened against our predefined inclusion criteria. The full texts of the selected articles were read, again using the inclusion and exclusion criteria. The following inclusion criteria were applied when reviewing the full articles: (1) CCE compared with colonoscopy; (2) Comparison of PDR; and (3) Available data for polyp detection in both colonoscopy and CCE in which a meta-analysis could be completed. The exclusion criteria included: Articles that lacked specific data (PDR) in which to conduct a systematic review and meta-analysis.
The following information was extracted from the studies: Study lead and publication date, study type, total number of patients, CCE patients, colonoscopy patients, PDR percentage for CCE, PDR percentage for colonoscopy, polyps detected by CCE, polyps detected by colonoscopy, colon capsule polyp size < 5 mm, colon capsule polyp size > 5 mm, colon capsule polyp size > 10 mm, colonoscopy polyp size < 5 mm, colonoscopy polyp size > 5 mm and colonoscopy polyp size > 10 mm.
The Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool assessed bias risk in the review. It examined patient selection, index test, reference standard, and flow and timing.
A meta-analysis compared PDR between CCE and TC using data from relevant studies compiled into a data frame with the 'meta' package in R version 4.4.1. The metaprop function pooled detection rates, showing results as proportions on the logit scale (PLO) with forest plots for visual summary. A direct comparison using the metabin function calculated the risk ratio (RR) for polyp detection, with results visualized in a forest plot, evaluating the relative effectiveness of both methods.
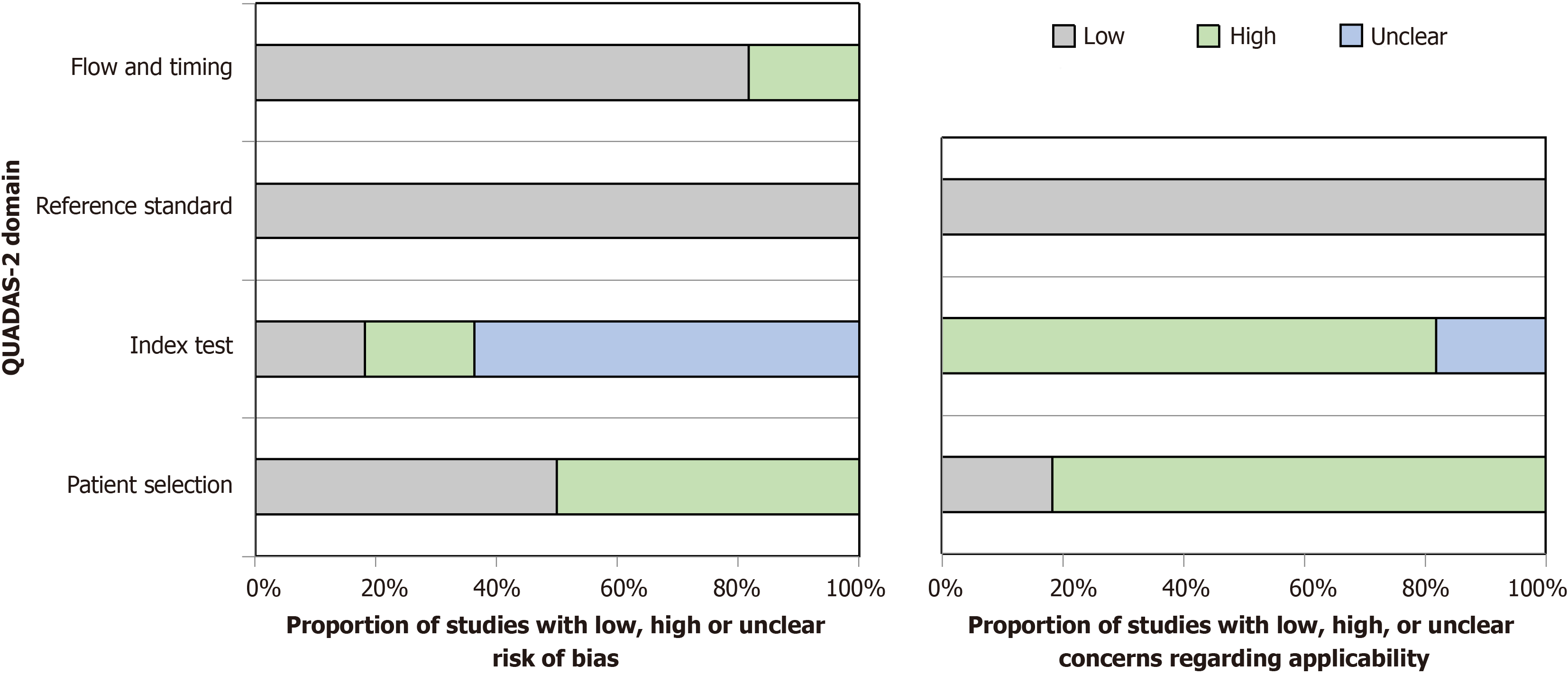
The review process began with 339 articles identified from four databases. After removing 128 duplicates and 15 non-English articles, 196 titles and abstracts were reviewed, excluding 172. Of the 32 full-text reviews, 23 did not meet the research criteria, resulting in 9 articles included in the final systematic review and meta-analysis. The PRISMA diagram (Figure 1) visually depicts the process from initial identification to final inclusion. Three studies had low bias risk[24-26], six had high bias risk, especially in patient selection[27-31], and Akyüz et al[32] was 'at risk of bias' due to limited patient selection and index testing details (Table 2 and Table 3). A graphical display for Quality Assessment of Diagnostic Accuracy Studies results (QUADAS) is presented in Figure 2.
| Ref. | Patient selection | Index test | Reference standard | Floe and timing |
| Koback-Larsen et al[24] | Low | Low | Low | Low |
| Spada et al[27] | High | Low | Low | Low |
| Eliakim et al[29] | High | Low | Low | Low |
| Pilz et al[30] | High | Low | Low | Low |
| Hagel et al[25] | Low | Low | Unclear | Low |
| Holleran et al[26] | Low | Low | Low | Low |
| Sacher-Huvelin et al[31] | High | Low | Low | Low |
| Akyüz et al[32] | Unclear | High | High | Low |
| Eliakim et al[28] | High | Low | Low | Low |
Among the 9 articles in the review, 4 were multicenter, 2 were single-center, and 3 were prospective comparative studies (Table 4 and Table 5). These studies, spanning from 2006 to 2017, included data from 1258 patients, analyzing PDR between CCE and TC. Eight studies used the latest CCE technology (CCE 2), while one, by Eliakim, used the older CCE 1 and included 98 patients[28]. The review highlights the evolution of CCE technology and its comparison to TC as a diag
| Ref. | Aims | Patient selection criteria stated in their paper |
| Koback-Larsen et al[24] | To determine the polyp detection rate for polyps > 9 mm by colon capsule endoscopy compared with colonoscopy, as well as the diagnostic accuracy of CCE | Individuals who had a positive immuno-fecal-occult blood test during screening |
| Spada et al[27] | To assess the feasibility, accuracy, and safety of CCE-2 in a head-to-head comparison with colonoscopy | Patients aged 18-80 who were scheduled to undergo colonoscopy for either known or suspected colonic disease |
| Eliakim et al[29] | To compare the second-generation PillCam with colonoscopy as an update on their earlier paper (first generation) | Patients aged 18-57, who were scheduled to undergo colonoscopy for either known or suspected colonic disease |
| Pilz et al[30] | To compare the efficacy of CCE against routine screening colonoscopy | Men and women above the age of 50 years without symptoms, or with lower- gastrointestinal signs and symptoms; and individuals 18-50 years with positive family history for CRC |
| Hagel et al[25] | To assess the PillCam Colon 2 capsule regarding feasibility, sensitivity, and specificity for the detection of colonic pathologies and additional recorded extracolonic findings | Patients scheduled to undergo CCE for known or suspected colonic diseases |
| Holleran et al[26] | To assess the sensitivity, specificity, and negative and positive predictive values of CCE compared with optical colonoscopy in a FIT-positive CRC screening cohort | Participants with a positive FIT result |
| Sacher-Huvelin et al[31] | To conduct a prospective, multi-center trial to compare CCE and colonoscopy in asymptomatic subjects enrolled in screening or surveillance program for the detection of colorectal neoplasia | Healthy, asymptomatic individual with a personal or family history of CRC or polyps |
| Akyüz et al[32] | To evaluate both the optimal cleaning regimen for CCE and the diagnostic value of test in the study group | Unclear. States 28 patients. Discounted patients with: “gastrointestinal dysmotility, suspected/known strictures, pregnancy, abdomen surgery in the past 6 months, life-threatening conditions.” |
| Eliakim et al[28] | Comparing PillCam (first-generation) colon capsule endoscopy with conventional colonoscopy for the detection of colonic disease | Patients aged 18-75 who were either suspected of having colonic disease and had been referred for conventional colonoscopy or who were being referred for colonoscopy for colorectal cancer screening |
| Ref. | CCE patients | Colonoscopy patients | Positive CCE | Positive colonoscopy |
| Koback-Larsen et al[24] | 253 | 253 | 187 | 162 |
| Spada et al[27] | 109 | 109 | 88 | 92 |
| Eliakim et al[29] | 98 | 98 | 45 | 43 |
| Pilz et al[30] | 56 | 56 | 35 | 28 |
| Hagel et al[25] | 23 | 23 | 14 | 16 |
| Holleran et al[26] | 62 | 62 | 43 | 36 |
| Sacher-Huvelin et al[31] | 545 | 545 | 251 | 311 |
| Akyüz et al[32] | 28 | 28 | 6 | 7 |
| Eliakim et al[28] | 84 | 84 | 64 | 67 |
The systematic review emphasizes advancements in CCE while noting concurrent developments from 2006 to 2017. Key improvements include high-definition endoscopes from Olympus, Fujinon, and Pentax, which were less covered in the studies[33]. Furthermore, AI integration in lower gastrointestinal endoscopy, as explored by Milluzzo et al[34], highlights ongoing technological evolution and potential research directions.
A study by Kobaek-Larsen et al[24] compared CCE and TC in patients with positive FIT. The study included 253 patients who underwent both procedures on the same day. CCE showed a significantly higher PDR (74%) compared to TC (64%). CCE detected 483 polyps, often larger, while TC identified 434 polyps, more frequently in the transverse colon. CCE reported more polyps in the right colon. The higher sensitivity of CCE led to 21% of patients needing a repeat TC for missed pathology.
Spada et al’s study involved a blinded comparison of CCE and TC performed within 10 hours or the next day[27]. CCE showed higher sensitivity for polyp detection: 84% for polyps > 6 mm and 88% for polyps > 10 mm, with specificities of 64% and 95%, respectively. Initially, 117 patients were enrolled, but 8 were excluded due to technical issues, inability to swallow the capsule, poor transit, or consent withdrawal. Bowel preparation was adequate in 81% of CCE patients and 92% of TC patients. 41% of patients had polyps > 6 mm, and 29% had polyps > 10 mm. Both methods detected 3 cancers, with overall detection rates of 84% for TC and 81% for CCE. The study suggests CCE is a viable tool for colorectal assessment.
Hagel et al’s study compared CCE with TC in symptomatic patients[25]. CCE detected 43 polyps while TC found 47, with higher bowel preparation rated for TC. Preparation was adequate in 90% of patients. In 23 cases, no polyps were found by either method. Of the 47 polyps, 4 found by TC were missed by CCE, while 3 polyps seen by CCE were missed by TC. One case remained unidentified even after unblinding and repeat TC. CCE had a sensitivity of 91% and specificity of 68% for polyp detection, concluding it as a safe and feasible alternative with acceptable detection rates and bowel cleanliness.
Pilz et al[30] compared CCE with TC under routine screening conditions. They found CCE less effective for polyp detection than TC, with an 11% miss rate. Bowel preparation was rated good in 27%, moderate in 54%, and poor in 20% of cases. 61% of patients had consistent bowel cleansing levels with both methods, while 39% did not. CCE showed a sensitivity of 79% and specificity of 54% for polyps of any size. The study concludes that although TC remains the gold standard, CCE could be useful for screening due to its high negative predictive value (NPV).
Holleran et al[26] evaluated CCE as a filter test to reduce dependence on TC for FIT screening. CCE was performed one day, followed by TC the next. The capsule was swallowed by all patients, with a completion rate of 73%. PDR were 68% for CCE and 58% for TC. The correlation between CCE and TC was described as good to excellent for detecting polyps of any size or with significant neoplasia. The study suggests CCE is safe and reliable, potentially reducing the need for TC by 71%.
Sacher-Huvelin et al[31] compared CCE and TC in asymptomatic patients undergoing surveillance or screening. They found TC detected more polyps (311, 57%) than CCE (249, 46%). TC identified 5 cases of CRC, while CCE identified 3. Rereading capsule videos did not reveal additional pathology. The study concluded that CCE cannot replace TC as the gold standard due to lower polyp detection and missed cancers.
Akyüz et al[32] evaluated the diagnostic value of CCE in patients with optimal bowel cleaning. They found that CCE was highly sensitive for detecting colonic polyps. Both CCE and TC had 100% completion rates in 28 patients who underwent both procedures.
For this systematic review, two studies by Eliakim et al[28] compared CCE and TC. The first study, a pilot, evaluated the first-generation CCE, demonstrating promising results for colonic evaluation and confirming its feasibility and safety. The second study assessed the second-generation capsule, showing a significant improvement in polyp detection compared to the first-generation capsule[29]. Eliakim et al[29] noted the need for further studies, but advancements in CCE have occurred over the past 15 years.
The nine studies in this review compared PDR between CCE and TC. PDR varied: CCE (21%-81%) and TC (25%-84%). Five studies favored TC, while four favored CCE. In four studies, the difference in detection rates was less than 5%.
In Koback-Larsen's study, 21% of patients needed repeat colonoscopy due to polyps found by CCE but missed during initial TC[24]. This led to the detection of 82 additional polyps, highlighting CCE's role as a complementary tool. Eliakim also noted discrepancies in polyp detection and reporting between CCE and TC[29].
Sacher-Huvelin et al[31] highlighted that colonoscopy had better diagnostic performance compared to CCE, including the detection of two large cancerous tumors (35 mm and 15 mm) missed by CCE despite adequate bowel preparation. Colonoscopy detected more polyps overall (57%) than CCE (46%).
Akyüz et al[32] focused on bowel cleansing and CCE's diagnostic value. In their study of 28 patients who had both CCE and TC, additional polyps were found in two patients during TC, and one polyp detected by CCE was missed by TC. They concluded that CCE had excellent diagnostic accuracy.
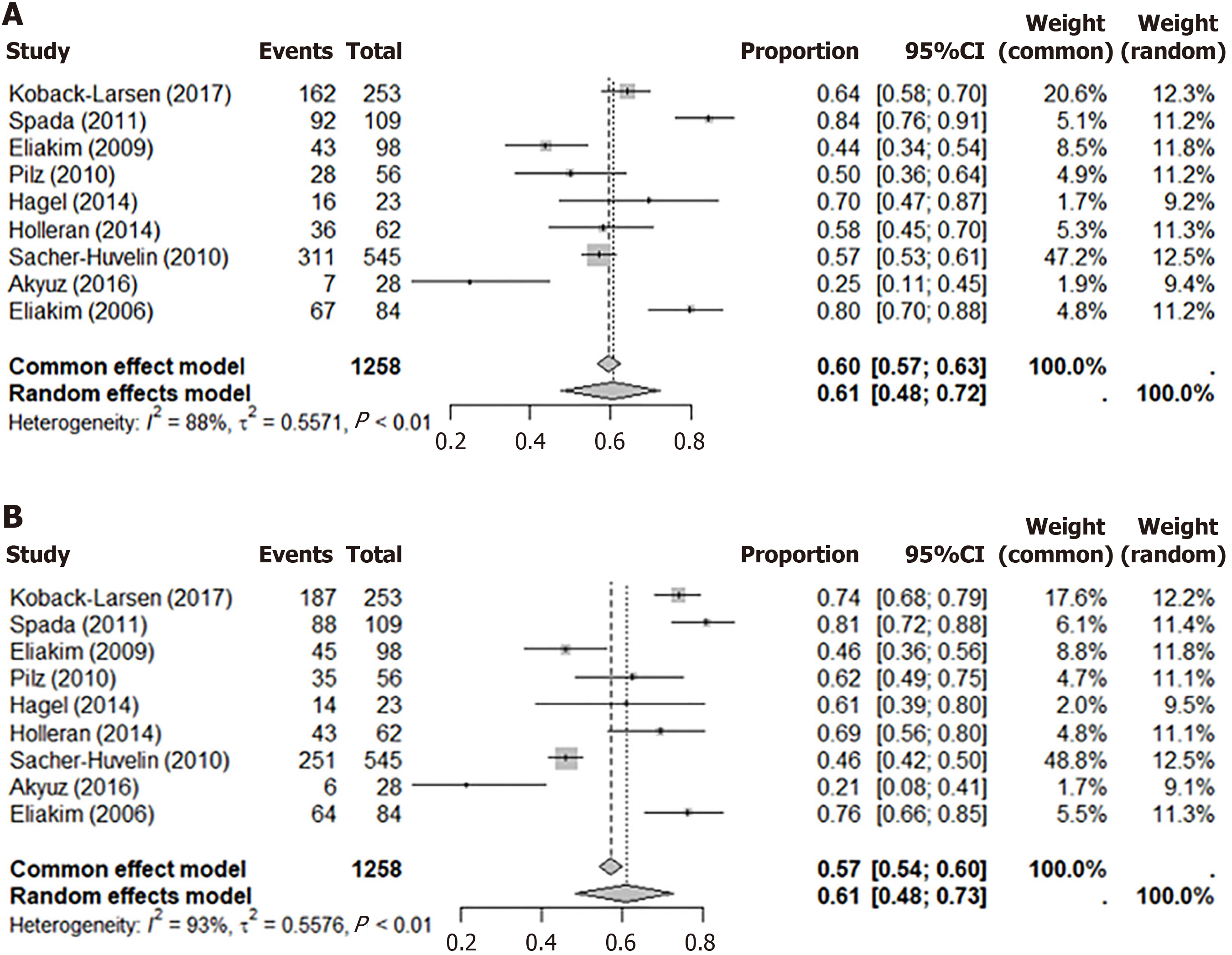
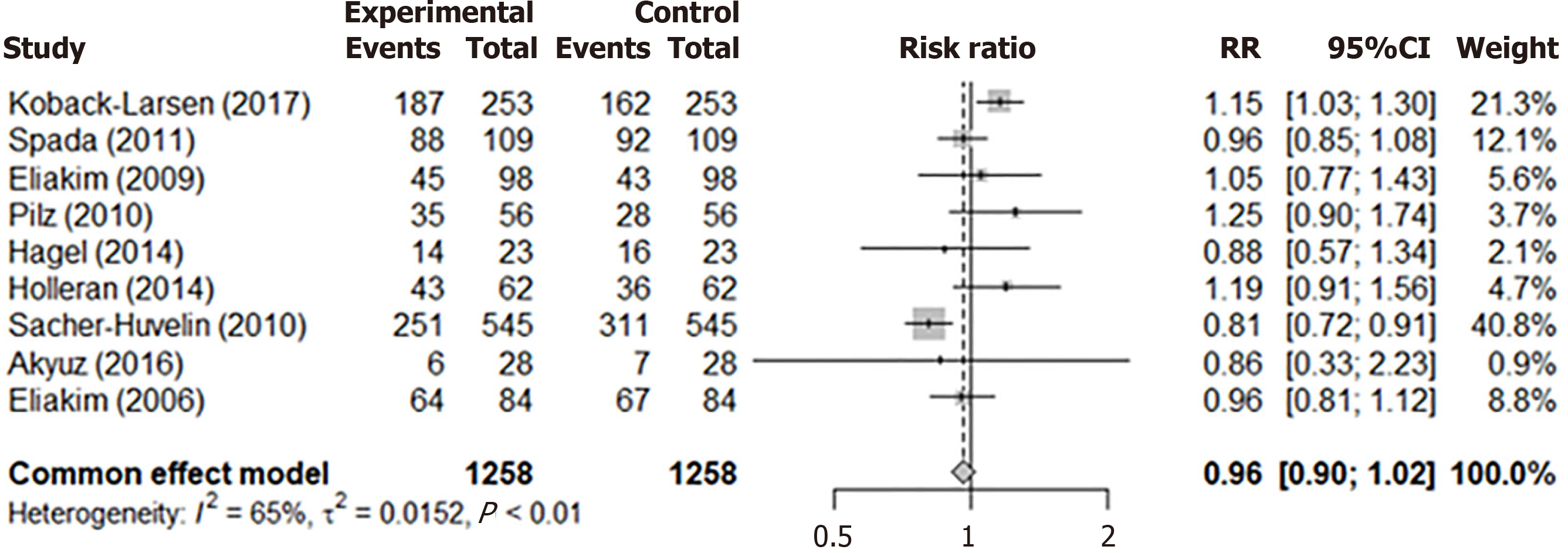
The systematic review included three meta-analyses. The first forest plot (Figure 3A) showed colonoscopy’s PDR in 9 studies with a common effects model proportion of 0.60 (95%CI: 0.57-0.63) and a random effects model proportion of 0.61 (95%CI: 0.48-0.72). The second plot (Figure 3B) presented CCE’s PDR: The common effects model proportion was 0.57 (95%CI: 0.54-0.60) and the random effects model was 0.61 (95%CI: 0.48-0.73). The third plot (Figure 4) compared PDR between CCE and colonoscopy, showing a RR of 0.96 (95%CI: 0.90-1.02), indicating negligible differences in performance.
In meta-analysis, the fixed effect model assumes a single true effect size, while the random effects model addresses within-study and between-study variability, providing a more accurate estimate under high heterogeneity[35]. The I² statistic quantifies variability across studies, with values over 50% indicating moderate to high heterogeneity, making the random effects model more appropriate[36]. A non-inferiority hypothesis tests if a new procedure is not worse than the standard (colonoscopy) and does not exceed the non-inferiority margin[37]. According to Schumi and Wittes[38], a new product should not be unacceptably worse and may offer benefits like fewer side effects, improved quality of life, or easier dosing[38]. CCE's benefits include pain tolerance, emotional ease, and fewer risks compared to colonoscopy[39,15].
CRCs are a leading cause of morbidity and mortality, making early diagnosis and treatment crucial[40-42]. This systematic review and meta-analysis aimed to compare PDRs between CCE and TC to determine the optimal diagnostic approach. Several studies investigated differences in PDR between CCE and TC, providing data for the meta-analysis.
This article belongs to a group of systematic reviews and meta-analyses comparing recent studies. The main goal was to assess the effectiveness of PDR when using CCE vs TC. The research emphasizes the need for alternative procedures that are both effective and more acceptable to patients, particularly for those who might avoid TC. This alternative is crucial in the global effort against CRC.
TC is globally recognized as the gold standard for large bowel assessment, with around 50 million cases performed annually[43]. It effectively detects and treats neoplastic and non-neoplastic conditions, reducing the need for further procedures. However, TC can be uncomfortable, emotionally challenging, and carries risks such as bleeding, perforation, and medication reactions[15].
CCE has been a safe method for large bowel diagnostics since its introduction in 2001[11]. It avoids sedation and analgesics, making it appealing for polyp diagnosis. However, its effectiveness depends on proper bowel preparation, as inadequate preparation can limit visualization. Koulaouzidis et al[44] highlighted that, despite CCE’s high-definition cameras and ability to navigate haustral folds, some areas of the colon may still be difficult to access. Therefore, opti
This meta-analysis revealed that both TC and CCE have similar PDRs across all sizes. Hagel et al[25] confirmed that CCE is technically feasible and safe, with acceptable sensitivity and specificity for PDR. Holleran et al[26] concurred, stating that "CCE is safe and effective". Their findings suggest CCE as a potential "filter test," reducing the need for TC. Spada et al[27], Kobaek-Larsen et al[24], and Akyüz et al[32] found CCE highly sensitive for detecting colonic pathology, making it a valuable tool in cancer screening programs. Eliakim et al[28,29] noted that CCE showed promise in 2006, and by 2009, improvements led to enhanced visualization and PDR.
Studies by Sacher-Huvelin et al[31] and Pilz et al[30] concluded that CCE does not match the gold standard set by TC, citing issues like bowel preparation quality and the capsule missing two cancers, which wasn't due to reader error. They noted that the difference between CCE and TC exceeds the maximal interval accepted for non-inferiority, differing from the findings of this systematic review. Spada et al[45] also acknowledged TC's superiority due to its interventional capabilities, but noted that CCE is appealing for being non-invasive, low-risk, and suitable for patients who might refuse other procedures.
Adverse events related to both CCE and TC were reported. Spada et al[27] noted vomiting, nausea, and abdominal pain from bowel preparation, and extreme fatigue due to the prolonged CCE procedure, as well as severe abdominal pain during TC. Pilz et al[30] also mentioned abdominal pain post-TC with polypectomy, though no perforation occurred. Kobaek-Larsen et al[24] found no serious adverse events related to either procedure, and Eliakim et al[28] similarly reported minimal issues, with one patient experiencing severe pain during TC. Hagel et al[25] reported a headache during bowel preparation, and Sacher-Huvelin et al[31] listed various adverse events without specifying the procedure.
Reflecting on the strengths and weaknesses of this systematic review and meta-analyses, the use of PRISMA guidelines was crucial for transparency, particularly in establishing a clear methodology that aids project development and helps peer reviewers assess replicability. The PICO approach was employed to develop a solid research question and guide the literature review. Strict inclusion and exclusion criteria were set according to PRISMA guidelines, further strengthening the study. However, a notable weakness was the assessment of bias using the QUADAS-2 tool, as it affected the final results.
An independent history of performing both TC and reading CCE data provided a strong foundation for this project, enhancing access to guidance and evidence. Improvements could have included reviewing grey literature, which might have highlighted different results or opinions. Paez introduces grey literature as evidence not commercially published, such as academic papers, dissertations, government reports, conference papers, and ongoing research, all of which could contribute to a systematic review[46].
The review also addressed other critical factors related to the study's main question: The adequacy of bowel preparation, findings related to CRC, adenoma detection, equipment issues/malfunctions, and completion rates of both procedures. The central question was whether CCE could be used as a first-line diagnostic procedure or if TC remains the gold standard for large bowel assessment. After reviewing all relevant articles and completing meta-analyses, the results showed that statistically, the PDR for CCE and TC was comparable. Wang et al[47] describe non-inferiority as a comparison where one treatment is “not worse” than another. Even though the new treatment may not show better efficacy, it could still offer advantages such as fewer side effects, less invasiveness, or reduced costs.
One key finding from the comparative studies in this systematic review is the critical role of good bowel preparation for both CCE and TC to achieve proper bowel visualization for assessing polyps and other abnormalities[48]. Pilz et al[30] sub-analyzed 15 patients with 'good' bowel preparation and 11 with 'poor' preparation, noting that while sensitivity did not change significantly, good prep led to 100% positive and NPV. Sacher-Huvelin et al[31] emphasized that poor bowel preparation in nearly half of the patients significantly impacted polyp detection and diagnostic performance. Akyüz et al[32] highlighted the disadvantage of CCE due to its inability to wash the mucosa during the procedure. The ability of TC to introduce water into the large bowel for mucosal cleansing via a water pump system was noted as a strength, although it was not thoroughly discussed in the comparative studies[27,29,32].
Various studies explored different bowel preparation regimens, concluding that no perfect regimen exists. Patel et al[49] identified risk factors for poor bowel preparation, such as previous poor prep, non-English-speaking populations in the United Kingdom, obesity, advanced age, male gender, and comorbidities like Parkinson’s, dementia, diabetes, and stroke. These factors should be considered individually during the referral stage and could influence the choice between TC, CCE, or alternative procedures like computerized tomography colonography[50,51].
All but one study in this systematic review extensively discusses various bowel preparations and booster regimens for CCE. Hagel et al[25] scheduled TC the day after CCE, with a cleansing regimen that included 4 senna tablets, a liquid diet, 2 L polyethylene glycol (PEG) in the afternoon, and another 2 L the next morning. After CCE ingestion, the capsule’s transit was monitored, with sodium phosphate and bisacodyl suppository boosters given to shorten procedure time. The evening before TC, an additional 2 L of PEG was prescribed. Kobaek-Larsen et al[24] used a similar pattern but with 1000 mg oral magnesium oxide and a clear fluid diet post-CCE. Holleran et al[26] also monitored patients until the capsule reached the duodenum, using a similar bowel prep regimen. Sacher-Huvelin et al[31] allowed a low-fiber snack between CCE and TC, which may have contributed to the 45% poor bowel preparation reported. Akyüz et al[32] tested three cleaning regimens, with Group C showing improved results: A liquid diet before CCE and 4 L PEG, followed by 45 mL sodium phosphate and 5 mg bisacodyl on the examination day. Eliakim et al[28,29] reported adequate cleanliness for both procedures, following a regimen similar to other studies.
Patient acceptability, preference, and comfort are critical when choosing a diagnostic procedure, especially if previous experiences have been negative[50]. Ismail et al[52] reported that 78% of patients preferred CCE for future procedures, even if a follow-up TC was needed. Fiorillo et al[53] found equal tolerance for both CCE and TC, with no significant differences in comfort. Voska et al[54] noted that patients who refuse TC often do so because of the bowel preparation rather than the procedure itself; over half would still prefer TC due to the risk of requiring a second prep after CCE. Sieg[55] highlighted that non-invasive options like CCE could appeal to patients avoiding TC due to discomfort or embarrassment. Ali et al[56] echoed this, suggesting that non-invasive methods could increase general population acceptability. Deding et al[57] observed that 58% of patients reported moderate to high discomfort during TC, compared to just 6% during CCE.
Cash et al[50] found that 69% of patients preferred TC over CCE due to its quicker procedure time, reduced bowel preparation, and the ability to convert to a therapeutic procedure. Patients favoring CCE cited its less invasive nature and no sedation requirement. Similarly, Adrián-de-Ganzo et al[58] reported better acceptance for TC than CCE.
Incomplete examinations pose challenges for both procedures. TC can be incomplete due to looping, patient discomfort, or anatomical issues, while CCE can be hindered by gastric and bowel mobility, battery life, and technical issues. Pilz et al[30] noted that the 8-10 hour battery life of CCE was insufficient for some patients, especially those with bowel motility disorders. Hagel et al[25] mentioned temporary frame loss due to transmission failure, which has been mitigated by improved sensor array placement. Current CCE sensors are integrated into a recorder belt, reducing risks, with the sensor array now generally used only in pediatric patients or those with smaller abdomens[59].
Missed pathology is a recognized issue, and patients are consented accordingly before TC, following BSG guidelines that highlight the potential of missing pathology[60]. Ahn et al[61] report that the risk of missing polyps during large bowel examination varies from 6% to 27%, especially smaller polyps during initial TC. The standard withdrawal time from the caecum, ranging from > 6 to > 10 minutes, is critical in enhancing PDR[16]. For CCE, small polyps could be missed if both cameras are viewed together or the image speed exceeds 12 frames per second[62].
Cost implications arise when polyps found during CCE require completion TC for therapeutic intervention. Kroijer et al[41] highlight that capsule data evaluation and report generation take about an hour, longer than most TC procedures, making it less cost-effective. Eliakim et al[29] found that 40% of capsule readings were completed in 30 minutes, 64% in 40 minutes, and over 75% within 50 minutes. Hassan et al's study on the cost-effectiveness of CCE for CRC screening concluded that its cost-effectiveness depends on its ability to improve compliance with CRC screening[63].
Advancements in medical procedures are crucial for improving diagnostic tests. Costigan et al[64] discuss the development of CCE procedures with future improvements including machine learning, AI for reading phases, and a cloud-based platform for remote and emergency readings. Seward et al[65] highlight ongoing improvements in TC, such as water-assisted insertion, water-assisted polypectomy, and tools like endocuff and endocap for enhanced PDR. For both CCE and TC, virtual reality (VR) training has emerged as a valuable tool, offering a simulated environment that enhances endoscopic skills and reduces procedure times. Studies indicate that VR improves accuracy and decreases patient discomfort, making it an effective supplement to traditional training methods and a promising avenue for future integration into endoscopy training[66,67].
In conclusion, CCE and TC are similar for PDR. CCE offers advantages such as non-invasiveness, greater comfort, and no sedation, making it less emotionally demanding. However, CCE has limitations, including no capability for therapeutic procedures, lack of irrigation for better mucosal visualization, and potential need for TC if pathology is detected. This review indicates that CCE is a modern, safe alternative to TC, suitable for diagnostic purposes, including polyp screening and for patients who prefer or require a non-invasive option.
We extend our appreciation to the Faculty of Life Sciences and Education at the University of South Wales for the Gastroenterology MSc program and their invaluable support in our work. We sincerely acknowledge the efforts of the University of South Wales and commend them for their commitment to providing life-long learning opportunities and advanced life skills to healthcare professionals.
| 1. | Meseeha M, Attia M. Colon Polyps. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2024. [PubMed] |
| 2. | Mathews AA, Draganov PV, Yang D. Endoscopic management of colorectal polyps: From benign to malignant polyps. World J Gastrointest Endosc. 2021;13:356-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (8)] |
| 3. | Neilson LJ, Rutter MD, Saunders BP, Plumb A, Rees CJ. Assessment and management of the malignant colorectal polyp. Frontline Gastroenterol. 2015;6:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Huck MB, Bohl JL. Colonic Polyps: Diagnosis and Surveillance. Clin Colon Rectal Surg. 2016;29:296-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Grahn SW, Varma MG. Factors that increase risk of colon polyps. Clin Colon Rectal Surg. 2008;21:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, Kuipers EJ. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 917] [Article Influence: 91.7] [Reference Citation Analysis (0)] |
| 7. | American Cancer Society. American Cancer Society Guideline for Colorectal Cancer Screening. Available from: https://www.cancer.org/cancer/types/colon-rectal-cancer/detection-diagnosis-staging/acs-recommendations. |
| 8. | World Cancer Research Fund International. Worldwide cancer data. Available from: https://www.wcrf.org/cancer-trends/worldwide-cancer-data/. |
| 9. | Asadzadeh Aghdaei H, Nazemalhosseini Mojarad E, Ashtari S, Pourhoseingholi MA, Chaleshi V, Anaraki F, Haghazali M, Zali MR. Polyp detection rate and pathological features in patients undergoing a comprehensive colonoscopy screening. World J Gastrointest Pathophysiol. 2017;8:3-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (2)] |
| 10. | Rutter MD, East J, Rees CJ, Cripps N, Docherty J, Dolwani S, Kaye PV, Monahan KJ, Novelli MR, Plumb A, Saunders BP, Thomas-Gibson S, Tolan DJM, Whyte S, Bonnington S, Scope A, Wong R, Hibbert B, Marsh J, Moores B, Cross A, Sharp L. British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England post-polypectomy and post-colorectal cancer resection surveillance guidelines. Gut. 2020;69:201-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 261] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 11. | Adler SN. The history of time for capsule endoscopy. Ann Transl Med. 2017;5:194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Kroijer R, Dyrvig AK, Kobaek-Larsen M, Støvring JO, Qvist N, Baatrup G. Booster medication to achieve capsule excretion in colon capsule endoscopy: a randomized controlled trial of three regimens. Endosc Int Open. 2018;6:E1363-E1368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Deding U, Kaalby L, Baatrup G, Kobaek-Larsen M, Thygesen MK, Epstein O, Bjørsum-Meyer T. The Effect of Prucalopride on the Completion Rate and Polyp Detection Rate of Colon Capsule Endoscopies. Clin Epidemiol. 2022;14:437-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Toskas A, Laskaratos FM, Coda S. Virtual Chromoendoscopy in Capsule Endoscopy: A Narrative Review. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Stauffer CM, Pfeifer C. Colonoscopy. 2023 Jul 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 16. | Rees CJ, Thomas Gibson S, Rutter MD, Baragwanath P, Pullan R, Feeney M, Haslam N; British Society of Gastroenterology, the Joint Advisory Group on GI Endoscopy, the Association of Coloproctology of Great Britain and Ireland. UK key performance indicators and quality assurance standards for colonoscopy. Gut. 2016;65:1923-1929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 232] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 17. | de Sousa Magalhães R, Arieira C, Boal Carvalho P, Rosa B, Moreira MJ, Cotter J. Colon Capsule CLEansing Assessment and Report (CC-CLEAR): a new approach for evaluation of the quality of bowel preparation in capsule colonoscopy. Gastrointest Endosc. 2021;93:212-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Office for Health Improvement and Disparities (2023) Public health profiles. Available from: https://fingertips.phe.org.uk/search/screening#page/6/gid/1/pat/159/par/K02000001/ati/15/are/E92000001/iid/92601/age/280/sex/4/cat/-1/ctp/-1/yrr/1/cid/4/tbm/1. |
| 19. | Cancer Research UK. Bowel cancer screening. Available from: https://www.cancerresearchuk.org/health-professional/screening/bowel-cancer-screening#BCscreening1. |
| 20. | Say RE, Thomson R. The importance of patient preferences in treatment decisions--challenges for doctors. BMJ. 2003;327:542-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 307] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 21. | Pring ET, Rai J, Wallace A, Gould LE, Mai DVC, Drami I, Jenkins JT. Colorectal polyps. Br Med J. 2024. |
| 22. | Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4127] [Cited by in RCA: 4697] [Article Influence: 1174.3] [Reference Citation Analysis (0)] |
| 23. | National Institute for Health and Care Research. About PROSPERO. Available from: https://www.crd.york.ac.uk/PROSPERO/#aboutpage. |
| 24. | Kobaek-Larsen M, Kroijer R, Dyrvig AK, Buijs MM, Steele RJC, Qvist N, Baatrup G. Back-to-back colon capsule endoscopy and optical colonoscopy in colorectal cancer screening individuals. Colorectal Dis. 2018;20:479-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Hagel AF, Gäbele E, Raithel M, Hagel WH, Albrecht H, de Rossi TM, Singer C, Schneider T, Neurath MF, Farnbacher MJ. Colon capsule endoscopy: detection of colonic polyps compared with conventional colonoscopy and visualization of extracolonic pathologies. Can J Gastroenterol Hepatol. 2014;28:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Holleran G, Leen R, O'Morain C, McNamara D. Colon capsule endoscopy as possible filter test for colonoscopy selection in a screening population with positive fecal immunology. Endoscopy. 2014;46:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Spada C, Hassan C, Munoz-Navas M, Neuhaus H, Deviere J, Fockens P, Coron E, Gay G, Toth E, Riccioni ME, Carretero C, Charton JP, Van Gossum A, Wientjes CA, Sacher-Huvelin S, Delvaux M, Nemeth A, Petruzziello L, de Frias CP, Mayershofer R, Amininejad L, Dekker E, Galmiche JP, Frederic M, Johansson GW, Cesaro P, Costamagna G. Second-generation colon capsule endoscopy compared with colonoscopy. Gastrointest Endosc. 2011;74:581-589.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 196] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 28. | Eliakim R, Fireman Z, Gralnek IM, Yassin K, Waterman M, Kopelman Y, Lachter J, Koslowsky B, Adler SN. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study. Endoscopy. 2006;38:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 222] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 29. | Eliakim R, Yassin K, Niv Y, Metzger Y, Lachter J, Gal E, Sapoznikov B, Konikoff F, Leichtmann G, Fireman Z, Kopelman Y, Adler SN. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy. 2009;41:1026-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 30. | Pilz JB, Portmann S, Peter S, Beglinger C, Degen L. Colon Capsule Endoscopy compared to Conventional Colonoscopy under routine screening conditions. BMC Gastroenterol. 2010;10:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Sacher-Huvelin S, Coron E, Gaudric M, Planche L, Benamouzig R, Maunoury V, Filoche B, Frédéric M, Saurin JC, Subtil C, Lecleire S, Cellier C, Coumaros D, Heresbach D, Galmiche JP. Colon capsule endoscopy vs. colonoscopy in patients at average or increased risk of colorectal cancer. Aliment Pharmacol Ther. 2010;32:1145-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Akyüz Ü, Yılmaz Y, İnce AT, Kaya B, Pata C. Diagnostic Role of Colon Capsule Endoscopy in Patients with Optimal Colon Cleaning. Gastroenterol Res Pract. 2016;2016:2738208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Tang Y, Anandasabapathy S, Richards-Kortum R. Advances in optical gastrointestinal endoscopy: a technical review. Mol Oncol. 2021;15:2580-2599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 34. | Milluzzo SM, Cesaro P, Grazioli LM, Olivari N, Spada C. Artificial Intelligence in Lower Gastrointestinal Endoscopy: The Current Status and Future Perspective. Clin Endosc. 2021;54:329-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Dettori JR, Norvell DC, Chapman JR. Fixed-Effect vs Random-Effects Models for Meta-Analysis: 3 Points to Consider. Global Spine J. 2022;12:1624-1626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 240] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 36. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46560] [Article Influence: 2116.4] [Reference Citation Analysis (3)] |
| 37. | Althunian TA, de Boer A, Groenwold RHH, Klungel OH. Defining the noninferiority margin and analysing noninferiority: An overview. Br J Clin Pharmacol. 2017;83:1636-1642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 38. | Schumi J, Wittes JT. Through the looking glass: understanding non-inferiority. Trials. 2011;12:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 298] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 39. | Riccioni ME, Urgesi R, Cianci R, Bizzotto A, Spada C, Costamagna G. Colon capsule endoscopy: Advantages, limitations and expectations. Which novelties? World J Gastrointest Endosc. 2012;4:99-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Alihosseini S, Aryankhesal A, Sabermahani A. Second-generation colon capsule endoscopy for detection of colorectal polyps: A meta-analysis. Med J Islam Repub Iran. 2020;34:81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 41. | Kroijer R, Kobaek-Larsen M, Qvist N, Knudsen T, Baatrup G. Colon capsule endoscopy for colonic surveillance. Colorectal Dis. 2019;21:532-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Parodi A, Vanbiervliet G, Hassan C, Hebuterne X, De Ceglie A, Filiberti RA, Spada C, Conio M. Colon capsule endoscopy to screen for colorectal neoplasia in those with family histories of colorectal cancer. Gastrointest Endosc. 2018;87:695-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Olympus Global Healthcare Data. Olympus Integrated Report 2021. Available from: https://www.olympus-global.com/ir/data/integratedreport/pdf/integrated_report_2021e_27.pdf. |
| 44. | Koulaouzidis A, Dabos K, Philipper M, Toth E, Keuchel M. How should we do colon capsule endoscopy reading: a practical guide. Ther Adv Gastrointest Endosc. 2021;14:26317745211001983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 45. | Spada C, Pasha SF, Gross SA, Leighton JA, Schnoll-Sussman F, Correale L, González Suárez B, Costamagna G, Hassan C. Accuracy of First- and Second-Generation Colon Capsules in Endoscopic Detection of Colorectal Polyps: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1533-1543.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 46. | Paez A. Gray literature: An important resource in systematic reviews. J Evid-Based Med. 2017;10:3. |
| 47. | Wang B, Wang H, Tu XM, Feng C. Comparisons of Superiority, Non-inferiority, and Equivalence Trials. Shanghai Arch Psychiatry. 2017;29:385-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 48. | Kjølhede T, Ølholm AM, Kaalby L, Kidholm K, Qvist N, Baatrup G. Diagnostic accuracy of capsule endoscopy compared with colonoscopy for polyp detection: systematic review and meta-analyses. Endoscopy. 2021;53:713-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 49. | Patel N, Kashyap S, Mori A. Bowel Preparation. 2023 Apr 17. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 50. | Cash BD, Fleisher MR, Fern S, Rajan E, Haithcock R, Kastenberg DM, Pound D, Papageorgiou NP, Fernández-Urién I, Schmelkin IJ, Rex DK. Multicentre, prospective, randomised study comparing the diagnostic yield of colon capsule endoscopy versus CT colonography in a screening population (the TOPAZ study). Gut. 2021;70:2115-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 51. | Schelde-Olesen B, Kaalby L, Deding U, Thygesen MMI, Andersen PV, Koulaouzidis A, Baatrup G, Bjørsum-Meyer T. Colon CApsule endoscopy compared to conventional COlonoscopy in patients with colonic DIverticulitis: the study protocol for a randomised controlled superiority trial (CACODI trial). BMJ Open. 2023;13:e073575. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 52. | Ismail MS, Murphy G, Semenov S, McNamara D. Comparing Colon Capsule Endoscopy to colonoscopy; a symptomatic patient's perspective. BMC Gastroenterol. 2022;22:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Fiorillo C, Quero G, Longo F, Mascagni P, Delvaux M, Mutter D. Capsule Endoscopy Versus Colonoscopy in Patients With Previous Colorectal Surgery: A Prospective Comparative Study. Gastroenterology Res. 2020;13:217-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 54. | Voska M, Zavoral M, Grega T, Majek O, Martinek J, Tacheci I, Benes M, Vojtechova G, Drastich P, Bures J, Spicak J, Buckova B, Ngo O, Suchanek S. Accuracy of Colon Capsule Endoscopy for Colorectal Neoplasia Detection in Individuals Referred for a Screening Colonoscopy. Gastroenterol Res Pract. 2019;2019:5975438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 55. | Sieg A. Capsule endoscopy compared with conventional colonoscopy for detection of colorectal neoplasms. World J Gastrointest Endosc. 2011;3:81-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 56. | Ali H, Pamarthy R, Sarfraz S, Ali E. Diagnostic Accuracy for Per-Patient Polyp Detection of Second-Generation Capsule Endoscopy Compared to Colonoscopy: A Meta-Analysis of Multicenter Studies. Cureus. 2021;13:e17560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Deding U, Bjørsum-Meyer T, Kaalby L, Kobaek-Larsen M, Thygesen MK, Madsen JB, Kroijer R, Baatrup G. Colon capsule endoscopy in colorectal cancer screening: Interim analyses of randomized controlled trial CareForColon2015. Endosc Int Open. 2021;9:E1712-E1719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Adrián-de-Ganzo Z, Alarcón-Fernández O, Ramos L, Gimeno-García A, Alonso-Abreu I, Carrillo M, Quintero E. Uptake of Colon Capsule Endoscopy vs Colonoscopy for Screening Relatives of Patients With Colorectal Cancer. Clin Gastroenterol Hepatol. 2015;13:2293-301.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 59. | Medtronic Covidien Products. Pillcam™ capsule endoscopy platform accessories. Available from: https://www.medtronic.com/covidien/en-gb/products/capsule-endoscopy/pillcam-capsule-endoscopy-platform-accessories.html#. |
| 60. | Public Health England. Bowel cancer screening: guidelines for colonoscopy. Available from: https://www.gov.uk/government/publications/bowel-cancer-screening-colonoscopy-quality-assurance/bowel-cancer-screening-guidelines-for-colonoscopy. |
| 61. | Ahn SB, Han DS, Bae JH, Byun TJ, Kim JP, Eun CS. The Miss Rate for Colorectal Adenoma Determined by Quality-Adjusted, Back-to-Back Colonoscopies. Gut Liver. 2012;6:64-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 62. | National Institute for Health and Care Excellence. PillCam COLON 2 for investigation of the colon through direct visualisation. Available from: https://www.nice.org.uk/guidance/gid-dg10083/documents/final-scope-2. |
| 63. | Hassan C, Zullo A, Winn S, Morini S. Cost-effectiveness of capsule endoscopy in screening for colorectal cancer. Endoscopy. 2008;40:414-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 64. | Costigan C, Walker C, O'Connell J, Omallao E, Manoharan T, Eagle N, Bailey Y, O'Hara F, Mc Namara D. Cloud technology and capsule endoscopy: A single-center users' experience of remote online video analysis and reporting. Endosc Int Open. 2024;12:E227-E230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 65. | Seward E. Recent advances in colonoscopy. F1000Res. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 66. | Siau K, Beintaris I. My approach to water-assisted colonoscopy. Frontline Gastroenterol. 2019;10:194-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | Dương TQ, Soldera J. Virtual reality tools for training in gastrointestinal endoscopy: A systematic review. Artif Intell Gastrointest Endosc. 2024;5. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (4)] |