Published online Sep 18, 2024. doi: 10.13105/wjma.v12.i3.95417
Revised: July 20, 2024
Accepted: July 29, 2024
Published online: September 18, 2024
Processing time: 156 Days and 15.3 Hours
One in every two individuals will experience a traumatic brain injury in their lifetime with significant impacts on the global economy and healthcare system each year. Neurovascular injury is a key aspect of neurotrauma to both the brain and the spinal cord and an important avenue of current and future research seeking innovative therapies. In this paper, we discuss primary and secondary neurotrauma, mechanisms of injury, the glymphatic system, repair and recovery. Each of these topics are directly connected to the vasculature of the central ner
Core Tip: Primary neurotrauma is an initial mechanical insult to the central nervous system. Secondary neurotrauma involves metabolic and cellular derangements that occur days to months after the initial insult. Together, this neurotrauma disrupts cerebral autoregulation and neurovascular coupling, leading to derangements in neurovascular flow and blood-brain barrier dysfunction. Similarly, the glymphatic system, responsible for clearing waste products through the perivascular space, becomes impaired following neurotrauma, leading to increased protein deposition and cognitive decline. Emerging therapeutics focus on reducing neuroedema, decreasing blood-brain barrier dysfunction, and promoting neuroregeneration.
- Citation: Willman J, Kurian AL, Lucke-Wold B. Mechanisms of vascular injury in neurotrauma: A critical review of the literature. World J Meta-Anal 2024; 12(3): 95417
- URL: https://www.wjgnet.com/2308-3840/full/v12/i3/95417.htm
- DOI: https://dx.doi.org/10.13105/wjma.v12.i3.95417
Statistics suggest that one in every two individuals will experience a traumatic brain injury (TBI) in their lifetimes and the ensuing cost to the global economy has grown upwards of 400 billion United States dollars per year[1]. As a result, TBI remains a grave social, economic, and clinical concern.
Disruption and damage to the vascular unit and perivascular glymphatic system may play integral roles in the mechanisms underlying neurotrauma in the immediate and long-term setting[2]. It follows that the vascular disturbances associated with neurotrauma represent a significant avenue of prognostication and treatment for the future of neurotrauma therapy in both TBI and traumatic spinal cord injuries (TSCI).
TBIs may occur in any demographic and range in intensity from severe to moderate to mild. However, the majority of TBI cases are mild and, amongst adult patients, most are male[3]. This sex difference is commonly attributed to an association between male sex and increased ‘risk-taking behavior’[4]. Similarly, TSCI most often occur in males and the elderly with less than one-third of cases resulting from motor vehicle collisions, less than one-third from occupation accidents, and the remainder from other causes[5-7]. The majority of TSCIs are cervical injuries which have the highest mortality rates when compared with thoracic or lumbar lesions[6]. In addition, TSCIs are associated with significant morbidity and medical costs, even years after the primary hospital stay with pressure ulcers and urological concerns being some of the most common causes of readmission[8]. Likewise, even mild TBI patients may report long-lasting symptoms. A subgroup of mild TBI patients report persistent symptoms of reduced cognitive ability and depressed mood months to years after the initial TBI[9-11]. The elderly and those with reduced cognitive reserve appear to be at highest risk for persistent symptoms[10,12]. Common chronic ailments associated with TBI include but are not limited to sleep disturbances, cognitive deficits, depression, post-traumatic stress disorder (PTSD), chronic pain, and increased risk of neurodegenerative diseases[13]. Studies of blast-related TBIs in soldiers appear to exhibit even higher rates of comorbidities such as headache, PTSD, and anxiety[14]. While recent advances in helmet design have reduced the number and severity of TBIs, there has been little reduction in the occurrence of SCIs[15]. Consequently, although admittedly less common, SCIs in the primary occurrence setting remain a largely unaddressed cause of morbidity and mortality. With the growing elderly population worldwide and corresponding higher rates, SCI burden is predicted to increase globally in the coming years[16].
Neurotrauma is divided into two stages: Primary trauma and secondary trauma. Primary trauma is defined by the initial neurological and vascular insult caused by direct mechanical forces acting on the central nervous system (CNS)[17]. The mechanism of trauma may vary from blunt to penetrating and may include projectile, nonprojectile, or blast-associated[17,18]. Neurotrauma may result in a number of immediate gross vascular consequences including epidural hematoma, subdural hematoma, subarachnoid hemorrhage, and intraparenchymal bleeds, in addition to neuronal lesions such as diffuse axonal injury (DAI)[17]. However, not all primary vascular injury is macroscopic. There is growing evidence that primary neurotrauma may cause significant microvascular injury which may lead directly to substantial secondary trauma[18]. A recent study by Reiter et al[19] examined how mechanical impacts displaced human and porcine neurons and vessels in brain tissue. They found that neurons surrounding blood vessels displaced further creating a heterogeneous interface between two entities, potentially generating an environment of substantial strain on the vessels[19]. Blast injuries, in particular, stress the microvessels and damage the endothelium and surrounding micro environment[2].
Cortical spreading depolarization (CSD) is a term that refers to electrical derangement, depolarization, neuronal swelling, and vascular constriction following a CNS insult such as neurotrauma[20]. This vasoconstriction in the setting of TBI may significantly exacerbate ischemia and contribute to morbidity[21]. As it has been estimated that over one-half of patients with TBI experience this sequence of events, CSD represents another significant avenue of future research in the field of neurotrauma vascular pathogenesis[20].
Primary neurotrauma may be further subdivided into primary TBI and primary SCI.
Based on the pattern of injury TBI may be further subdivided into focal and diffuse injuries[22]. Focal injuries are predominantly due to the linear acceleration of an impact[23]. The skull may warp or fracture on the “coup” side (the same side as the impact) causing vascular trauma with associated contusions or meningeal arterial trauma with an associated epidural hematoma[22-25]. On the opposite side of the brain from the linear impact, a “contrecoup” injury may occur due to the rebound of the brain within the skull[24]. These injuries may cause damage to the bridging cortical veins with a resultant subdural hematoma[24].
Diffuse injuries, on the other hand, are more strongly associated with rotational or angular acceleration[23]. This type of acceleration is often caused by oblique impacts and may cause significant white matter and microvascular deformation[26]. The growing body of evidence suggests that, while previously overlooked, rotational acceleration may cause as much or more trauma as linear acceleration[26,27]. Specifically, rotational acceleration may cause both diffuse vascular injury and DAI through sheering forces[25,28-31]. This DAI is characterized by extensive microtubule and axonal trauma with resultant synapse failure and apoptosis[32-34]. This DAI results in extensive real-world consequences both economically and in patient outcomes with chronic neurological deficits[34]. One study by Lota et al[35] examined impacts in combat sports and found high rates of rotational acceleration in mixed martial arts, boxing, and taekwondo, yet headgear use alone, much less headgear designed to mitigate rotational acceleration, remains contentious.
The field of TBI therapy research is quickly changing. For example, Mesenchymal stem cell-derived extracellular vesicles is one promising treatment that, in the lab setting, appears to stabilize vascular trauma, reduce inflammation, and promote neuroprotection[36,37]. However, further research is needed to clarify real-world benefits and ideal routes of delivery. Virtual reality-based therapy represents another novel treatment that may represent a useful tool for alleviating cognitive and mood dysfunctions post-TBI[38,39]. In short, there are numerous studies examining disparate routes of treatment for TBI.
TSCI may cause autonomic dysfunction, motor inhibition with damage to the corticospinal tract, and loss of sensation with damage to the spinothalamic tract or gracillis-cuneatus fasciculi of the dorsal columns[40-42]. In addition, TSCI may include damage or pressure on structures surrounding the cord, including ligaments, vertebrae, and vessels[40]. Particularly vulnerable vessels include the single anterior spinal artery and the duo of posterior spinal arteries[40]. Radicular arteries also play a vital role with the artery of Adamkiewicz contributing to the vascular supply of the lower segments of the spinal cord[40,41].
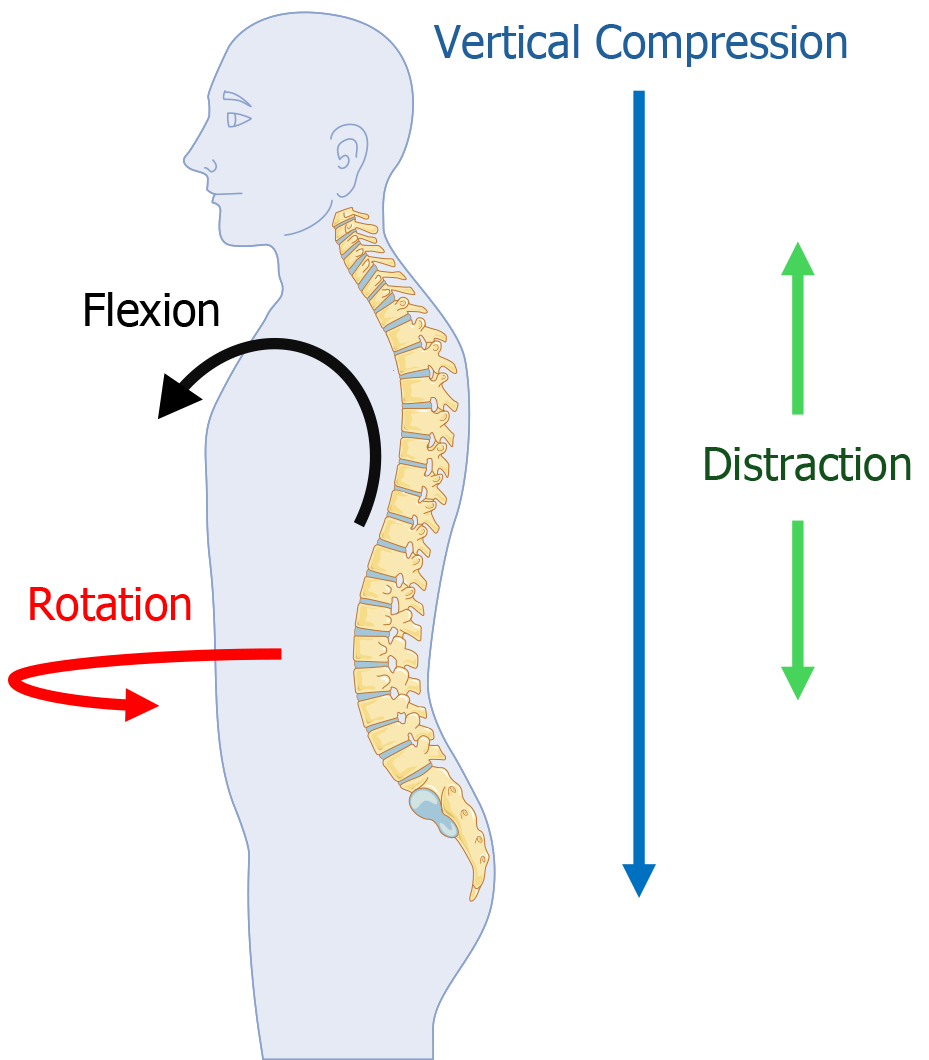
There are four primary mechanisms of force delivery to the spine[43]. These include axial compression, flexion compression, distraction, and rotational force[43]. Axial or vertical compression is commonly associated with burst fractures, especially of the thoracolumbar region[43-45]. Similarly, flexion compression is often associated with compression fractures of the anterior spinal column and a distinct “wedge fracture” [43,46,47]. Distraction force, which is essentially stretching force acting on the spine, has been associated with significant rates of blunt vascular trauma especially in the cervical region[48,49]. This force must often be significant to overpower spinal ligaments[50]. Rotational force especially when combined with other forces may result in significant damage to the cord and may cause vascular dissection, especially in the cervical region[43,51].
Furthermore, there are four main categories of TSCI[52]. These include TSCI with transient compression of the cord in which the initial cause of the compression is acutely self-corrected, TSCI with persistent compression of the cord in which the lesion remains uncorrected, distraction injury in which the spine remains extended due to a persistent lesion, and lastly direct laceration or transection in which the neural and vascular pathways are transected[52]. Compression, distraction, and laceration may all cause axonal and vascular disruption with ischemia and significant sequela[53].
In the setting of acute TSCI and traumatic stenosis, urgent surgery must often be performed with the goal decom
The immediate effects of primary neurotrauma are followed by secondary neurotrauma which is a collection of metabolic and cellular derangements that occur for days to months after the initial force event[17]. This cascade includes ischemia, excitotoxicity, oxidative stress, mitochondrial and endoplasmic reticulum dysfunction, vasogenic and cytotoxic edema, immune cell derangement, and neuronal cell death[17,55-58]. Excitotoxicity refers to the early release and failure of clearance of high concentrations of excitatory neurotransmitters such as glutamate and the consequent spreading depolarization or activation that occurs as a result[56]. Excitotoxicity is a neuronal derangement that has been identified in many CNS ailments from ischemic strokes to Alzheimer's disease[59].
The cell lysis from primary neurotrauma causes an increase in extracellular glutamate which binds N-methyl-D-aspartate and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors receptors which causes an influx of both calcium and sodium ions[60,61]. This influx of ions leads to deleterious effects on both the endoplasmic reticulum and the mitochondria[60]. Under normal conditions, mitochondria contain a complex and delicate network of membranes and channels that allow for oxidation and reduction through the electron transport chain[62,63]. The increased levels of intracellular calcium activate enzymes that damage the mitochondrial membrane, which in turn increases intra-organelle calcium levels, uncouples the mitochondrial electron transport chain, releases free radicals including reactive oxygen species, and triggers the activation of pro-apoptotic factors, and ultimately cell death[56,64,65]. As free radical formation and ischemia increase due to neurotrauma, there is a resultant increase in misfolded proteins within the endoplasmic reticulum (ER), a major site of protein production[57,66,67]. This causes an activation of the unfolded protein response (UPR)[68]. Persistent ER stress, free radical formation, and failed resolution of elevated protein misfolding cause the three sensor elements of the UPR, inositol-requiring protein 1 (IRE1), protein kinase RNA-like ER kinase (PERK), and activating transcription factor-6 (ATF6) to dissociate from the stabilizing protein GRP78[68]. IRE1 ultimately activates apoptotic protease-activating factor 1 (Apaf1)-dependent caspase, while PERK and ATF6 induce the pro-apoptotic factor C/EBP homologous protein[68]. Together, these proteins induce cell death[68].
Immune cell derangement is common post-neurotrauma. Levels of neutrophils in particular are elevated within CNS tissue immediately post neurotrauma, possibly as a result of elevated catecholamine concentrations[69]. These neu
There are two forms of edema that occur as a consequence of neurotrauma: vasogenic and cytotoxic edema. Vasogenic edema is the expansion of extracellular fluid as a result of CNS vascular injury[58]. Conversely, cytotoxic edema is intracellular edema caused by the pathologic accumulation of ions such as sodium within cells, post neurotrauma[58].
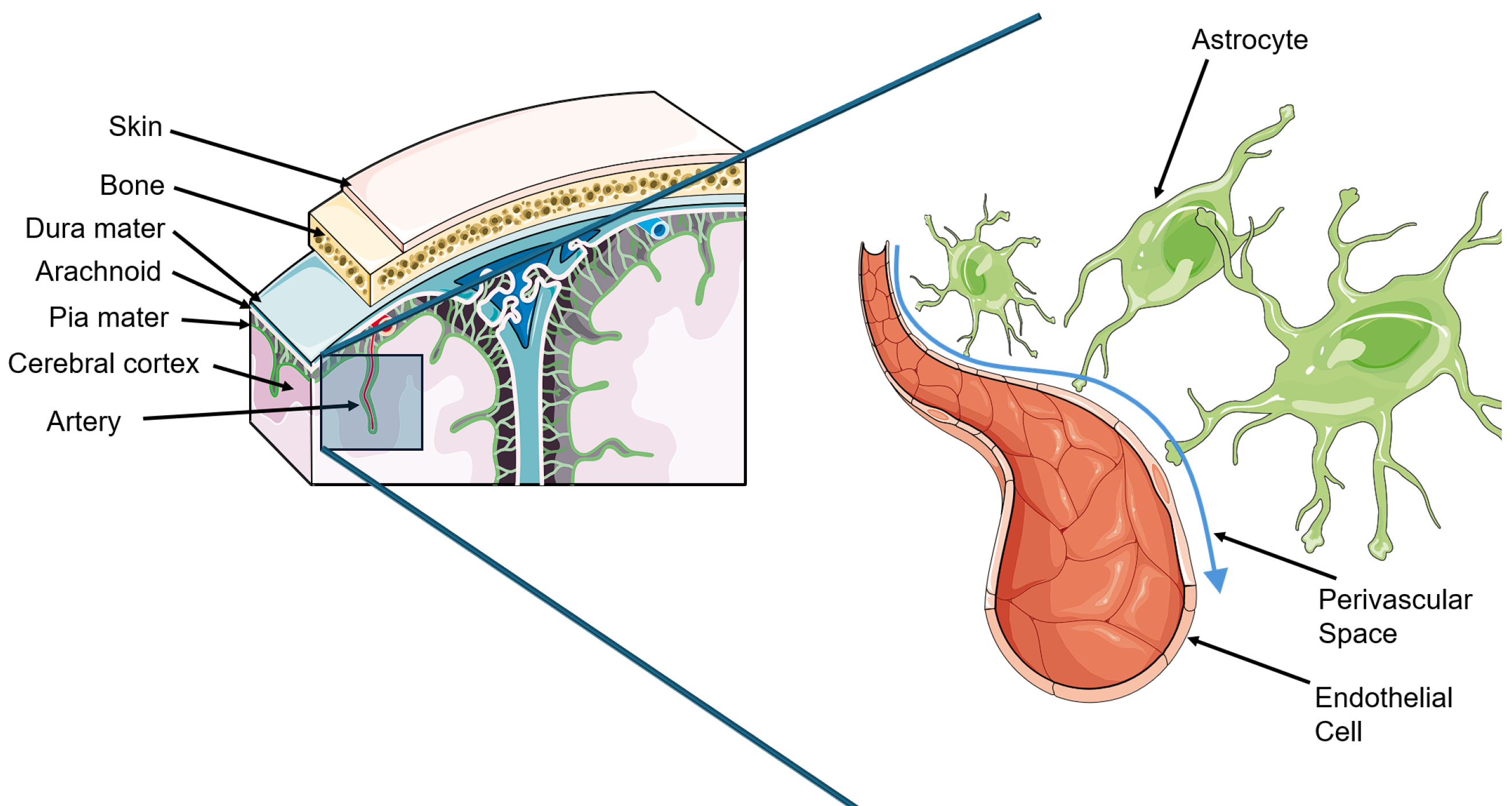
While it had long been believed that cerebral spinal fluid (CSF) ultimately drained into the lymphatic system, it was not until 2012 with the publication of a seminal paper by Iliff, Nedergaard, and colleagues that the CNS connection to the lymphatic system was discovered[70-72]. In their paper, Iliff et al[72] coined the term “glymphatic” by combining glial and lymphatic. This discovery has had substantial effects on the field since.
The glymphatic system is a perivascular network of astrocyte foot processes that create a space beneath the BBB where CSF and interstitial fluid between neuronal cells may cycle[70,73]. This is chiefly accomplished through aquaporin 4 (AQP4) water channels and allows for the clearance of waste products from neuronal tissue[73]. Ultimately, the CSF is absorbed by the arachnoid granulations or cervical lymphatics and is thus cleared from the CNS[70]. Following neurotrauma, the AQP4 channels become depolarized, and glymphatic system clearance becomes greatly impaired[74,75]. This appears to cause an increase in detrimental protein deposition including tau and amyloid beta and may account for some of the cognitive depression following TBI[74]. In addition, as the glymphatic system is naturally most active during sleep, there is some evidence that sleep disturbances caused by neurotrauma may contribute to a negative cycle of reduced sleep quality and worsening glymphatic function[70,76].
Consequently, the theoretical benefits of future therapeutics that might target glymphatic system malfunction in neurotrauma could include enhanced clearance of toxic excitatory or inflammatory byproducts and improved recovery (Figure 2).
Cerebrovascular disruption in CNS injuries can have significant effects due to impairment of cerebral autoregulation, a key factor influencing cerebral perfusion pressure, even when vascular injury is not apparent.
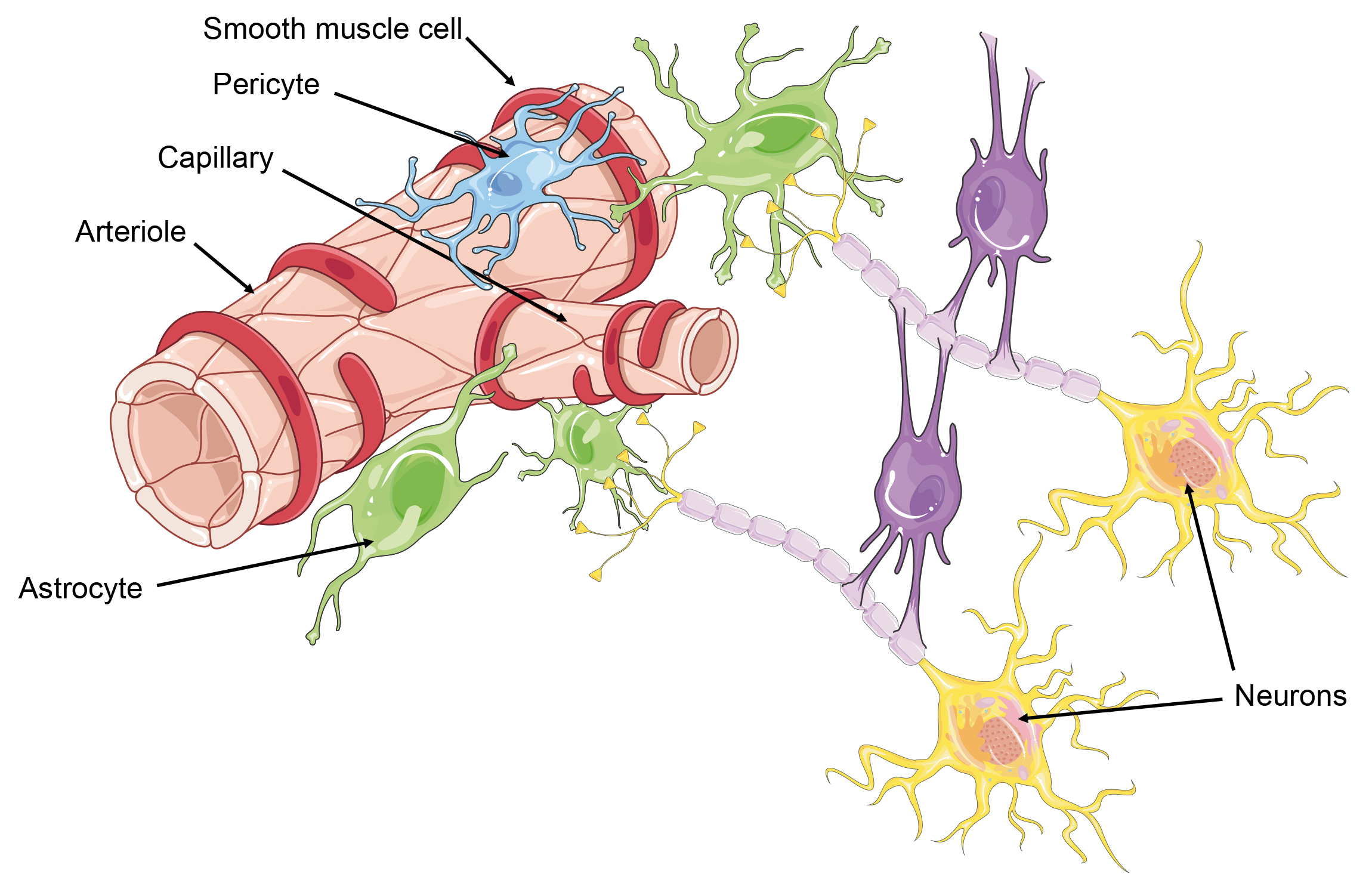
The CNS relies heavily on a consistent blood supply that dynamically adjusts to its metabolic demands through neurovascular units (NVUs)[77]. NVUs consist of endothelial cells, smooth muscle cells, pericytes, neurons, and perivascular astrocytes, working together to regulate cerebral blood flow, vascular permeability, and nutrient supply[77]. The endothelial cells form part of the BBB, which tightly controls the exchange of substances between the systemic circulation and CNS tissue[77]. NVUs communicate in a process known as neurovascular coupling to ensure consistent blood flow in response to neuronal activity[77]. However, following brain injury, communication within the NVU can be disrupted, leading to inappropriate changes in cerebral blood flow and BBB dysfunction[77]. This disruption can result in protein and electrolyte leakage into the brain parenchyma, triggering downstream processes such as microglia activation, that persist long after the primary injury, potentially contributing to ongoing neuropathology[77].
The brain relies heavily on consistent cerebral blood flow (CBF) to function optimally, unlike other organs, as it cannot withstand prolonged blood supply shortages. CBF is intricately linked to neuronal activity and is regulated by cerebral autoregulation, which maintains cerebral perfusion pressure (CPP) within a specific range to safeguard neural tissues from ischemia and subsequent neurodegeneration[78]. CPP is primarily determined by the difference between mean arterial pressure and intracranial pressure and it is maintained between 70–90 mmHg in healthy patients[79]. Cerebral autoregulation involves several interconnected processes, including neurogenic, myogenic, vasogenic, and metabolic mechanisms, mediated by various cellular components such as endothelial cells, smooth muscle cells, neurons, and glial cells[57]. These mechanisms adjust CBF in response to changes in systemic blood pressure, with different brain regions exhibiting distinct regulatory patterns[57].
TBI can impair cerebral autoregulation, which is vital during low systemic blood pressure scenarios, leading to diminished CBF[80]. Even mild TBIs (mTBI) can lead to cerebrovascular injury[81]. While cerebral autoregulation can maintain CPP between a wide range of pressures, neurotrauma can hinder this ability. This can manifest as symptoms ranging from a headache to chronic seizures or neuroendocrine abnormalities[82]. TBI can induce functional disruptions and neurodegenerative changes that impact CBF, potentially leading to lasting alterations in CBF regulation and post-traumatic complications.
Cerebral vascular dysfunction can arise from direct injury to vascular components or from secondary cascades initiated post-injury; as a result, cerebral vascular dysfunction can manifest either before or after neuronal damage in TBIs[83]. Reductions in CBF and microvascular circulation can lead to the formation of microthrombi, causing tissue hypoxia[77]. Microthrombi aggregation can then lead to secondary injuries such as hematoma formation and edema[77]. Vasogenic edema can cause hemispheric expansion and midline shift[77]. These effects of microvasculature disturbances, endothelial irregularities, dysmorphic capillaries, and disruption of pericytes and perivascular astrocytes, are seen even when models are used to simulate mTBIs[77].
Additionally, cerebrovascular reactivity (CVR) is a dynamic assessment of microvascular function that can serve as a sensitive biomarker of traumatic cerebrovascular injury (TCVI) in individuals with chronic moderate to severe TBIs[84]. CVR can be assessed with either functional near-infrared spectroscopy (fNIRS) or functional magnetic resonance imaging (fMRI), each offering its own set of advantages and drawbacks[84]. While fMRI stands as the benchmark due to its high resolution and compatibility with structural imaging, fNIRS presents superior temporal resolution, lower cost, and greater ease of use in outpatient settings[84]. Employing either imaging modality for CVR assessment not only holds potential as an additional diagnostic tool for evaluating TBI, but also emerges as a promising predictive and pharmacodynamic biomarker for interventions aimed at addressing TCVI[84].
Additionally, vascular risk factors such as hypertension, diabetes, hyperlipidemia, and especially smoking, lead to worse outcomes after TBI[85]. This necessitates aggressive treatment to improve patient outcomes for those with these risk factors[85] (Figure 3).
As shown through microdialysis, positron emission tomography, and magnetic resonance spectroscopy studies, TBIs can trigger a state of hypermetabolism characterized by increased glucose demand following biochemical brain injury, leading to neurotransmitter release and subsequent cellular responses[86]. This hyperactivity, aimed at restoring cellular and ionic homeostasis against progressing neuronal injury, can induce apoptotic events and mitochondrial impairment[86]. This causes a decrease in available energy for the body, which combined with the pre-existing microvascular isc
In TBI, the inherent mechanisms responsible for cerebrovascular repair remain incompletely understood. However, studies have shown that hypoxia-inducible factor 1α (HIF-1) plays a pivotal role in both natural recuperation and safeguarding against neurological damage following TBI[88]. HIF-1 is a vital regulator of homeostasis that can induce angiogenesis, erythropoiesis, as well as anti-apoptotic cascades[88,89].
Additionally, vascular endothelial growth factor-A (VEGF-A) is also released, increasing angiogenesis, vascular permeability, and vasogenic edema[90]. Moreover, it has been shown that veterans with blast-related mTBIs show persistently elevated levels of plasma VEGF-A, indicating continued cerebrovascular dysfunction following injury[91]. Animal studies have shown that levels of VEGF-A reach their highest levels at 24 hours after a TBI. The same study showed that bevacizumab, a common antitumor drug, can be given immediately after TBI to suppress VEGF-A expression and decrease cerebral edema[90].
Reducing cerebral edema is a key factor in the treatment of TBI. For example, the astrocytic water channel AQP-4 system plays a critical role in brain edema and transport across the BBB[92]. Animal studies have shown that minocycline, an antibiotic, could be used in a vascular-protective fashion against TBI because it reduced AQP-4 levels, thereby decreasing cerebral edema to optimize BBB integrity and astrocyte function[92]. Minocycline was also shown to be neuroprotective by reducing apoptosis of neurons and inflammation[92]. In the same way, stromal vascular fraction was also shown to ameliorate motor skills and slow memory regression in animals after TBI[93]. Yet another study showed a promising approach for TBI treatment with a novel biological scaffold containing heparin, collagen, and VEGF that could improve motor and cognitive function by generating an optimal microenvironment for the regeneration of injured nerve tissue[94].
VEGF-C is another of the vascular endothelial growth factors, which regulates microglia polarization and reduces cell apoptosis. Administration of VEGF-C has been shown to improve motor and neurologic function after TBI in animal models[95]. Administration of VEGI recombinant protein also showed similar results[96].
Moreover, these growth factors have been used as biomarkers to monitor outcomes in patients following TBI in the Transforming Research and Clinical Knowledge in TBI Pilot Study. This study showed that higher levels of thrombomodulin, angiopoietin-2, von Willebrand factor, and P-selectin were associated with more critical injuries while higher levels of angiopoietin-1, VEGF-C, and basic fibroblast growth factor were associated with less critical injuries[97].
Lastly, exercise has been shown to improve balance and walking abilities after neurotrauma[98]. Exercise can be done with physical therapists or even with robotic devices or virtual reality to progress neurorehabilitation[98].
While many animal studies over the years have initially shown promising results, later translation in human trials has often been less impressive[99,100]. This highlights the continued importance of pursuing innovative, new models and species that better capture human biology and choosing models that are best suited for the intricacies of the individual study.
The future of treatment options and the direction of future research for neurotrauma are poised for significant advancements aimed at improving patient outcomes and quality of life. Emerging therapeutic avenues are expected to focus on reducing cerebral edema, decreasing blood-brain barrier breakdown, and decreasing neuronal cell death. Additionally, there will be a growing emphasis on multidisciplinary collaboration, bringing together experts from various fields including neuroscience, neurology, neurosurgery, rehabilitation medicine, and bioengineering.
The development of novel pharmacological agents targeting specific molecular pathways implicated in neuroinflammation, excitotoxicity, and neuroregeneration holds promise for more effective treatments. These therapies may include neuroprotective agents, anti-inflammatory drugs, stem cell therapies, and gene therapies, among others.
Moreover, the integration of neurostimulation technologies, including transcranial magnetic stimulation, deep brain stimulation, and non-invasive brain stimulation techniques, offers exciting prospects for modulating neuronal activity and promoting neural plasticity to aid in recovery and rehabilitation post-neurotrauma. Additionally, advancements in neurorehabilitation strategies, such as robot-assisted therapy, virtual reality training, and brain-computer interfaces, are expected to play a crucial role in facilitating functional recovery and enhancing patient independence.
In terms of research directions, there will be a continued focus on elucidating the underlying pathophysiology of neurotrauma, including the complex interplay between genetic, environmental, and lifestyle factors. Large-scale prospective clinical studies and translational research efforts will be essential for validating novel therapeutic approaches and establishing evidence-based guidelines for optimal patient management. Furthermore, initiatives aimed at enhancing public awareness, education, and prevention strategies for neurotrauma will remain paramount in reducing the burden of these devastating injuries on individuals and society.
Overall, the future of treatment options and research directions for neurotrauma holds great promise, driven by advancements in technology, interdisciplinary collaboration, and a deeper understanding of the underlying mechanisms of brain injury and repair. These efforts aim to improve outcomes, enhance quality of life, and ultimately transform the landscape of neurotrauma care.
| 1. | Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, Bragge P, Brazinova A, Büki A, Chesnut RM, Citerio G, Coburn M, Cooper DJ, Crowder AT, Czeiter E, Czosnyka M, Diaz-Arrastia R, Dreier JP, Duhaime AC, Ercole A, van Essen TA, Feigin VL, Gao G, Giacino J, Gonzalez-Lara LE, Gruen RL, Gupta D, Hartings JA, Hill S, Jiang JY, Ketharanathan N, Kompanje EJO, Lanyon L, Laureys S, Lecky F, Levin H, Lingsma HF, Maegele M, Majdan M, Manley G, Marsteller J, Mascia L, McFadyen C, Mondello S, Newcombe V, Palotie A, Parizel PM, Peul W, Piercy J, Polinder S, Puybasset L, Rasmussen TE, Rossaint R, Smielewski P, Söderberg J, Stanworth SJ, Stein MB, von Steinbüchel N, Stewart W, Steyerberg EW, Stocchetti N, Synnot A, Te Ao B, Tenovuo O, Theadom A, Tibboel D, Videtta W, Wang KKW, Williams WH, Wilson L, Yaffe K; InTBIR Participants and Investigators. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 983] [Cited by in RCA: 1605] [Article Influence: 200.6] [Reference Citation Analysis (0)] |
| 2. | Elder GA, Gama Sosa MA, De Gasperi R, Perez Garcia G, Perez GM, Abutarboush R, Kawoos U, Zhu CW, Janssen WGM, Stone JR, Hof PR, Cook DG, Ahlers ST. The Neurovascular Unit as a Locus of Injury in Low-Level Blast-Induced Neurotrauma. Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 3. | Nguyen R, Fiest KM, McChesney J, Kwon CS, Jette N, Frolkis AD, Atta C, Mah S, Dhaliwal H, Reid A, Pringsheim T, Dykeman J, Gallagher C. The International Incidence of Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Can J Neurol Sci. 2016;43:774-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 298] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 4. | Duncan KA, Garijo-Garde S. Sex, Genes, and Traumatic Brain Injury (TBI): A Call for a Gender Inclusive Approach to the Study of TBI in the Lab. Front Neurosci. 2021;15:681599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Jain NB, Ayers GD, Peterson EN, Harris MB, Morse L, O'Connor KC, Garshick E. Traumatic spinal cord injury in the United States, 1993-2012. JAMA. 2015;313:2236-2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 461] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 6. | Barbiellini Amidei C, Salmaso L, Bellio S, Saia M. Epidemiology of traumatic spinal cord injury: a large population-based study. Spinal Cord. 2022;60:812-819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 118] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 7. | Ding W, Hu S, Wang P, Kang H, Peng R, Dong Y, Li F. Spinal Cord Injury: The Global Incidence, Prevalence, and Disability From the Global Burden of Disease Study 2019. Spine (Phila Pa 1976). 2022;47:1532-1540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 221] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 8. | Gabbe BJ, Nunn A. Profile and costs of secondary conditions resulting in emergency department presentations and readmission to hospital following traumatic spinal cord injury. Injury. 2016;47:1847-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Barker-Collo S, Jones K, Theadom A, Starkey N, Dowell A, McPherson K, Ameratunga S, Dudley M, Te Ao B, Feigin V; BIONIC Research Group. Neuropsychological outcome and its correlates in the first year after adult mild traumatic brain injury: A population-based New Zealand study. Brain Inj. 2015;29:1604-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Rabinowitz AR, Li X, McCauley SR, Wilde EA, Barnes A, Hanten G, Mendez D, McCarthy JJ, Levin HS. Prevalence and Predictors of Poor Recovery from Mild Traumatic Brain Injury. J Neurotrauma. 2015;32:1488-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 11. | Dikmen S, Machamer J, Temkin N. Mild Traumatic Brain Injury: Longitudinal Study of Cognition, Functional Status, and Post-Traumatic Symptoms. J Neurotrauma. 2017;34:1524-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 12. | Oldenburg C, Lundin A, Edman G, Nygren-de Boussard C, Bartfai A. Cognitive reserve and persistent post-concussion symptoms--A prospective mild traumatic brain injury (mTBI) cohort study. Brain Inj. 2016;30:146-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Cifu DX. Clinical research findings from the long-term impact of military-relevant brain injury consortium-Chronic Effects of Neurotrauma Consortium (LIMBIC-CENC) 2013-2021. Brain Inj. 2022;36:587-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 14. | Dismuke-Greer C, Hirsch S, Carlson K, Pogoda T, Nakase-Richardson R, Bhatnagar S, Eapen B, Troyanskaya M, Miles S, Nolen T, Walker WC. Health Services Utilization, Health Care Costs, and Diagnoses by Mild Traumatic Brain Injury Exposure: A Chronic Effects of Neurotrauma Consortium Study. Arch Phys Med Rehabil. 2020;101:1720-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Donnan J, Walsh S, Fortin Y, Gaskin J, Sikora L, Morrissey A, Collins K, MacDonald D. Factors associated with the onset and progression of neurotrauma: A systematic review of systematic reviews and meta-analyses. Neurotoxicology. 2017;61:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;6:309-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 414] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 17. | Salasky VR, Chang WW. Neurotrauma Update. Emerg Med Clin North Am. 2023;41:19-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Das AS, Vicenty-Padilla JC, Chua MMJ, Jeelani Y, Snider SB, Regenhardt RW, Al-Mufti F, Du R, Izzy S. Cerebrovascular injuries in traumatic brain injury. Clin Neurol Neurosurg. 2022;223:107479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 19. | Reiter N, Paulsen F, Budday S. Mechanisms of mechanical load transfer through brain tissue. Sci Rep. 2023;13:8703. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Andrew RD, Hartings JA, Ayata C, Brennan KC, Dawson-Scully KD, Farkas E, Herreras O, Kirov SA, Müller M, Ollen-Bittle N, Reiffurth C, Revah O, Robertson RM, Shuttleworth CW, Ullah G, Dreier JP. The Critical Role of Spreading Depolarizations in Early Brain Injury: Consensus and Contention. Neurocrit Care. 2022;37:83-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 21. | Zhao HT, Tuohy MC, Chow D, Kozberg MG, Kim SH, Shaik MA, Hillman EMC. Neurovascular dynamics of repeated cortical spreading depolarizations after acute brain injury. Cell Rep. 2021;37:109794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Vande Vyvere T, Pisică D, Wilms G, Claes L, Van Dyck P, Snoeckx A, van den Hauwe L, Pullens P, Verheyden J, Wintermark M, Dekeyzer S, Mac Donald CL, Maas AIR, Parizel PM. Imaging Findings in Acute Traumatic Brain Injury: a National Institute of Neurological Disorders and Stroke Common Data Element-Based Pictorial Review and Analysis of Over 4000 Admission Brain Computed Tomography Scans from the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) Study. J Neurotrauma. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 23. | Li Y, Adanty K, Vakiel P, Ouellet S, Vette AH, Raboud D, Dennison CR. Review of Mechanisms and Research Methods for Blunt Ballistic Head Injury. J Biomech Eng. 2023;145. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Tsiouris AJ, Lui YW. Neuroimaging Update on Traumatic Brain Injury. IDKD Springer Series. 2024;. [DOI] [Full Text] |
| 25. | Hanna ME, Pfister BJ. Advancements in in vitro models of traumatic brain injury. Curr Opin Biomed Eng. 2023;25:100430. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Goutnik M, Goeckeritz J, Sabetta Z, Curry T, Willman M, Willman J, Currier Thomas T, Lucke-wold B. Neurotrauma Prevention Review: Improving Helmet Design and Implementation. Biomechanics. 2022;2:500-512. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Naumenko Y, Yuryshinetz I, Zabenko Y, Pivneva T. Mild traumatic brain injury as a pathological process. Heliyon. 2023;9:e18342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 28. | Stewart MKG, Shkrum MJ, McClafferty KJ, Mao H, Zhang Q. A Neuropathological Study of Diffuse Vascular Injury in Fatal Motor Vehicle Collisions. J Neuropathol Exp Neurol. 2022;81:88-96. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Umfress A, Chakraborti A, Priya Sudarsana Devi S, Adams R, Epstein D, Massicano A, Sorace A, Singh S, Iqbal Hossian M, Andrabi SA, Crossman DK, Kumar N, Shahid Mukhtar M, Luo H, Simpson C, Abell K, Stokes M, Wiederhold T, Rosen C, Lu H, Natarajan A, Bibb JA. Cdk5 mediates rotational force-induced brain injury. Sci Rep. 2023;13:3394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 30. | Tierney G. Concussion biomechanics, head acceleration exposure and brain injury criteria in sport: a review. Sports Biomech. 2021;1-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 31. | Losurdo M, Davidsson J, Sköld MK. Diffuse Axonal Injury in the Rat Brain: Axonal Injury and Oligodendrocyte Activity Following Rotational Injury. Brain Sci. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Baskurt O, Aydin S, Avci I, Ozay Nayir P, Aydin MV. Evaluation of neurogranin levels in a rat model of diffuse axonal injury. Acta Neurobiol Exp (Wars). 2024;84:80-88. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Hajiaghamemar M, Seidi M, Margulies SS. Head Rotational Kinematics, Tissue Deformations, and Their Relationships to the Acute Traumatic Axonal Injury. J Biomech Eng. 2020;142:0310061-03100613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Frank D, Melamed I, Gruenbaum BF, Grinshpun J, Kuts R, Shvartsur R, Azab AN, Assadi MH, Vinokur M, Boyko M. Induction of Diffuse Axonal Brain Injury in Rats Based on Rotational Acceleration. J Vis Exp. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Lota KS, Malliaropoulos N, Blach W, Kamitani T, Ikumi A, Korakakis V, Maffulli N. Rotational head acceleration and traumatic brain injury in combat sports: a systematic review. Br Med Bull. 2022;141:33-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 36. | Kodali M, Madhu LN, Reger RL, Milutinovic B, Upadhya R, Attaluri S, Shuai B, Shankar G, Shetty AK. A single intranasal dose of human mesenchymal stem cell-derived extracellular vesicles after traumatic brain injury eases neurogenesis decline, synapse loss, and BDNF-ERK-CREB signaling. Front Mol Neurosci. 2023;16:1185883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 37. | Xiong Y, Mahmood A, Chopp M. Mesenchymal stem cell-derived extracellular vesicles as a cell-free therapy for traumatic brain injury via neuroprotection and neurorestoration. Neural Regen Res. 2024;19:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 38. | De Luca R, Bonanno M, Marra A, Rifici C, Pollicino P, Caminiti A, Castorina MV, Santamato A, Quartarone A, Calabrò RS. Can Virtual Reality Cognitive Rehabilitation Improve Executive Functioning and Coping Strategies in Traumatic Brain Injury? A Pilot Study. Brain Sci. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 39. | Calabrò RS, Bonanno M, Torregrossa W, Cacciante L, Celesti A, Rifici C, Tonin P, De Luca R, Quartarone A. Benefits of Telerehabilitation for Patients With Severe Acquired Brain Injury: Promising Results From a Multicenter Randomized Controlled Trial Using Nonimmersive Virtual Reality. J Med Internet Res. 2023;25:e45458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 40. | Patek M, Stewart M. Spinal cord injury. Anaesth Intensive Care Med. 2023;24:406-411. [DOI] [Full Text] |
| 41. | Diaz E, Morales H. Spinal Cord Anatomy and Clinical Syndromes. Semin Ultrasound CT MR. 2016;37:360-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | Smith AC, Weber KA 2nd, O'Dell DR, Parrish TB, Wasielewski M, Elliott JM. Lateral Corticospinal Tract Damage Correlates With Motor Output in Incomplete Spinal Cord Injury. Arch Phys Med Rehabil. 2018;99:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 43. | Zanza C, Tornatore G, Naturale C, Longhitano Y, Saviano A, Piccioni A, Maiese A, Ferrara M, Volonnino G, Bertozzi G, Grassi R, Donati F, Karaboue MAA. Cervical spine injury: clinical and medico-legal overview. Radiol Med. 2023;128:103-112. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 44. | Woo JH, Lee HW, Choi HJ, Kwon YM. Are "Unstable" Burst Fractures Really Unstable? J Korean Neurosurg Soc. 2021;64:944-949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Giorgi PD, Pallotta ML, Legrenzi S, Nardi M, Andrea M, Schirò GR. Spinal cord compression in thoracolumbar burst fractures: application of high-definition three-dimensional exoscope in minimally invasive lateral surgery. Eur J Orthop Surg Traumatol. 2023;33:2173-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Landham PR, Baker-Rand HL, Gilbert SJ, Pollintine P, Annesley-Williams DJ, Adams MA, Dolan P. Is kyphoplasty better than vertebroplasty at restoring form and function after severe vertebral wedge fractures? Spine J. 2015;15:721-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 47. | Soultanis K, Thano A, Soucacos PN. "Outcome of thoracolumbar compression fractures following non-operative treatment". Injury. 2021;52:3685-3690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Vilela MD, Kim LJ, Bellabarba C, Bransford RJ. Blunt cerebrovascular injuries in association with craniocervical distraction injuries: a retrospective review of consecutive cases. Spine J. 2015;15:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Wu J, Xue J, Huang R, Zheng C, Cui Y, Rao S. A rabbit model of lumbar distraction spinal cord injury. Spine J. 2016;16:643-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Kaplan NB, Molinari C, Molinari RW. Nonoperative Management of Craniocervical Ligamentous Distraction Injury: Literature Review. Global Spine J. 2015;5:505-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | van Den Hauwe L, Sundgren PC, Flanders AE. Spinal Trauma and Spinal Cord Injury (SCI). 2020 Feb 15. In: Diseases of the Brain, Head and Neck, Spine 2020–2023: Diagnostic Imaging [Internet]. Cham (CH): Springer; 2020. [PubMed] |
| 52. | Kaur J, Mojumdar A. A mechanistic overview of spinal cord injury, oxidative DNA damage repair and neuroprotective therapies. Int J Neurosci. 2023;133:307-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Yu M, Wang Z, Wang D, Aierxi M, Ma Z, Wang Y. Oxidative stress following spinal cord injury: From molecular mechanisms to therapeutic targets. J Neurosci Res. 2023;101:1538-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 54. | Adegeest CY, Ter Wengel PV, Peul WC. Traumatic spinal cord injury: acute phase treatment in critical care. Curr Opin Crit Care. 2023;29:659-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 55. | Kempuraj D, Ahmed ME, Selvakumar GP, Thangavel R, Raikwar SP, Zaheer SA, Iyer SS, Govindarajan R, Nattanmai Chandrasekaran P, Burton C, James D, Zaheer A. Acute Traumatic Brain Injury-Induced Neuroinflammatory Response and Neurovascular Disorders in the Brain. Neurotox Res. 2021;39:359-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 56. | Rauchman SH, Zubair A, Jacob B, Rauchman D, Pinkhasov A, Placantonakis DG, Reiss AB. Traumatic brain injury: Mechanisms, manifestations, and visual sequelae. Front Neurosci. 2023;17:1090672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 57. | Ali HT, Sula I, AbuHamdia A, Elejla SA, Elrefaey A, Hamdar H, Elfil M. Nervous System Response to Neurotrauma: A Narrative Review of Cerebrovascular and Cellular Changes After Neurotrauma. J Mol Neurosci. 2024;74:22. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 58. | Freire MAM, Rocha GS, Bittencourt LO, Falcao D, Lima RR, Cavalcanti JRLP. Cellular and Molecular Pathophysiology of Traumatic Brain Injury: What Have We Learned So Far? Biology (Basel). 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 59. | Neves D, Salazar IL, Almeida RD, Silva RM. Molecular mechanisms of ischemia and glutamate excitotoxicity. Life Sci. 2023;328:121814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 70] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 60. | Baracaldo-Santamaría D, Ariza-Salamanca DF, Corrales-Hernández MG, Pachón-Londoño MJ, Hernandez-Duarte I, Calderon-Ospina CA. Revisiting Excitotoxicity in Traumatic Brain Injury: From Bench to Bedside. Pharmaceutics. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 61. | Hoffe B, Holahan MR. Hyperacute Excitotoxic Mechanisms and Synaptic Dysfunction Involved in Traumatic Brain Injury. Front Mol Neurosci. 2022;15:831825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 62. | Vekaria HJ, Kalimon OJ, Prajapati P, Velmurugan GV, Sullivan PG. An efficient and high-throughput method for the evaluation of mitochondrial dysfunction in frozen brain samples after traumatic brain injury. Front Mol Biosci. 2024;11:1378536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 63. | Pandya JD, Musyaju S, Modi HR, Cao Y, Flerlage WJ, Huynh L, Kociuba B, Visavadiya NP, Kobeissy F, Wang K, Gilsdorf JS, Scultetus AH, Shear DA. Comprehensive evaluation of mitochondrial redox profile, calcium dynamics, membrane integrity and apoptosis markers in a preclinical model of severe penetrating traumatic brain injury. Free Radic Biol Med. 2023;198:44-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 64. | Lamade AM, Anthonymuthu TS, Hier ZE, Gao Y, Kagan VE, Bayır H. Mitochondrial damage & lipid signaling in traumatic brain injury. Exp Neurol. 2020;329:113307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 65. | Benaroya H. Brain energetics, mitochondria, and traumatic brain injury. Rev Neurosci. 2020;31:363-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 66. | Sun D, Wang J, Liu X, Fan Y, Yang M, Zhang J. Dexmedetomidine attenuates endoplasmic reticulum stress-induced apoptosis and improves neuronal function after traumatic brain injury in mice. Brain Res. 2020;1732:146682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 67. | Mi L, Min X, Shi M, Liu L, Zhang Y, Zhu Y, Li P, Chai Y, Chen F, Deng Q, Zhang S, Zhang J, Chen X. Neutrophil extracellular traps aggravate neuronal endoplasmic reticulum stress and apoptosis via TLR9 after traumatic brain injury. Cell Death Dis. 2023;14:374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 68. | Wang L, Liu Y, Zhang X, Ye Y, Xiong X, Zhang S, Gu L, Jian Z, Wang H. Endoplasmic Reticulum Stress and the Unfolded Protein Response in Cerebral Ischemia/Reperfusion Injury. Front Cell Neurosci. 2022;16:864426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 69. | Alam A, Thelin EP, Tajsic T, Khan DZ, Khellaf A, Patani R, Helmy A. Cellular infiltration in traumatic brain injury. J Neuroinflammation. 2020;17:328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 70. | Gao Y, Liu K, Zhu J. Glymphatic system: an emerging therapeutic approach for neurological disorders. Front Mol Neurosci. 2023;16:1138769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 71. | Al Masri M, Corell A, Michaëlsson I, Jakola AS, Skoglund T. The glymphatic system for neurosurgeons: a scoping review. Neurosurg Rev. 2024;47:61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 72. | Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2426] [Cited by in RCA: 3672] [Article Influence: 306.0] [Reference Citation Analysis (0)] |
| 73. | Benveniste H, Elkin R, Heerdt PM, Koundal S, Xue Y, Lee H, Wardlaw J, Tannenbaum A. The glymphatic system and its role in cerebral homeostasis. J Appl Physiol (1985). 2020;129:1330-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 74. | Peters ME, Lyketsos CG. The glymphatic system's role in traumatic brain injury-related neurodegeneration. Mol Psychiatry. 2023;28:2707-2715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 75. | Li L, Chopp M, Ding G, Davoodi-Bojd E, Zhang L, Li Q, Zhang Y, Xiong Y, Jiang Q. MRI detection of impairment of glymphatic function in rat after mild traumatic brain injury. Brain Res. 2020;1747:147062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 76. | Piantino JA, Iliff JJ, Lim MM. The Bidirectional Link Between Sleep Disturbances and Traumatic Brain Injury Symptoms: A Role for Glymphatic Dysfunction? Biol Psychiatry. 2022;91:478-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 77. | Sandsmark DK, Bashir A, Wellington CL, Diaz-Arrastia R. Cerebral Microvascular Injury: A Potentially Treatable Endophenotype of Traumatic Brain Injury-Induced Neurodegeneration. Neuron. 2019;103:367-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 78. | Charkviani M, Muradashvili N, Lominadze D. Vascular and non-vascular contributors to memory reduction during traumatic brain injury. Eur J Neurosci. 2019;50:2860-2876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 79. | Acharya D, Ruesch A, Schmitt S, Yang J, Smith MA, Kainerstorfer JM. Changes in neurovascular coupling with cerebral perfusion pressure indicate a link to cerebral autoregulation. J Cereb Blood Flow Metab. 2022;42:1247-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 80. | Silverman A, Petersen NH. Physiology, Cerebral Autoregulation. 2023 Mar 15. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. [PubMed] |
| 81. | Zhao ZA, Yan L, Wen J, Satyanarayanan SK, Yu F, Lu J, Liu YU, Su H. Cellular and molecular mechanisms in vascular repair after traumatic brain injury: a narrative review. Burns Trauma. 2023;11:tkad033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 82. | Cifu DX, Diaz-Arrastia R, Williams RL, Carne W, West SL, McDougal M, Dixon K. The VA/DoD Chronic Effects of Neurotrauma Consortium: An Overview at Year 1. Fed Pract. 2015;32:44-48. [PubMed] |
| 83. | Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci. 2016;19:771-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 797] [Article Influence: 99.6] [Reference Citation Analysis (0)] |
| 84. | Amyot F, Kenney K, Spessert E, Moore C, Haber M, Silverman E, Gandjbakhche A, Diaz-Arrastia R. Assessment of cerebrovascular dysfunction after traumatic brain injury with fMRI and fNIRS. Neuroimage Clin. 2020;25:102086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 85. | Schneider ALC, Barber J, Temkin N, Gardner RC, Manley G, Diaz-Arrastia R, Sandsmark D. Associations of Preexisting Vascular Risk Factors With Outcomes After Traumatic Brain Injury: A TRACK-TBI Study. J Head Trauma Rehabil. 2023;38:E88-E98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 86. | Jalloh I, Carpenter KL, Helmy A, Carpenter TA, Menon DK, Hutchinson PJ. Glucose metabolism following human traumatic brain injury: methods of assessment and pathophysiological findings. Metab Brain Dis. 2015;30:615-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 87. | Farajzadeh Khosroshahi S, Yin X, K Donat C, McGarry A, Yanez Lopez M, Baxan N, J Sharp D, Sastre M, Ghajari M. Multiscale modelling of cerebrovascular injury reveals the role of vascular anatomy and parenchymal shear stresses. Sci Rep. 2021;11:12927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 88. | Umschweif G, Alexandrovich AG, Trembovler V, Horowitz M, Shohami E. Hypoxia-inducible factor 1 is essential for spontaneous recovery from traumatic brain injury and is a key mediator of heat acclimation induced neuroprotection. J Cereb Blood Flow Metab. 2013;33:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 89. | Ziello JE, Jovin IS, Huang Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med. 2007;80:51-60. [PubMed] |
| 90. | Wu M, Gong Y, Jiang L, Zhang M, Gu H, Shen H, Dang B. VEGF regulates the bloodbrain barrier through MMP9 in a rat model of traumatic brain injury. Exp Ther Med. 2022;24:728. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 91. | Meabon JS, Cook DG, Yagi M, Terry GE, Cross DJ, Muzi M, Pagulayan KF, Logsdon AF, Schindler AG, Ghai V, Wang K, Fallen S, Zhou Y, Kim TK, Lee I, Banks WA, Carlson ES, Mayer C, Hendrickson RC, Raskind MA, Marshall DA, Perl DP, Keene CD, Peskind ER. Chronic elevation of plasma vascular endothelial growth factor-A (VEGF-A) is associated with a history of blast exposure. J Neurol Sci. 2020;417:117049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Lu Q, Xiong J, Yuan Y, Ruan Z, Zhang Y, Chai B, Li L, Cai S, Xiao J, Wu Y, Huang P, Zhang H. Minocycline improves the functional recovery after traumatic brain injury via inhibition of aquaporin-4. Int J Biol Sci. 2022;18:441-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 93. | Berman S, Uhlendorf TL, Berman M, Lander EB. Effective Treatment of Traumatic Brain Injury in Rowett Nude Rats with Stromal Vascular Fraction Transplantation. Brain Sci. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 94. | Zhang J, Wang RJ, Chen M, Liu XY, Ma K, Xu HY, Deng WS, Ye YC, Li WX, Chen XY, Sun HT. Collagen/heparan sulfate porous scaffolds loaded with neural stem cells improve neurological function in a rat model of traumatic brain injury. Neural Regen Res. 2021;16:1068-1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 95. | Ju S, Xu C, Wang G, Zhang L. VEGF-C Induces Alternative Activation of Microglia to Promote Recovery from Traumatic Brain Injury. J Alzheimers Dis. 2019;68:1687-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 96. | Zhou Z, Gao S, Li Y, Peng R, Zheng Z, Wei W, Zhao Z, Liu X, Li L, Zhang J. VEGI Improves Outcomes in the Early Phase of Experimental Traumatic Brain Injury. Neuroscience. 2020;438:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 97. | Schneider ALC, Huie JR, Jain S, Sun X, Ferguson AR, Lynch C, Yue JK, Manley GT, Wang KKW, Sandsmark DK, Campbell C, Diaz-Arrastia R. Associations of Microvascular Injury-Related Biomarkers With Traumatic Brain Injury Severity and Outcomes: A Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) Pilot Study. J Neurotrauma. 2023;40:1625-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 98. | Bonanno M, De Luca R, De Nunzio AM, Quartarone A, Calabrò RS. Innovative Technologies in the Neurorehabilitation of Traumatic Brain Injury: A Systematic Review. Brain Sci. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 99. | Freeman-Jones E, Miller WH, Work LM, Fullerton JL. Polypathologies and Animal Models of Traumatic Brain Injury. Brain Sci. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 100. | Kundu S, Singh S. What Happens in TBI? A Wide Talk on Animal Models and Future Perspective. Curr Neuropharmacol. 2023;21:1139-1164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |