Published online Mar 6, 2021. doi: 10.12998/wjcc.v9.i7.1741
Peer-review started: November 18, 2020
First decision: December 30, 2020
Revised: January 5, 2021
Accepted: January 21, 2021
Article in press: January 21, 2021
Published online: March 6, 2021
Processing time: 102 Days and 16.3 Hours
Vasculitis, a systemic disorder with inflammation of blood vessel walls, can develop broad spectrum of signs and symptoms according to involvement of various organs, and therefore, early diagnosis of vasculitis is challenging. We herein describe a patient who developed a special case of systemic vasculitis with mononeuropathy multiplex, rectal perforation and antiphospholipid syndrome (APS) presented with pulmonary embolism.
A 61-year-old woman visited hospital with complaints of myalgia and occasional fever. She was initially diagnosed as proctitis and treated with antibiotics, however, there was no improvement. In addition, she also complained right foot drop with hypesthesia, and left 2nd and 3rd finger tingling sensation. She underwent nerve conduction study for evaluation, and it revealed sensorimotor polyneuropathy in the left arm and bilateral legs. Subsequent sural nerve biopsy strongly suggested vasculitic neuropathy. Based on nerve biopsy and clinical manifestation, she was diagnosed with vasculitis and treated with immuno-suppressive therapy. During treatment, sudden rectal perforation and pulmonary thromboembolism occurred, and further laboratory study suggested probable concomitant APS. Emergency Hartmann operation was performed for rectal perforation, and anti-coagulation therapy was started for APS. After few cycles of immunosuppressive therapy, tingling sensation and weakness in her hand and foot had been partially recovered and vasculitis was considered to be stationary.
Vasculitis can be presented with a variety of signs and symptoms, therefore, clinicians should always consider the possibility of diagnosis.
Core Tip: We present a special case of systemic vasculitis with initially developed a mononeuropathy multiplex, followed by rectal perforation, and antiphospholipid syndrome presented with pulmonary embolism. Systemic vasculitis can be presented with a variety of signs and symptoms, therefore, clinicians should always consider the possibility of diagnosis.
- Citation: Chae HJ, Kim JW, Lee YL, Park JH, Lee SY. Mononeuropathy multiplex associated with systemic vasculitis: A case report. World J Clin Cases 2021; 9(7): 1741-1747
- URL: https://www.wjgnet.com/2307-8960/full/v9/i7/1741.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i7.1741
Vasculitis is a systemic disorder caused by inflammation of blood vessel walls, and classified into different types according to size of involved vessels and presence of fibrinoid necrosis or granulomas[1]. Among various signs and symptoms in vasculitis, peripheral nerve involvement is common and can be presented as mononeuropathy multiplex or distally polyneuropathy[2]. This case report describes a case of initial clinical manifestation of mononeuropathy multiplex associated with systemic vasculitis, followed by rectal perforation, and antiphospholipid syndrome (APS) presented with pulmonary embolism.
A previously healthy 61-year-old woman was referred to a tertiary hospital presented with myalgia and occasional high fever from one month ago.
She was treated with intravenous ceftriaxone (1 g qd) for a week under the diagnosis of proctitis, but her symptoms did not improve. With myalgia, she also reported right foot drop with hypesthesia, left 2nd and 3rd finger tingling sensation, and urinary and fecal incontinence before few days visiting hospital.
The patient had a free previous medical history.
The patient had a free family history.
The neurological examination showed weakness in her right leg involving tibialis anterior and extensor hallucis muscles. The dorsiflexion and big toe extension power of the right foot were both graded 1/5 by manual muscle testing. Further sensory examination revealed hypesthesia in her right foot and left fingers.
Blood analysis revealed a mild leukocytosis (white blood cell count: 11580/μL; normal: 4000-9600/μL) and elevated C-reactive protein (16.53 mg/dL; normal: ≤ 0.30 mg/dL), but there was neither eosinophilia nor any abnormalities in the level of creatine kinase or antibodies including antineutrophil cytoplasmic antibody (ANCA), rheumatoid factor, anti-cyclic citrullinated peptide, anti-double stranded DNA antibody and anti-nuclear antibodies.
She underwent whole spine magnetic resonance imaging for evaluation of spinal cord, and there was no definite abnormality.
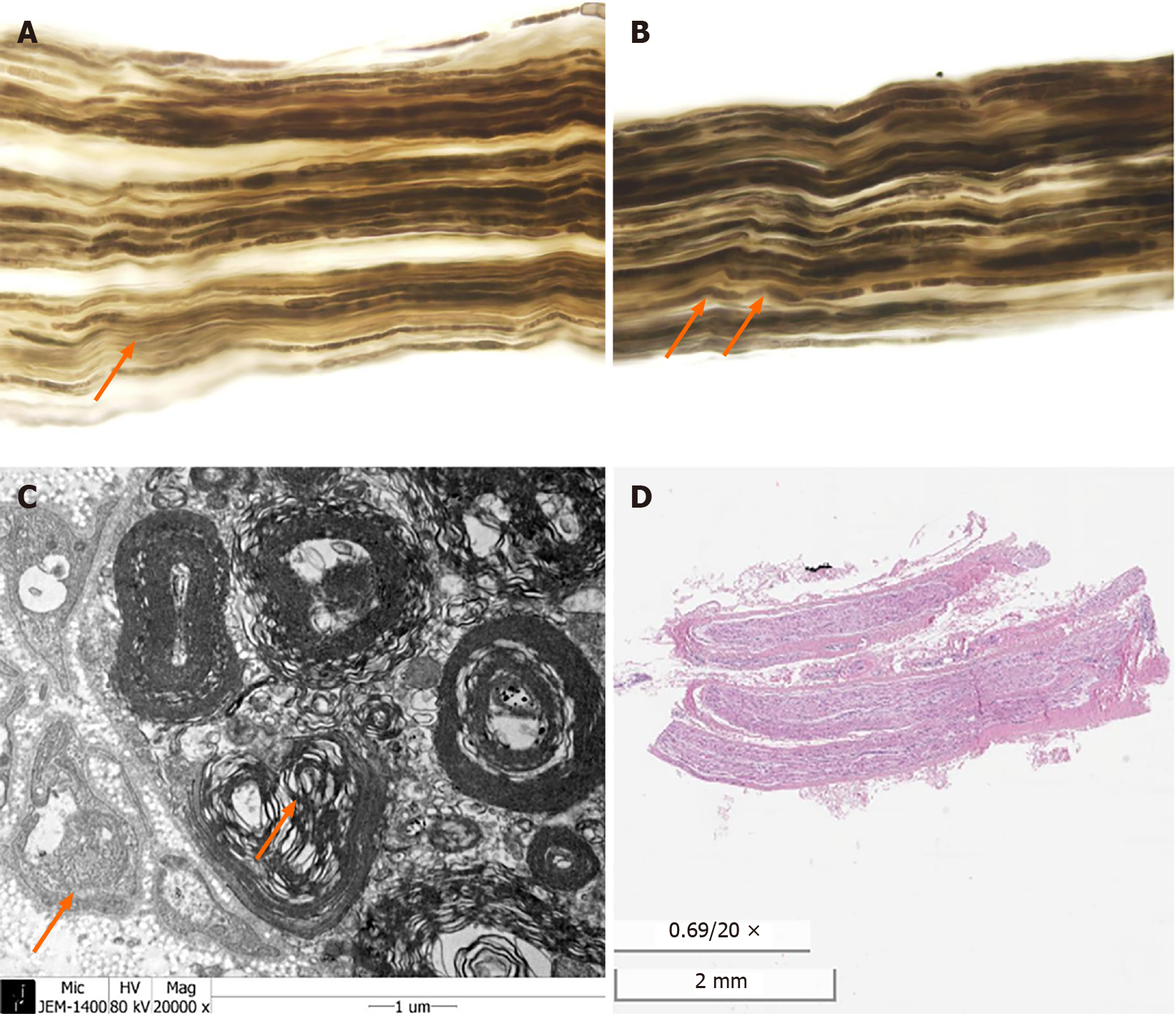
The patient was further evaluated with nerve conduction study (NCS), and it revealed sensorimotor polyneuropathy in the left arm and bilateral legs (Tables 1-3). Mononeuropathy multiplex of inflammatory cause, in particular vasculitic neuropathy, was suspected, and sural nerve biopsy was performed. In biopsy, although vasculitis was not present, diffuse sequential axonal degeneration and secondary demyelination strongly suggested vasculitic neuropathy (Figure 1).
| Initial (1 d after onset) | Follow-up (4 mo after onset) | |||||
| Nerve | Stimulation/recording site | Latency (ms) | Amplitude (µV) | Stimulation/recording site | Latency (ms) | Amplitude (µV) |
| Rt. median | Digit II/III/Wrist | Not done | Wrist/Digit II | 2.45 | 27.5 | |
| Lt. median | 2.5 | 12.1 | NR | NR | ||
| Rt. ulnar | Digit IV/V/Wrist | Not done | Wrist/Digit V | 2.66 | 42.9 | |
| Lt. ulnar | 2.7 | 15.1 | 2.40 | 9.9 | ||
| Rt. superficial peroneal | Not done | Lateral leg/Foot | NR | NR | ||
| Lt. superficial peroneal | NR | |||||
| Rt. sural | Calf/Lat. Malleolus | 2.8 | 1.6 | Calf/Lat. Malleolus | 2.03 | 5.6 |
| Lt. sural | 3.0 | 5.5 | 1.67 | 3.4 | ||
| Initial (1 d after onset) | Follow-up (4 mo after onset) | ||||||
| Nerve (recorded muscle) | Stimulation site | Latency (ms) | Amplitude (mV) | Conduction velocity (m/s) | Latency (ms) | Amplitude (mV) | Conduction velocity (m/s) |
| Rt. median(APB) | Wrist | Not done | 3.23 | 12.1 | |||
| Elbow | 6.35 | 7.5 | 57.6 | ||||
| Lt. median (APB) | Wrist | 3.0 | 0.9 | 3.23 | 2.7 | ||
| Elbow | NR | NR | 9.74 | 0.6 | 27.6 | ||
| Rt. Ulnar (ADM) | Wrist | Not done | 2.45 | 10.7 | |||
| B. Elbow | 5.57 | 9.1 | 67.2 | ||||
| Lt. ulnar (ADM) | Wrist | 2.5 | 7.7 | 2.81 | 5.4 | ||
| B. Elbow | 6.2 | 7.3 | 57.0 | 6.30 | 5.2 | 60.2 | |
| Rt. common peroneal (EDB) | Ankle | 4.3 | 0.5 | NR | NR | ||
| Fibular head | 9.4 | 0.4 | 53.0 | NR | NR | ||
| Lt. common peroneal (EDB) | Ankle | 3.2 | 3.8 | 3.80 | 2.6 | ||
| Fibular head | 8.7 | 3.5 | 48.0 | 9.97 | 2.0 | 50.1 | |
| Rt. common peroneal (TA) | Fibular head | 3.8 | 1.1 | 4.64 | 0.1 | ||
| Post. knee | 7.2 | 1.1 | 28.0 | 5.73 | 0.1 | 36.6 | |
| Lt. common peroneal (TA) | Fibular head | 2.8 | 9.6 | 2.14 | 4.2 | ||
| Post. knee | 4.4 | 9.6 | 63.0 | 2.71 | 4.0 | 69.8 | |
| Rt. tibial (AH) | Ankle | NR | NR | NR | NR | ||
| Post. knee | NR | NR | NR | NR | |||
| Lt. tibial (AH) | Ankle | NR | NR | NR | NR | ||
| Post. knee | NR | NR | NR | NR | |||
| H reflex | Initial | Follow up | F wave | Initial | Follow up | ||
| Nerve | Latency (ms) | Amplitude (µV) | Latency (ms) | Amplitude (µV) | Nerve | Latency (ms) | Latency (ms) |
| Rt. tibial | NR | NR | NR | NR | Rt. median | Not done | 24.43 |
| Lt. median | NR | 26.35 | |||||
| Rt. ulnar | Not done | 23.02 | |||||
| Lt. tibial | NR | NR | NR | NR | Lt. ulnar | NR | 27.24 |
| Rt. tibial | NR | NR | |||||
| Lt. tibial | NR | NR | |||||
Based on nerve biopsy findings and clinical manifestation, she was diagnosed with systemic vasculitis.
The patient underwent immunosuppressive therapies with initial intravenous methylprednisolone (500 mg qd) for three days, and was switched to oral steroids (starting at prednisolone 30 mg bid), followed by a tapered dose. After early treatment with steroid, she received total 6 cycles of intravenous cyclophosphamide pulse therapies (750 mg qd) at one-month intervals.
After first cycle of cyclophosphamide pulse therapy, sudden rectal perforation occurred and emergency Hartmann operation was performed. Histological examination of the resected colon showed segmental transmural ischemia and perforation with thrombotic obliteration of subserosal arteries.
Meanwhile, acute multifocal pulmonary thromboembolism and deep vein thrombosis were simultaneously found during admission. She underwent echocardiography to rule out possibility of amyloidosis which could have caused proctitis, and oscillating large mass in main pulmonary trunk bifurcation was incidentally found. Since the possibility of massive venous embolism is low in the case of vasculitis, additional blood tests were performed to rule out accompanied APS, and both anti-cardiolipin antibody (aCL) and lupus anticoagulant (LA) were positive. She started anti-coagulation therapy with subcutaneous injection of enoxaparine (60 mg bid) for one month, and was switched to oral warfarin with 2-3 target range of prothrombin time international normalized ratio. After 1 year later, both aCL and LA were changed to negative in follow-up examination.
After cyclophosphamide pulse therapies at one-month interval, tingling sensation and weakness in her hand and foot had been partially recovered. Four months later, follow-up electrodiagnostic study was performed, and it showed slightly aggravation in left median, ulnar, and bilateral peroneal nerves compared to previous NCS (Tables 1-4). With improving clinical symptoms, vasculitis was considered to be stationary, and she is now under observation after end of 6 cycles of cyclophosphamide therapies (750 mg qd).
| Needle EMG | Follow-up (4 mo after onset) | ||||||
| Muscle | Spontaneous | MUAP | Interference | ||||
| IA | Fib | PSW | Amp | Dur | PPP | Pattern | |
| Lt. first dorsal interosseous | Normal | None | 2+ | Normal | Normal | Normal | Reduced |
| Lt. extensor carpi radialis longus | Normal | None | None | Normal | Normal | Normal | Complete |
| Lt. biceps | Normal | None | None | Normal | Normal | Normal | Complete |
| Rt. tibialis anterior | Normal | 3+ | 3+ | Normal | Long | Increased | Single |
| Rt. gastrocnemius (medial) | Normal | 3+ | 3+ | None | |||
| Rt. abductor hallucis | Normal | 1+ | 1+ | None | |||
| Lt. tibialis anterior | Normal | None | None | Normal | Normal | Normal | Complete |
| Lt. gastrocnemius (medial) | Increased | None | None | Normal | Normal | Normal | Complete |
Vasculitis may involve skin, lungs, kidneys, and other various organs, but it also can affect the peripheral nervous system as inflammation of epineural arteries of the nerve causes thrombosis and subsequent ischemic nerve damage. Peripheral nerve involvement of vasculitis is known as up to 60%-70%, especially common in small or medium-sized vessel vasculitides, and classically presents with mononeuropathy multiplex or distally polyneuropathy[2]. In this case, the patient was presented with mononeuropathy multiplex in the left arm and bilateral legs, confirmed with NCS. Although there was no definite vessel wall or perivascular inflammation in sural nerve biopsy, predominant axonal degeneration and secondary demyelination strongly suggested vasculitic neuropathy. However, because additional laboratory studies including ANCA were negative and specific organ involvement was absent, further specific diagnosis of small or medium-sized vessel vasculitis was limited.
APS is an autoimmune disease, characterized by recurrent arterial and venous thrombosis, and presence of antiphospholipid antibodies (aPL) including aCL and LA[3]. Prevalence of APS occurring in systemic vasculitis is known to ranges from 0.7% to 6%, and APS can occur with all kinds of vasculitis affecting small, medium, and large-vessels[4,5]. In this case, the patient was initially diagnosed with probable APS concurrent with vasculitis, however, both aCL and LA were changed to negative after 1 year later. This can be explained by either seroconversion of aPL in APS or initially transient presence of aPL associated with infection. However, considering associated massive thrombosis, seroconversion of aPL in APS is more plausible[6]. Meanwhile, the causal relationship of concurrent vasculitis and APS is still blurred, however, one possible theory is that endothelial cell disruption in vasculitis stimulate antiphospholipid antibody as phospholipids flipped to the outer side of endothelial cells[7].
The gastrointestinal (GI) involvement can be found in both vasculitis and APS. In vasculitis, small or medium-sized vessel vasculitides are more likely affected than large-sized vessel vasculitis, and reported rates of GI involvement are 30%-60%[8-10]. One of severe complications is bowel perforation, which can be caused by advanced nature of vasculitis or immunosuppressive therapy[11]. Pagnoux et al[12] reported GI involvement in 62 patients with systemic vasculitides, and only 9 of 62 patients (15%) had bowel perforation. Moreover, even though bowel perforation was induced by vasculitis, histological evidence of vasculitis was present in only half of cases with bowel perforation[13-15]. In APS, GI involvement is rare, and usually presents as bowel infarction caused by thrombosis of mesenteric vessels. Thrombosis in GI arteries or arterioles can lead to bowel perforation, however, is less common than venous thrombosis. Among previous reported cases of GI involvement in patients with APS, only a few cases are found with bowel perforation[16].
In this case, plausible reason for rectal perforation is advanced proctitis caused by vasculitis, however, bowel ischemia or necrosis induced by APS is also possible regarding thrombotic obliteration of subserosal arteries in resected rectum.
Vasculitis can be initially presented with vasculitic neuropathy, therefore, clinicians should always consider the possibility of diagnosis.
Manuscript source: Unsolicited manuscript
Specialty type: Neurosciences
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Meng FB S-Editor: Zhang H L-Editor: A P-Editor: Li X
| 1. | Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CG, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DG, Specks U, Stone JH, Takahashi K, Watts RA. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4416] [Cited by in F6Publishing: 3966] [Article Influence: 360.5] [Reference Citation Analysis (0)] |
| 2. | Gwathmey KG, Burns TM, Collins MP, Dyck PJ. Vasculitic neuropathies. Lancet Neurol. 2014;13:67-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 3. | Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, DE Groot PG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4924] [Cited by in F6Publishing: 4513] [Article Influence: 250.7] [Reference Citation Analysis (0)] |
| 4. | Rees JD, Lança S, Marques PV, Gómez-Puerta JA, Moco R, Oliveri C, Khamashta MA, Hughes GR, D'Cruz DP. Prevalence of the antiphospholipid syndrome in primary systemic vasculitis. Ann Rheum Dis. 2006;65:109-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Cervera R, Piette JC, Font J, Khamashta MA, Shoenfeld Y, Camps MT, Jacobsen S, Lakos G, Tincani A, Kontopoulou-Griva I, Galeazzi M, Meroni PL, Derksen RH, de Groot PG, Gromnica-Ihle E, Baleva M, Mosca M, Bombardieri S, Houssiau F, Gris JC, Quéré I, Hachulla E, Vasconcelos C, Roch B, Fernández-Nebro A, Boffa MC, Hughes GR, Ingelmo M; Euro-Phospholipid Project Group. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019-1027. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1425] [Cited by in F6Publishing: 1301] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 6. | Asherson RA, Cervera R. Antiphospholipid antibodies and infections. Ann Rheum Dis. 2003;62:388-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 157] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Norden DK, Ostrov BE, Shafritz AB, Von Feldt JM. Vasculitis associated with antiphospholipid syndrome. Semin Arthritis Rheum. 1995;24:273-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Fraioli P, Barberis M, Rizzato G. Gastrointestinal presentation of Churg Strauss syndrome. Sarcoidosis. 1994;11:42-45. [PubMed] [Cited in This Article: ] |
| 9. | Gayraud M, Guillevin L, le Toumelin P, Cohen P, Lhote F, Casassus P, Jarrousse B; French Vasculitis Study Group. Long-term followup of polyarteritis nodosa, microscopic polyangiitis, and Churg-Strauss syndrome: analysis of four prospective trials including 278 patients. Arthritis Rheum. 2001;44:666-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 10. | Pinkney JH, Clarke G, Fairclough PD. Gastrointestinal involvement in Wegener's granulomatosis. Gastrointest Endosc. 1991;37:411-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Kronzer VL, Larson DP, Crowson CS, Warrington KJ, Ytterberg SR, Makol A, Koster MJ. Occurrence and aetiology of gastrointestinal perforation in patients with vasculitis. Clin Exp Rheumatol. 2019;37 Suppl 117:32-39. [PubMed] [Cited in This Article: ] |
| 12. | Pagnoux C, Mahr A, Cohen P, Guillevin L. Presentation and outcome of gastrointestinal involvement in systemic necrotizing vasculitides: analysis of 62 patients with polyarteritis nodosa, microscopic polyangiitis, Wegener granulomatosis, Churg-Strauss syndrome, or rheumatoid arthritis-associated vasculitis. Medicine (Baltimore). 2005;84:115-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 280] [Cited by in F6Publishing: 239] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 13. | Levine SM, Hellmann DB, Stone JH. Gastrointestinal involvement in polyarteritis nodosa (1986-2000): presentation and outcomes in 24 patients. Am J Med. 2002;112:386-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Eriksson P, Segelmark M, Hallböök O. Frequency, Diagnosis, Treatment, and Outcome of Gastrointestinal Disease in Granulomatosis with Polyangiitis and Microscopic Polyangiitis. J Rheumatol. 2018;45:529-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Storesund B, Gran JT, Koldingsnes W. Severe intestinal involvement in Wegener's granulomatosis: report of two cases and review of the literature. Br J Rheumatol. 1998;37:387-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 73] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Al-Daqal S, Mansouri M, Qari MH, Sibiany A. Recurrent intestinal perforations as a presentation of antiphospholipid syndrome. Ann Saudi Med. 2006;26:52-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |