Published online Mar 6, 2021. doi: 10.12998/wjcc.v9.i7.1682
Peer-review started: October 7, 2020
First decision: November 8, 2020
Revised: November 23, 2020
Accepted: January 22, 2021
Article in press: January 22, 2021
Published online: March 6, 2021
Processing time: 144 Days and 12.7 Hours
Solid pseudopapillary neoplasm (SPN) is a rare tumor that was first described by Frantz in 1959. Although this tumor is benign, some may have malignant potential that can be predicted based on demographics, imaging characteristics, and pathologic evaluation. This case series presents 3 SPN cases with discussion on gender differences, preoperative predictors of malignancy, and a suggested algorithm for diagnostic approach as well as post-surgical follow up.
Three adult patients in a tertiary hospital found to have SPN, one elderly male and two young females. Each of the cases presented with abdominal pain and were discovered incidentally. Two cases underwent endoscopic ultrasound with fine needle aspiration and biopsy to assess tumor markers and immuno-histochemical staining (which were consistent with SPN before undergoing surgery), and one case underwent surgery directly after imaging. The average tumor size was 5 cm. Diagnosis was confirmed by histology. Two patients had post-surgical complications requiring intervention.
Demographic and imaging characteristics can be sufficient to establish diagnosis for SPN, while malignant cases require pre-operative evaluation with endoscopic ultrasound fine needle aspiration/fine needle biopsy.
Core Tip: Solid pseudopapillary neoplasm accounts for approximately 0.7% of pancreatic cystic tumors. It is benign in 85% of the cases, while 15% can have malignant behavior. The advancement of cross sectional imaging has led to increased detection. Demographic and characteristic imaging findings might be sufficient to make a diagnosis and predict malignant behavior, which can direct the need towards further testing or to proceed directly with surgery. Definitive treatment is surgical resection and the type of surgery depends on location and presence of peripancreatic invasion.
- Citation: Abudalou M, Vega EA, Dhingra R, Holzwanger E, Krishnan S, Kondratiev S, Niakosari A, Conrad C, Stallwood CG. Solid pseudopapillary neoplasm-diagnostic approach and post-surgical follow up: Three case reports and review of literature. World J Clin Cases 2021; 9(7): 1682-1695
- URL: https://www.wjgnet.com/2307-8960/full/v9/i7/1682.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i7.1682
Solid pseudopapillary tumor (SPN) of the pancreas is a rare tumor representing 1%-2% of all exocrine pancreatic neoplasms[1]. SPN occurs in females in the second or third decade of life in about 90% of cases, with a female to male ratio is (9.78:1)[2]. Its pathogenesis involves the activation of Wnt-β-catenin, Hedgehoc and Androgen signaling pathway. There is also an increased expression of CTNNB1 protein and other proteins that belong to the Wnt-β-catenin pathway (i.e., DKK4, Fine needle 1, SELENBP1, DDX5, YWHAZ, NONO and Fused in sarcoma)[3,4].
The most common symptom is abdominal pain, occurring in 65% of cases; other symptoms can be nausea and vomiting or a palpable abdominal mass. Of note, 15%-19% of cases are asymptomatic[2,5] and the tumor is discovered incidentally on imaging for other clinical reasons. The presence of cystic and solid components with hemorrhage without internal septation on computed tomography (CT) or magnetic resonance imaging (MRI) is characteristic for SPN[6]. 61% of cases are in the tail or body of the pancreas, while 34% are found in the head[2]. The main management is surgical with 5-year overall survival of 95%[2].
On macroscopic evaluation, the tumor is grossly well circumscribed or partially encapsulated. Color varies from tan to yellow. There are irregular cystic cavities lined by soft, friable tissue. Foci of hemorrhage is common. Microscopically, they demonstrate solid areas alternating with pseudopapillary formations, accompanied by innumerable capillary sized vessels. There is also evidence of cellular degeneration including cholesterol clefts and aggregates or foamy histocyte and nuclear grooves[7].
Most of the time, resection of the tumor carries a good prognosis[2]. However, these tumors can still have malignant tendency and risk of recurrence even after surgical resection, necessitating the need for post-surgical follow up[6,8,9].
The aim of this case series is to discuss malignant potential and gender differences of these tumors, and based on reviewed literature, propose a stepwise approach towards diagnosis and surveillance. The literature review was conducted from PubMed based on following criteria: Using SPN term and choosing peer reviewed English literature that has 10 or more reviewed case series of adults ≥ 18 years old based on relevance to our topic.
Cases 1 and 2: Abdominal pain.
Case 3: Flank pain.
Case 1: A 30-year-old female who was seen in outpatient clinic because of right lower abdominal pain and vomiting several days after a night of moderate alcohol consumption.
Case 2: A 64-year-old male who was evaluated in the hospital for severe diffuse abdominal pain, nausea and vomiting.
Case 3: A 25 years old female who presented to the emergency department for evaluation of right flank pain after antibiotic treatment for urinary tract infection.
Cases 1 and 3: Patients have no past medical problems.
Case 2: Patient is known to have a history of atrial fibrillation and chronic obstructive pulmonary disease as well as hyperlipidemia and tobacco dependence.
Case 1: Vital signs were normal and physical exam was unremarkable.
Case 2: Vital signs revealed a blood pressure of 161/129 mmHg and mild tachycardia of 97 beats per minute (bpm). On exam, abdomen was soft but diffusely tender.
Case 3: Patient was afebrile with stable vital signs. Abdomen was soft, without abdominal or flank tenderness.
Cases 1-3: Labs including complete blood count, kidney function test, lipase, amylase, liver function test and Ca19-9 were normal.
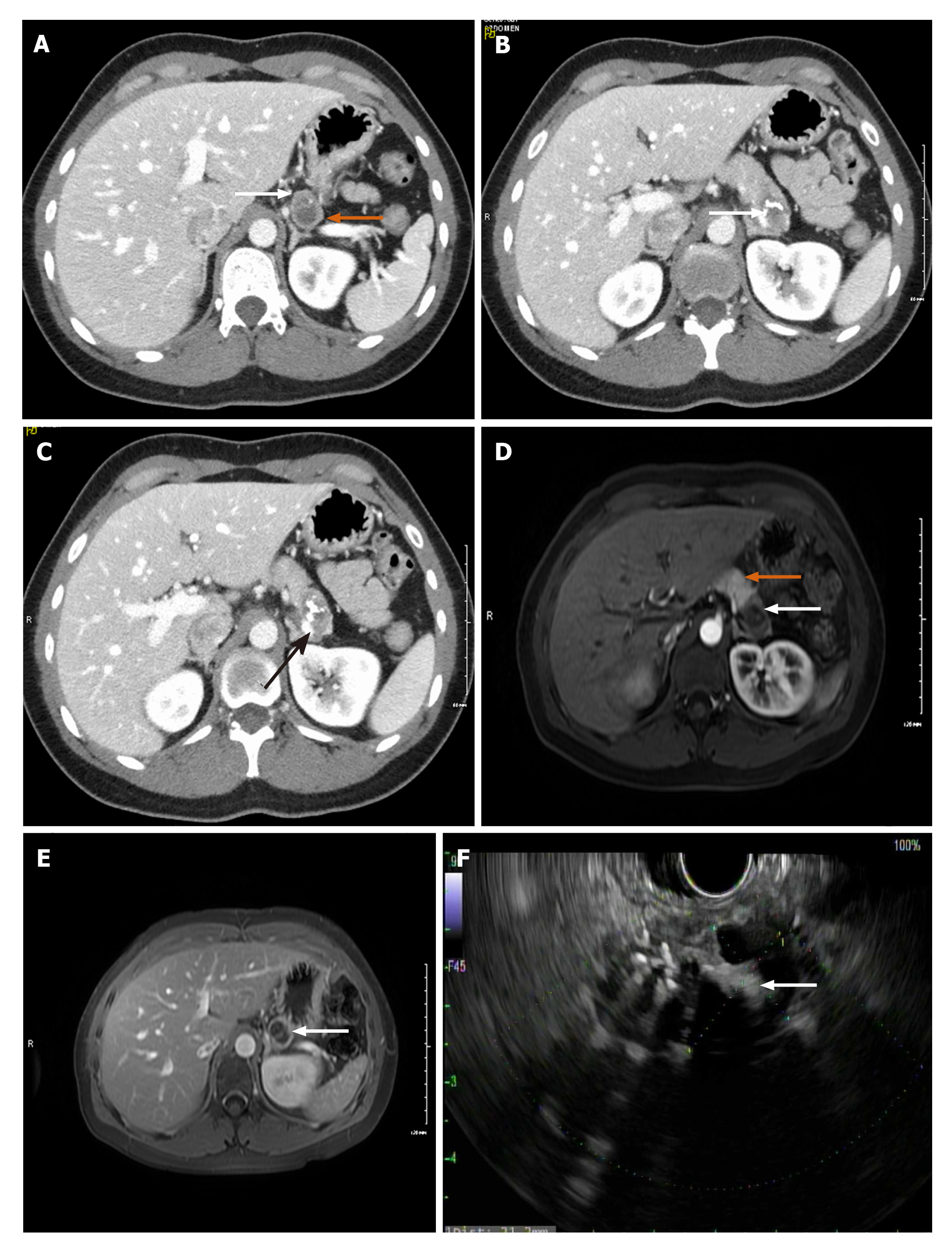
Case 1: CT of abdomen/pelvis revealed a normal appendix but an incidental finding of a bi-lobed hypodense lesion in the tail of the pancreas, measuring 3.2 cm × 2.8 cm × 1.8 cm with superior and inferior components (Figure 1A). The superior component showed several punctate foci of calcification while the inferior component had a dense curvilinear calcification (Figure 1B). On post-contrast imaging there were several enhancing septations and faint amorphous enhancement. There was an involvement of splenic vein due to mass effect (Figure 1C).
Magnetic resonance cholangiopancreatography (MRCP) also showed a bilobed exophytic lesion in the tail of the pancreas measuring 3.1 cm × 3.2 cm × 1.8 cm (Figure 1D and E). The superior component appeared cystic, T2 hyperintense and T1 hypointense with small punctate calcifications of the wall and on post-gadolinium imaging, it demonstrated central stellate shaped enhancement. The inferior component was heterogeneously hyperintense on T2 with central hypointensity corresponding to dense curvilinear calcifications. On post-gadolinium, it demonstrated peripheral enhancement. There was no evidence of metastasis.
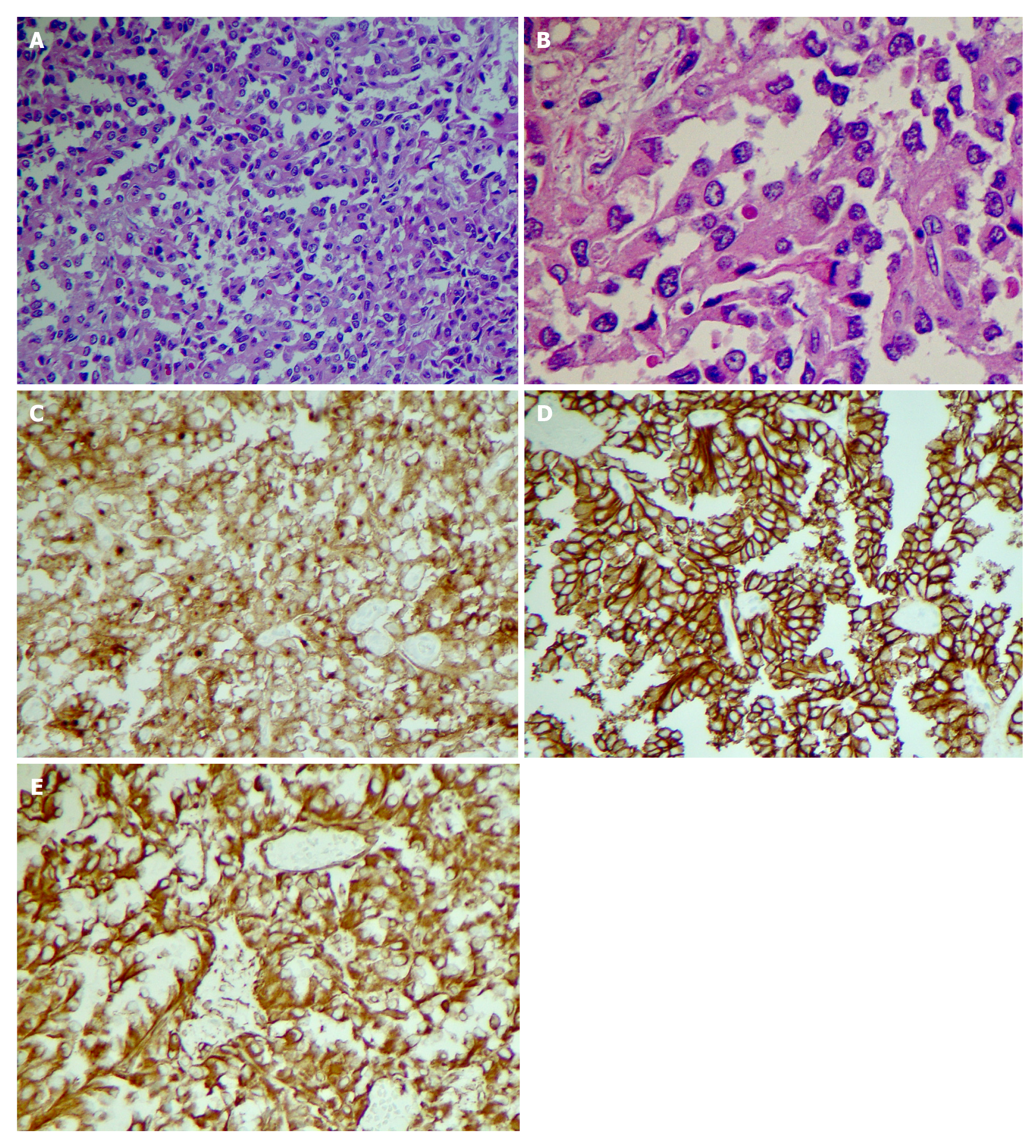
Endoscopic Ultrasound (EUS) was performed next, which noted the mass to have both solid and cystic components (Figure 1F), and measuring 3.1 cm × 2.0 cm. Fine needle aspiration (FNA) and biopsy was performed using a 22-gauge biopsy needle. The differential diagnosis included mucinous or serous cystic neoplasm, SPN, and intrapapillary mucinous neoplasm. Cystic fluid carcinoembryonic antigen (CEA) was 1.4 ng/mL (normal < 4.7) making a mucinous neoplasm less likely. Additionally, fluid amylase was not elevated making a pseudocyst also less likely. Pathology showed morphologic features consistent with SPN (Figure 2A and B). The tumor was positive for CD10 (Figure 2C), CD56 (Figure 2D), CD117, vimentin (Figure 2E), and β-catenin, and negative for synaptophysin, chromogranin, CK7, and CD20 by immuno-histochemistry, confirming the diagnosis of SPN.
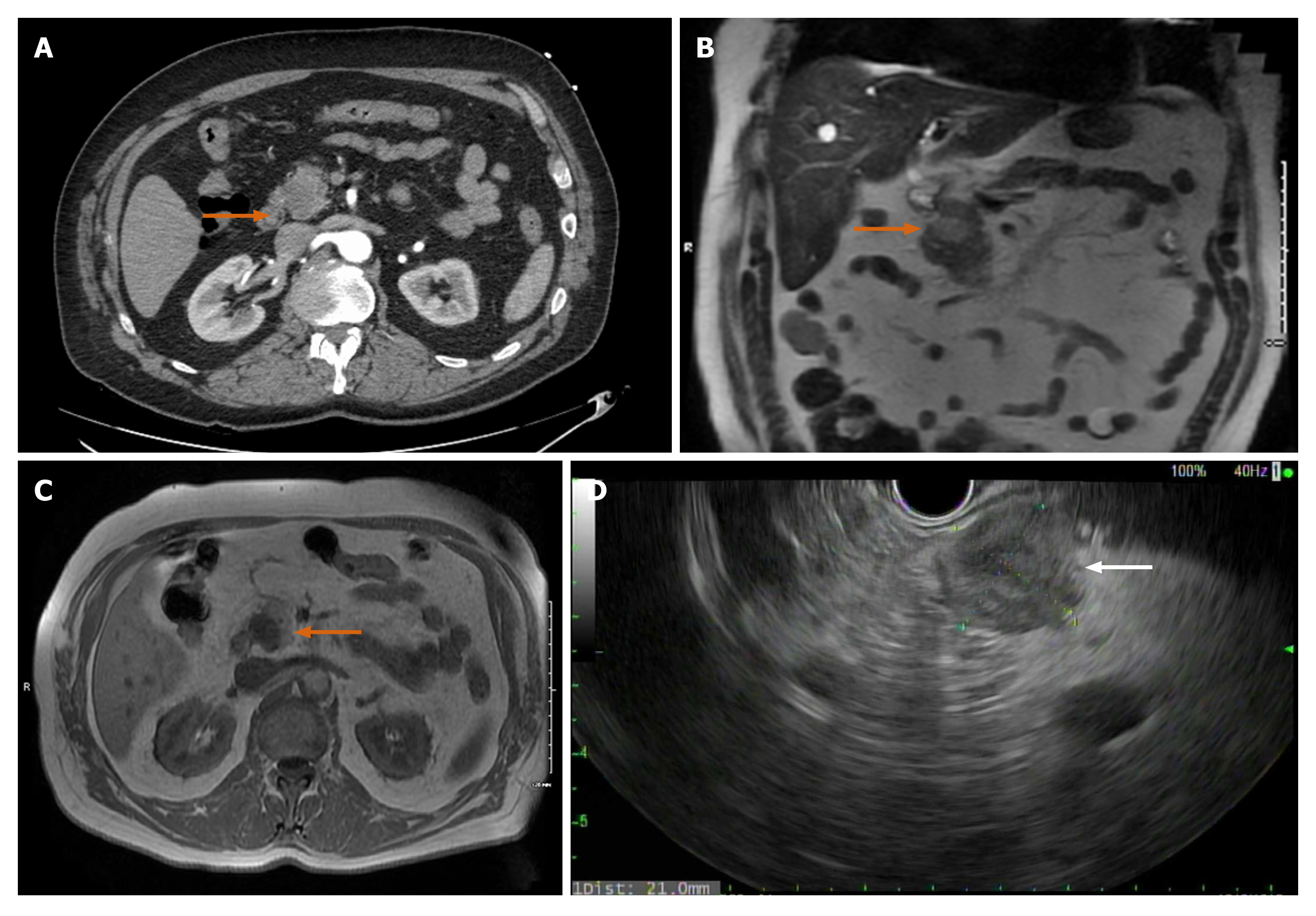
Case 2: Initial concern was for ischemic colitis, hence an abdominal computed tomography angiography was obtained. The CT revealed patent vasculature but showed an incidental finding of 2.3 cm hypodense lesion in the pancreatic head (Figure 3A).
On MRCP, the mass measured 1.7 cm × 2.1 cm × 1.8 cm. It was bright on T2 suggesting a solid component (Figure 3B). On T1 the mass appeared dark (Figure 3C). Lipase, liver enzymes and Ca19-9 were all within normal levels.
The patient underwent EUS-Fine needle biopsy (FNB), which was notable for a 2.1 cm × 2.1 cm hypoechoic pancreatic head mass (Figure 3D). FNB using a 25-gauge needle was performed through a trans-duodenal approach and adequate tissue was obtained. The tumor showed histologic features consistent with SPN and was positive for CD10, CD56, vimentin, androgen receptor, and cyclin D1, and negative for pancytokeratin, PAX8, CK20, chromogranin, and synaptophysin by immuno-histochemistry. Ki-67 demonstrated a low proliferative labeling index, 3%. The results were consistent with the diagnosis of SPN.
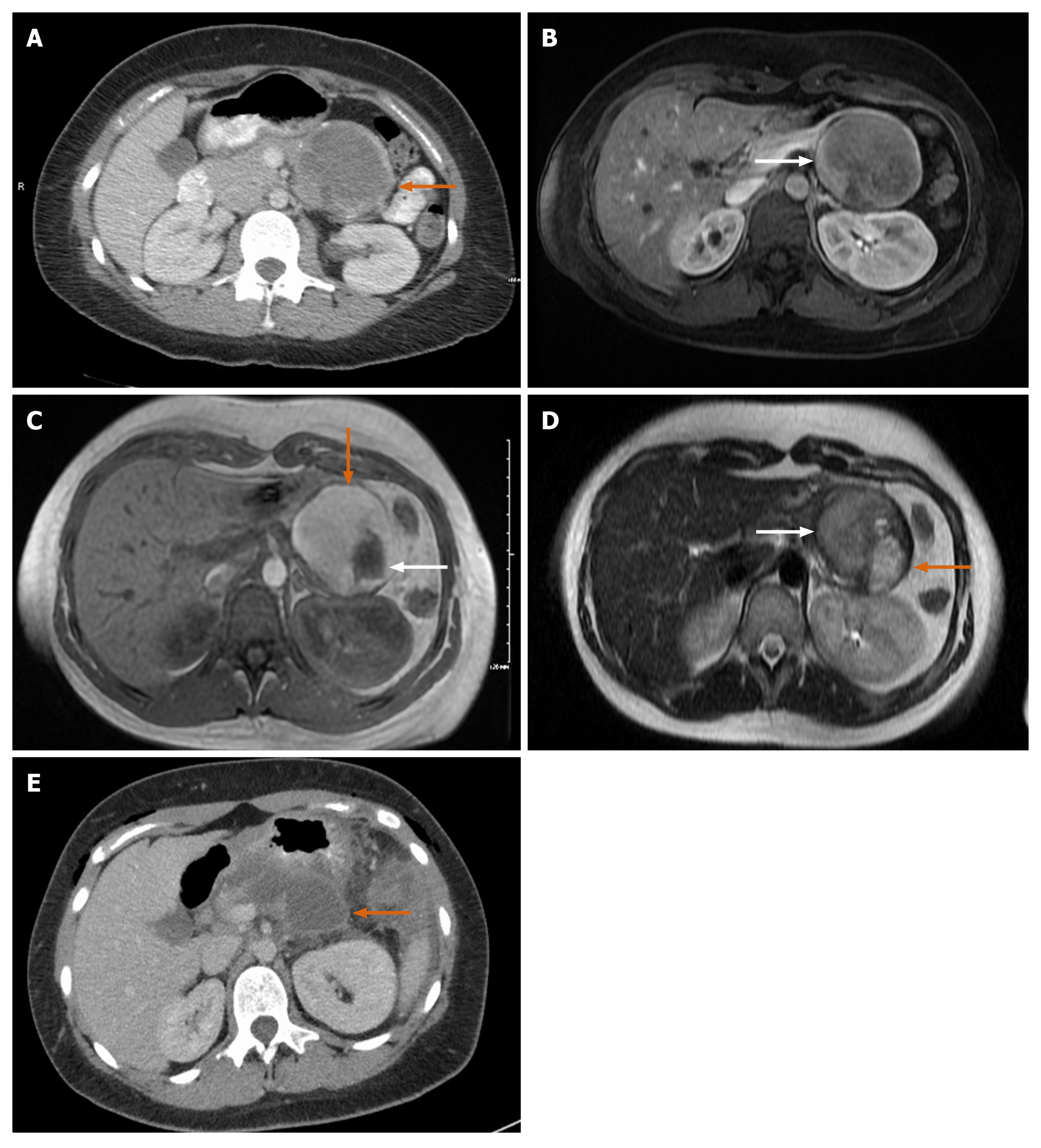
Case 3: CT of abdomen showed normal kidneys and ureters but an incidental finding of a large mass in tail of pancreas with rim of calcification measuring 8.6 cm × 6.8 cm × 6.2 cm (Figure 4A and B).
A follow up MRCP showed a well-defined and encapsulated rounded mass measuring 7.5 cm × 5.8 cm × 8 cm. No evidence of metastasis was seen. There was a bright T1 hyperintense signal and T2 heterogenous signal, both T1 and T2 appearance likely representing hemorrhage (Figure 4C and D) with a few scattered peripheral nodular lesions with faint enhancement. The nodule along outer margin measured 2.5 cm × 2.3 cm while the inner margin nodule measured 3.3 cm × 1.8 cm.
An EUS-FNA/FNB was planned but was not performed due to consensus agreement for a surgical approach to diagnosis and treatment.
SPN.
Case 1: The patient underwent an uncomplicated distal pancreatectomy with spleen preservation and regional lymph node resection. SPN diagnosis was confirmed on pathologic evaluation, with the mass measuring 5.2 cm × 3.2 cm × 3.2 cm. There was retroperitoneal soft tissue invasion and perineural invasion. No peripancreatic lymph nodes involvement was identified.
Case 2: Patient subsequently underwent an elective robotic pancreaticoduodenectomy (“Whipple”) with regional lymph node resection. Pathologic evaluation confirmed the diagnosis of SPN (Figure 2A and B). The tumor measured 1.7 cm × 1.5 cm × 1.3 cm in size. Peripancreatic adipose tissue and perineural invasion were noted. There was no lympho-vascular or peripancreatic lymph node involvement. Immunohistochemical stains were positive for CD 56 (Figure 2C), CD 10 (Figure 2D), vimentin (Figure 2E) and negative for CK7, CK20, Pankeratin, synaptophysin and Chromogranin.
Case 3: The patient underwent a laparoscopic spleen preserving distal pancreatectomy and regional lymph node resection. The size of the tumor was 8.5 cm in greatest dimension. Histologic findings were consistent with SPN (Figure 2A and B).
Tumor was positive for CD 10 (Figure 2C), CD56 (Figure 2D), vimentin (Figure 2E), Cyclin D1, and negative for CEA and chromogranin A by immunohistochemical staining. No peripancreatic adipose tissue invasion or perineural invasion was identified. There was no regional lymph node involvement. Patient had an uneventful hospital course.
Case 1: Patient had no post-surgical complications during the hospitalization or after discharge.
Case 2: Post-operatively, peritoneal fluid amylase and bilirubin from the surgical drain were persistently elevated. An anastomotic leak was seen on imaging, and the patient was taken back to the operating room for laparoscopic surgical repair of an inadvertent bowel injury. He was eventually discharged with surgical drains, which were removed 40 d after discharge.
Case 3: Three weeks after discharge, the patient presented to the emergency department with abdominal pain due to a peripancreatic fluid collection seen on imaging (Figure 4E). The collection was drained, revealing a high amylase content. The patient had no complications afterwards. She had a regular post-operative follow up as outpatient with oncology (due to the large tumor size) and surgery at 3 and 9 mo. She had a full recovery 4 mo after surgery without chemotherapy treatment.
All patients had a regular follow up at 2 wk post discharge from hospital, 3 mo and 9 mo after surgery.
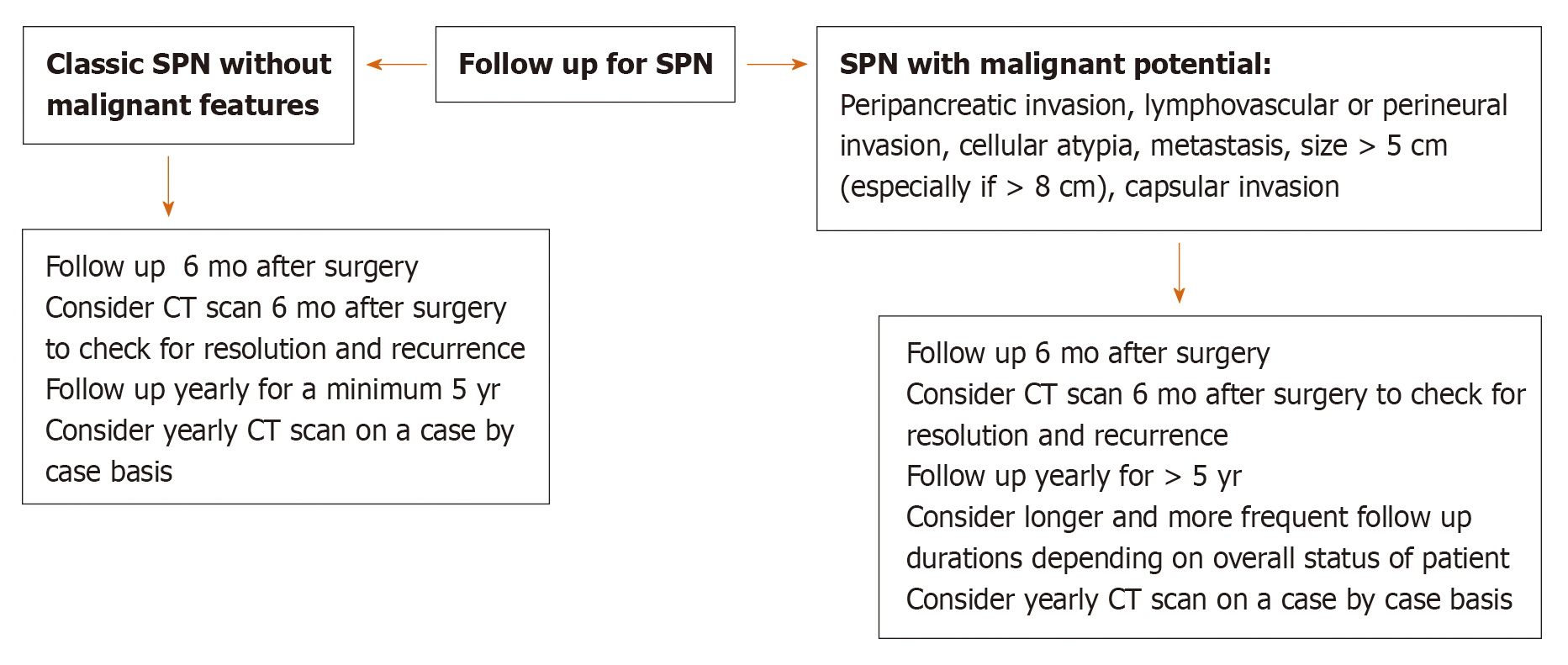
Given the large tumor size in the case 3 patient, the patient had a follow up CT scan at 3 and 6 mo after surgery, both negative for recurrence. An MRI was also performed 9 mo after the operation and no recurrence was seen. She will have another follow up in 6 mo after last visit and will continue to be followed for the next 5 years at minimum.
SPN is an uncommon tumor of the pancreas. It was first described by pathologist Virginia Frantz in 1959 as “Papillary Tumors of The Pancreas”. She reviewed three cases of cystic tumors that were resected from the pancreas in two young women and a child[10]. It was noted that these tumors were rounded with smooth borders and areas of calcification with cavities that may contain debris or blood. In 2010, the World Health Organization defined SPN as benign tumors with low malignant potential[1]. SPN is included under the entity of pancreatic cystic tumors accounting for approximately 1%-2% of all exocrine pancreatic tumors[1].
SPN has a strong gender association, with nearly 90% of cases occurring in adult females[11]. The tumor usually presents at a young age, in the second or third decade of life[2,11]. Interestingly, males with this condition tend to present at an older age in the fifth decade of life[12,13]. Abdominal pain is the most common symptom and other symptoms include nausea, vomiting, weight loss and less often a palpable abdominal mass. About one-third of patients are asymptomatic, with a mass incidentally identified on imaging ordered for other clinical reasons[5,9,11]. The location can be anywhere in the pancreas, but most commonly found in the body and tail[2,5,14]. Only, 1% of these tumors can have extrapancreatic localization[2]. Metastasis is seen in 19% of cases, with predilection to the liver and lymph nodes[2].
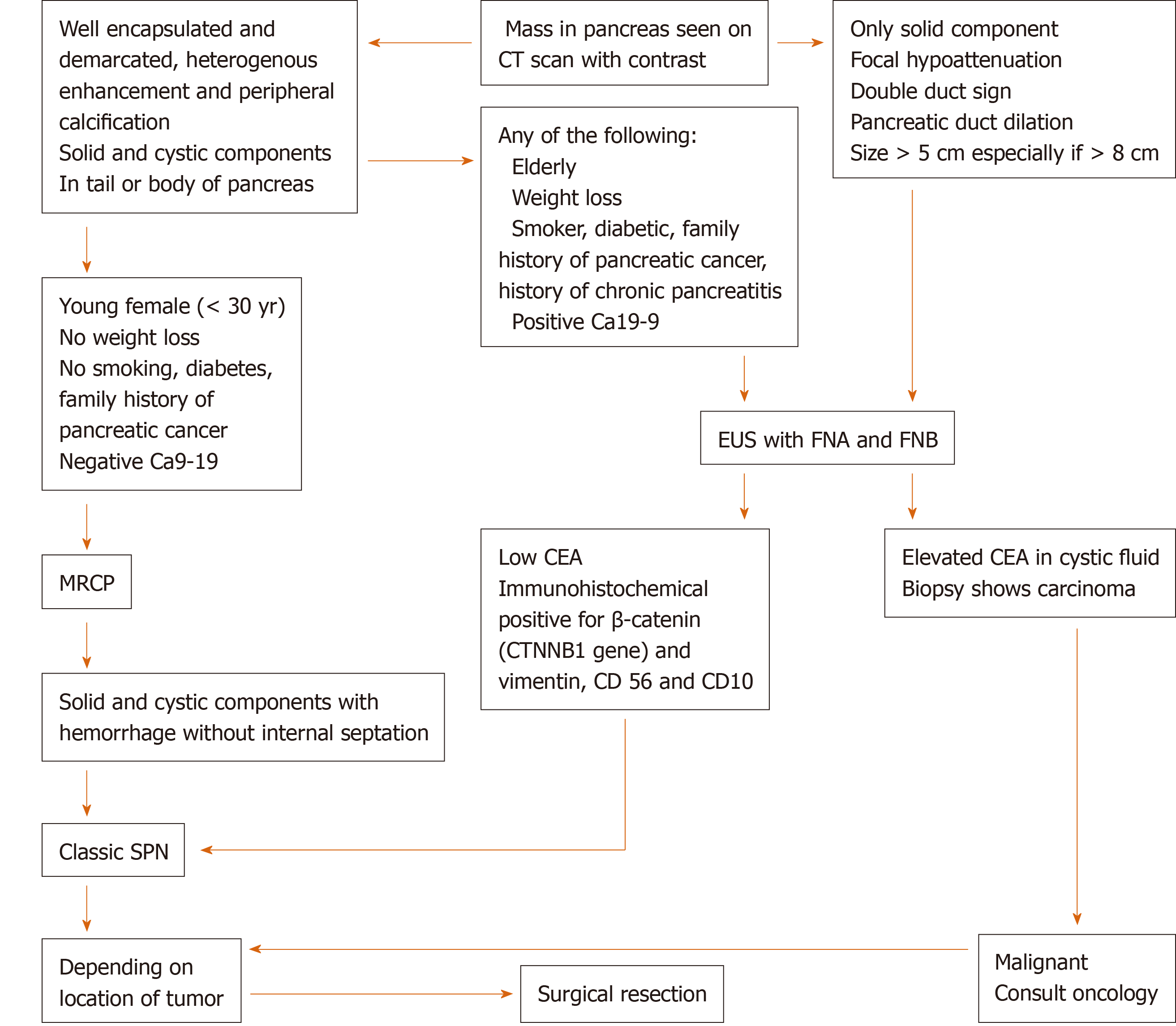
Different imaging modalities, including CT, MRI, and EUS can be used for diagnosis. On CT, the tumor usually appears well demarcated and encapsulated with mixed solid and cystic components[14]. Calcification can be present in some cases[15]. In the Yang et al[16] study, calcification was found in two of the 26 cases[16]. On MRI, there is often a heterogeneous appearance both on T1 and T2. Hyperintensity on T1 and hypointensity on T2 correlate with areas of hemorrhage, which is characteristic for SPN (Figure 4C and D). Additionally, the presence of solid and cystic components on MRI with hemorrhage but without internal septation should highly raise suspicion for SPN. SPN can sometimes have a completely solid or cystic component. In a review of 26 cases, seven had a completely solid component and two had a completely cystic component on imaging (CT or MRI)[17]. In Jani et al[15] 50% of cases were completely solid and 11% were cystic. Endoscopic ultrasound with FNA and biopsy is often performed to differentiate SPN from other cystic lesions should the diagnosis remain unclear after initial evaluation with CT and/or MRI[9,18] (Figure 5). If the presentation and evaluation is classic for SPN, surgical resection may be considered without prior biopsy (as in case 3).
Immunohistochemical staining of neoplastic cells from FNB sample can pre-operatively confirm the diagnosis. Almost all SPN tumors express β-catenin (CTNNB1 gene), CD10 (Figure 2C) and CD56 (Figure 2D), vimentin (Figure 2E), and most express CD99, α1-antitrypsin, neuron specific enolase and progesterone receptors (Figure 2). Some express synaptophysin but usually do not express chromogranin A[19-21]. Males have a stronger expression of androgen receptors[22]. Serum CEA and Ca9-19 levels are usually within normal limits but can be slightly elevated in a minority of cases[16,23].
Many studies have investigated different pre-operative clinical features and molecular markers that can classify pancreatic cysts pre-operatively and detect malignant potential[24]. Springer et al[25] identified molecular markers in cystic fluid samples. In 10 SPN samples, genome sequencing detected the presence of CTNNB1 mutation while KRAS, GNAS, RNF43 mutations and loss of heterozygosity were absent. This composite was noted to have a sensitivity and specificity of 100% as it was detected in all 10 SPN, which was confirmed by pathologic evaluation after resection.
Cohen et al[26] assessed prognostic factors associated with systemic metastasis. Among clinical features, a larger tumor size was seen in patients with metastasis compared to localized tumors (8.13 ± 1.03 cm in metastatic tumors and 5.20 ± 3.78 cm in localized tumors, range 7–9 cm, P < 0.012). Larger tumor size was also associated with a higher likelihood of malignant pathology and recurrence[27]. This finding was also seen in the Estrella et al[5] study, which again noted that larger tumor size (mean 10.5 cm) was significantly associated with metastasis and risk of recurrence (P < 0.002)[5]. Moreover, the study found that muscular vessel invasion (presence of tumor cells in the luminal spaces of blood vessels with circumferential smooth muscle layers), invasion to adjacent organs, and presence of metastasis regardless of T or N category findings were significant independent predictive factors of disease specific survival on univariate analysis (P ≤ 0.001)[5].
Histological features in Cohen and Estrella et al[5] including invasiveness (cellular atypia, peripancreatic fat invasion, lymph node metastasis, capsular invasion, perineural or lymphovascular invasion) and immunohistochemical stains were not associated with metastatic disease[5,26]. However, WHO suggest that perineural behavior, angioinvasion and deep invasion into surrounding tissue can predict malignant behavior[1]. In addition, Kang et al[28] found that pancreatic fat tissue invasion, capsular invasion, cellular atypia, perineural invasion, and lymph node metastasis define malignant behavior of SPN tumors. Yu et al[6], reviewed 26 cases from 1981 to 2005, 9 of these were considered malignant based on invasion of surrounding tissue. Of these nine cases, 4 developed duodenal invasions, two had portal venous invasion and one had both portal venous and superior mesenteric artery invasion. The tendency of these malignant tumors to be located in the head of pancreas was also noted (6 of 9 cases).
On a molecular level, there was a higher expression of certain microRNAs (miR-10a, miR-887, and miR-184) in metastatic tumors, which can potentially be used to predict tumor behavior[26]. However, larger studies are needed to confirm this finding. One study investigated the relationship of inflammatory marker neutrophil to lymphocyte ratio (NLR) with malignant potential in a retrospective review of 113 patients with SPN in a single center[29]. The study found that NLR above 3.22 is a significant predictive factor of a malignant SPN (OR 6.871, 95%CI: 1.482-31.864, P = 0.014). Furthermore, in another study Wang et al[30] demonstrated a tumor size > 7 cm (P = 0.003), high Ki-67 ≥ 5% (P = 0.044) and negative progesterone receptor (PR) result were significantly associated with recurrence and distant metastasis (P = 0.001). Of note, tumor size > 7 cm and PR negative were also associated with a worse survival (P < 0.001)[30].
Machado et al[8] reviewed 34 patients with SPN (27 females and 7 males). Recurrence after resection occurred in 2 patients (1 female and 1 male). One patient underwent systemic chemotherapy with combination of 5-FU and cisplatin, and he remained alive 39 mo after initial operation. The other patient (whose initial surgery was subtotal pancreatectomy with resection of the portal vein) developed disseminated liver metastasis and died of the disease 24 mo after the initial procedure. In Yu et al[9], three hundred and five patients were followed for a mean time of 49.2 mo after radical resection. The mean disease free survival was 45.1 mo. Of these 11 patients (3.6%) had local recurrence and 8 underwent a second resection. Hepatic metastasis occurred in 2 patients at 3 and 46 mo after surgery. The estimated 1-, 3-, and 5- year survival rate was 99.4%, 97.5% and 96.9% respectively.
In another study, 26 patients were followed for a median of 32.5 mo. 25/26 patients survived, two developed local recurrence and liver metastasis. These two were found to have a high Ki-67 immunoreactivity (> 25%) when compared with other patients (< 5%)[16].
Finally, in a study of 467 cases, 31 patients (6.6%) developed recurrence. Metastasis to the liver and lymph nodes were most common[2].
Studies also evaluated differences between male and female presentations. Two studies evaluated differences between gender based on imaging (MRI and CT) and found that there is a significant difference in tumor shape, as the tumors in males are noted to be more lobulated vs tumors in females, which are noted to be more oval[12]. In addition, tumors in males tend to have more solid component and calcification[12]. On the other hand, there was no significant difference in tumor margin, capsule, pancreatic duct dilation, parenchymal atrophy, MR T1 and T2 signal intensities, tumor site or clinical manifestations. There was discrepancy of whether there is difference in tumor size as one study showed no difference[12], another showed a smaller median diameter in males while another showed a larger diameter in males (6.3 cm vs 4.6 cm, P = 0.0413). Also, the age of presentation for males was significantly older than females in multiple studies, with average age noted to be 40 to 50 years old for males[12,13,31]. Finally, men may have a greater propensity for aggressive behavior (portal vein involvement) and conservative surgery (i.e. spleen preserving distal pancreatectomy, central pancreatectomy and enucleation) was less likely in the male population[24].
Surgical resection remains the mainstay of treatment for SPN with an excellent prognosis, as 5-year survival is 95%[2]. The most common post-operative complication is pancreatic fistula. Other complications including pancreatitis, steatorrhea, wound infection, biliary fistula, prolonged gastric emptying, gastrointestinal bleeding, diabetes mellitus and ileus were less common[9,14].
At our institution, we perform partial pancreatectomy for solid pseudopapillary tumor with standard lymph node dissection.
The patients in cases 1 and 3 underwent distal pancreatectomy with spleen preservation since the tumor was located in tail of the pancreas. The patient in case 2 underwent a Whipple surgery. Enucleation surgery rather than resection was considered in all cases. However, enucleation is generally reserved for cystic tumors of small size < 3.5 cm[32-35]. The tumor size in case 1 and 3 precluded enucleation. While the tumor size was small in case 2, other factors (age, male, smoker) were concerning for malignancy, so more aggressive surgical management was recommended.
Based on the demographic and imaging characteristics of the SPN tumors, we created an algorithm for the diagnosis, management, and post-surgical follow-up in order to avoid unnecessary testing and misdiagnosis of a malignant neoplasm in preoperative evaluation (Figures 5 and 6).
From the previous literature review, a young female patient[2,24] without significant risk factors for pancreatic cancer (tobacco use, heavy alcohol use, chronic pancreatitis, obesity, diabetes[36,37]) and without unintentional weight loss, along with a CT/MRI showing solid and cystic mass with hemorrhage[18], size ≤ 5 cm[5,26-28] without invasion or metastasis can be considered a classic presentation for SPN and less likely to have a metastatic potential. This population can undergo surgical resection depending on location of tumor without undergoing EUS with FNA/FNB.
On the contrary, the presence of any of the following characteristics is concerning for either SPN with malignant potential or malignant neoplasm and will require an EUS-FNA/FNB prior to surgery in order to determine a histologic diagnosis: Male gender or elderly (> 40 years), risk factors for pancreatic cancer, imaging showing only a solid component, focal hypoattenuation, double duct sign or pancreatic duct dilation[38-40], large tumor size > 5 cm[28] (especially if > 8 cm[5,26,28]), invasion to surrounding tissue or metastasis. Should it found to be malignant (i.e., ductal adenocarcinoma) on biopsy, early oncologic consultation should be considered for neoadjuvant chemotherapy[41].
The paradigm of pancreatic adenocarcinoma has changed in the last decade, with neoadjuvant chemotherapy most often now recommended. The importance of neoadjuvant chemotherapy stems from the fact that one-third of patients with pancreatic cancer were found to have metastasis at the time of the operation[42], but up to one-half of these patients had not received neoadjuvant chemotherapy[43-45]. Neoadjuvant chemotherapy, even in patients with stage 1 pancreatic adenocarcinoma, was found to confer a significant advantage for overall survival compared with upfront surgery alone (HR: 0.784, P = 0.002) and showed a trend toward improved survival when compared to post-operative chemotherapy[41]. Pancreatic ductal adenocarcinoma can develop cystic changes due to central necrosis, which can sometimes be mistaken for SPN[46].
There have been concerns that FNA-FNB of pancreatic cysts might cause dissemination and peritoneal seeding[47]. However, in the Yoon et al[48] study, 175 patients with intraductal papillary neoplasm who underwent resection with previous sampling by EUS-FNA (EUS-FNA group) were compared to 68 patients who underwent resection with no previous sampling (no sampling group). Four patients (2.3%) in the EUS-FNA group developed peritoneal seeding, whereas three patients (4.4%) developed peritoneal seeding in the no sampling group (P = 0.403), a finding that was not statistically significant.
Regarding surveillance, the few studies in the literature suggest long-term surveillance is important[2,5,6,9,16]. Therefore, we suggest that patients be followed for a minimum of 5 years, with longer follow up for SPN showing malignant behavior[1,18,28] (i.e., Peripancreatic invasion, cellular atypia, vascular invasion, metastasis) on pathology.
SPN is a tumor of low malignant potential that is generally found in young females, but can also be seen in males (with more aggressive behavior in males). Factors that predict malignancy preoperatively have been studied and when combined with demographic characteristics, the tumor can be diagnosed based solely on age, gender, presence of risk factors along with imaging features without undergoing EUS-FNA/FNB. Tumors that are suspicious for a malignant behavior should be worked up with EUS-FNA/FNB, and might require longer follow up after discharge.
The relatively rare occurrence of SPN makes it challenging to develop consensus guidelines on diagnosis and work up of these tumors. Most studies are either single-center or a review of previous literature, as opposed to randomized, controlled trials. In the future, a multi-center randomized controlled trial would be helpful in developing such guidelines.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Liu T, Sampath S, Suzuki S S-Editor: Fan JR L-Editor: A P-Editor: Wang LYT
| 1. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. 4th ed. Geneva: Wolrd Health Organization; 2010. Available from: https://www.researchgate.net/publication/285348862_WHO_Classification_of_Tumors_of_the_Digestive_System. |
| 2. | Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 536] [Article Influence: 26.8] [Reference Citation Analysis (1)] |
| 3. | Cavard C, Audebourg A, Letourneur F, Audard V, Beuvon F, Cagnard N, Radenen B, Varlet P, Vacher-Lavenu MC, Perret C, Terris B. Gene expression profiling provides insights into the pathways involved in solid pseudopapillary neoplasm of the pancreas. J Pathol. 2009;218:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Park M, Lim JS, Lee HJ, Na K, Lee MJ, Kang CM, Paik YK, Kim H. Distinct Protein Expression Profiles of Solid-Pseudopapillary Neoplasms of the Pancreas. J Proteome Res. 2015;14:3007-3014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Estrella JS, Lei L, Rashid A, Wang H, Wang H. Solid Pseudopapillary Neoplasm of the Pancreas. Am J Surg Pathol. 2014;38:147-157. [RCA] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Yu CC, Tseng JH, Yeh CN, Hwang TL, Jan YY. Clinicopathological study of solid and pseudopapillary tumor of pancreas: emphasis on magnetic resonance imaging findings. World J Gastroenterol. 2007;13:1811-1815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (11)] |
| 7. | Martin RC, Klimstra DS, Brennan MF, Conlon KC. Solid-pseudopapillary tumor of the pancreas: a surgical enigma? Ann Surg Oncol. 2002;9:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 283] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Machado MC, Machado MA, Bacchella T, Jukemura J, Almeida JL, Cunha JE. Solid pseudopapillary neoplasm of the pancreas: distinct patterns of onset, diagnosis, and prognosis for male versus female patients. Surgery. 2008;143:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Yu PF, Hu ZH, Wang XB, Guo JM, Cheng XD, Zhang YL, Xu Q. Solid pseudopapillary tumor of the pancreas: a review of 553 cases in Chinese literature. World J Gastroenterol. 2010;16:1209-1214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 189] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | Frantz VK. Tumors of the Pancreas. In: Atlas of Tumor Pathology, Section VII, Fascicles 27 and 28. Washington, DC: Armed Forces Institute of Pathology; 1959. |
| 11. | Wang P, Wei J, Wu J, Xu W, Chen Q, Gao W, Jiang K, Miao Y. Diagnosis and treatment of solid-pseudopapillary tumors of the pancreas: A single institution experience with 97 cases. Pancreatology. 2018;18:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Sur YK, Lee JH, Kim JK, Park MJ, Kim B, Park MS, Choi JY, Kim YB, Lee D. Comparison of MR imaging features of solid pseudopapillary neoplasm of pancreas between male and female patients. Eur J Radiol. 2015;84:2065-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Shi S, Zhou Y, Hu C. Clinical manifestations and multi-slice computed tomography characteristics of solid pseudopapillary neoplasms of the pancreas between males and females. BMC Med Imaging. 2019;19:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Lubezky N, Papoulas M, Lessing Y, Gitstein G, Brazowski E, Nachmany I, Lahat G, Goykhman Y, Ben-Yehuda A, Nakache R. Solid pseudopapillary neoplasm of the pancreas: Management and long-term outcome. Eur J Surg Oncol. 2017;43:1056-1060. [RCA] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Jani N, Dewitt J, Eloubeidi M, Varadarajulu S, Appalaneni V, Hoffman B, Brugge W, Lee K, Khalid A, McGrath K. Endoscopic ultrasound-guided fine-needle aspiration for diagnosis of solid pseudopapillary tumors of the pancreas: a multicenter experience. Endoscopy. 2008;40:200-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Yang F, Jin C, Long J, Yu XJ, Xu J, Di Y, Li J, Fu de L, Ni QX. Solid pseudopapillary tumor of the pancreas: a case series of 26 consecutive patients. Am J Surg. 2009;198:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Lee JH, Yu JS, Kim H, Kim JK, Kim TH, Kim KW, Park MS, Kim JH, Kim YB, Park C. Solid pseudopapillary carcinoma of the pancreas: differentiation from benign solid pseudopapillary tumour using CT and MRI. Clin Radiol. 2008;63:1006-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Mima K, Hirota M, Abe S, Iwatsuki M, Imamura H, Tsuruzoe S, Chikamoto A, Tanaka H, Takamori H, Kanemitsu K, Tagami H, Honda Y, Iyama K, Baba H. Small solid pseudopapillary tumor of the pancreas in a 32-year-old man: report of a case. Surg Today. 2010;40:772-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Notohara K, Hamazaki S, Tsukayama C, Nakamoto S, Kawabata K, Mizobuchi K, Sakamoto K, Okada S. Solid-pseudopapillary tumor of the pancreas: immunohistochemical localization of neuroendocrine markers and CD10. Am J Surg Pathol. 2000;24:1361-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 168] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Stömmer P, Kraus J, Stolte M, Giedl J. Solid and cystic pancreatic tumors. Clinical, histochemical, and electron microscopic features in ten cases. Cancer. 1991;67:1635-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Tanaka Y, Kato K, Notohara K, Hojo H, Ijiri R, Miyake T, Nagahara N, Sasaki F, Kitagawa N, Nakatani Y, Kobayashi Y. Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res. 2001;61:8401-8404. [PubMed] |
| 22. | Zou Y, Huang Y, Hong B, Xiang X, Zhou B, Wei S. Comparison of the clinicopathological features of pancreatic solid pseudopapillary neoplasms between males and females: gender does matter. Histol Histopathol. 2020;35:257-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 23. | Hongjian X, Dujuan L, Shuang X, Yuewu Z, Lingfei K. Solid Pseudopapillary Tumor of the Pancreas in a 50-Year-Old Man: A Case Report and Review of the Literature. Case Rep Pancreat Cancer. 2016;2:23-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Masica DL, Dal Molin M, Wolfgang CL, Tomita T, Ostovaneh MR, Blackford A, Moran RA, Law JK, Barkley T, Goggins M, Irene Canto M, Pittman M, Eshleman JR, Ali SZ, Fishman EK, Kamel IR, Raman SP, Zaheer A, Ahuja N, Makary MA, Weiss MJ, Hirose K, Cameron JL, Rezaee N, He J, Joon Ahn Y, Wu W, Wang Y, Springer S, Diaz LL Jr, Papadopoulos N, Hruban RH, Kinzler KW, Vogelstein B, Karchin R, Lennon AM. A novel approach for selecting combination clinical markers of pathology applied to a large retrospective cohort of surgically resected pancreatic cysts. J Am Med Inform Assoc. 2017;24:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Springer S, Wang Y, Dal Molin M, Masica DL, Jiao Y, Kinde I, Blackford A, Raman SP, Wolfgang CL, Tomita T, Niknafs N, Douville C, Ptak J, Dobbyn L, Allen PJ, Klimstra DS, Schattner MA, Schmidt CM, Yip-Schneider M, Cummings OW, Brand RE, Zeh HJ, Singhi AD, Scarpa A, Salvia R, Malleo G, Zamboni G, Falconi M, Jang JY, Kim SW, Kwon W, Hong SM, Song KB, Kim SC, Swan N, Murphy J, Geoghegan J, Brugge W, Fernandez-Del Castillo C, Mino-Kenudson M, Schulick R, Edil BH, Adsay V, Paulino J, van Hooft J, Yachida S, Nara S, Hiraoka N, Yamao K, Hijioka S, van der Merwe S, Goggins M, Canto MI, Ahuja N, Hirose K, Makary M, Weiss MJ, Cameron J, Pittman M, Eshleman JR, Diaz LA Jr, Papadopoulos N, Kinzler KW, Karchin R, Hruban RH, Vogelstein B, Lennon AM. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology. 2015;149:1501-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 328] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 26. | Cohen SJ, Papoulas M, Graubardt N, Ovdat E, Loewenstein S, Kania-Almog J, Pasmanik-Chor M, Brazowski E, Cagnano E, Nachmany I, Lahat G, Klausner JM, Lubezky N. Micro-RNA Expression Patterns Predict Metastatic Spread in Solid Pseudopapillary Neoplasms of the Pancreas. Front Oncol. 2020;10:328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Kang CM, Kim KS, Choi JS, Kim H, Lee WJ, Kim BR. Solid pseudopapillary tumor of the pancreas suggesting malignant potential. Pancreas. 2006;32:276-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Kang CM, Choi SH, Kim SC, Lee WJ, Choi DW, Kim SW; Korean Pancreatic Surgery Club. Predicting recurrence of pancreatic solid pseudopapillary tumors after surgical resection: a multicenter analysis in Korea. Ann Surg. 2014;260:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 29. | Yang F, Bao Y, Zhou Z, Jin C, Fu D. Preoperative neutrophil-to-lymphocyte ratio predicts malignancy and recurrence-free survival of solid pseudopapillary tumor of the pancreas. J Surg Oncol. 2019;120:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Wang F, Meng Z, Li S, Zhang Y, Wu H. Prognostic value of progesterone receptor in solid pseudopapillary neoplasm of the pancreas: evaluation of a pooled case series. BMC Gastroenterol. 2018;18:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Cai YQ, Xie SM, Ran X, Wang X, Mai G, Liu XB. Solid pseudopapillary tumor of the pancreas in male patients: report of 16 cases. World J Gastroenterol. 2014;20:6939-6945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Hackert T, Hinz U, Fritz S, Strobel O, Schneider L, Hartwig W, Büchler MW, Werner J. Enucleation in pancreatic surgery: indications, technique, and outcome compared to standard pancreatic resections. Langenbecks Arch Surg. 2011;396:1197-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 33. | Cauley CE, Pitt HA, Ziegler KM, Nakeeb A, Schmidt CM, Zyromski NJ, House MG, Lillemoe KD. Pancreatic enucleation: improved outcomes compared to resection. J Gastrointest Surg. 2012;16:1347-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Talamini MA, Moesinger R, Yeo CJ, Poulose B, Hruban RH, Cameron JL, Pitt HA. Cystadenomas of the pancreas: is enucleation an adequate operation? Ann Surg. 1998;227:896-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 131] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Kiely JM, Nakeeb A, Komorowski RA, Wilson SD, Pitt HA. Cystic pancreatic neoplasms: enucleate or resect? J Gastrointest Surg. 2003;7:890-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Becker AE, Hernandez YG, Frucht H, Lucas AL. Pancreatic ductal adenocarcinoma: risk factors, screening, and early detection. World J Gastroenterol. 2014;20:11182-11198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 200] [Cited by in RCA: 230] [Article Influence: 20.9] [Reference Citation Analysis (5)] |
| 37. | Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, Petersen GM. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129:504-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 369] [Article Influence: 18.5] [Reference Citation Analysis (1)] |
| 38. | Ahn SS, Kim MJ, Choi JY, Hong HS, Chung YE, Lim JS. Indicative findings of pancreatic cancer in prediagnostic CT. Eur Radiol. 2009;19:2448-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Larena JA, Astigarraga E, Saralegui I, Merino A, Capelastegui A, Calvo MM. Magnetic resonance cholangiopancreatography in the evaluation of pancreatic duct pathology. Br J Radiol. 1998;71:1100-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Gangi S, Fletcher JG, Nathan MA, Christensen JA, Harmsen WS, Crownhart BS, Chari ST. Time interval between abnormalities seen on CT and the clinical diagnosis of pancreatic cancer: retrospective review of CT scans obtained before diagnosis. AJR Am J Roentgenol. 2004;182:897-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 173] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 41. | Vega EA, Kutlu OC, Salehi O, James D, Alarcon SV, Herrick B, Krishnan S, Kozyreva O, Conrad C. Preoperative Chemotherapy for Pancreatic Cancer Improves Survival and R0 Rate Even in Early Stage I. J Gastrointest Surg. 2020;24:2409-2415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Lim KH, Chung E, Khan A, Cao D, Linehan D, Ben-Josef E, Wang-Gillam A. Neoadjuvant therapy of pancreatic cancer: the emerging paradigm? Oncologist. 2012;17:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 43. | Mackay TM, Smits FJ, Roos D, Bonsing BA, Bosscha K, Busch OR, Creemers GJ, van Dam RM, van Eijck CHJ, Gerhards MF, de Groot JWB, Groot Koerkamp B, Haj Mohammad N, van der Harst E, de Hingh IHJT, Homs MYV, Kazemier G, Liem MSL, de Meijer VE, Molenaar IQ, Nieuwenhuijs VB, van Santvoort HC, van der Schelling GP, Stommel MWJ, Ten Tije AJ, de Vos-Geelen J, Wit F, Wilmink JW, van Laarhoven HWM, Besselink MG; Dutch Pancreatic Cancer Group. The risk of not receiving adjuvant chemotherapy after resection of pancreatic ductal adenocarcinoma: a nationwide analysis. HPB (Oxford). 2020;22:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 44. | Merkow RP, Bilimoria KY, Tomlinson JS, Paruch JL, Fleming JB, Talamonti MS, Ko CY, Bentrem DJ. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014;260:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 334] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 45. | Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, Sinn M, Hinke A, Riess H. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1361] [Article Influence: 113.4] [Reference Citation Analysis (0)] |
| 46. | Kosmahl M, Pauser U, Peters K, Sipos B, Lüttges J, Kremer B, Klöppel G. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch. 2004;445:168-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 253] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 47. | Hirooka Y, Goto H, Itoh A, Hashimoto S, Niwa K, Ishikawa H, Okada N, Itoh T, Kawashima H. Case of intraductal papillary mucinous tumor in which endosonography-guided fine-needle aspiration biopsy caused dissemination. J Gastroenterol Hepatol. 2003;18:1323-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 48. | Yoon WJ, Daglilar ES, Fernández-del Castillo C, Mino-Kenudson M, Pitman MB, Brugge WR. Peritoneal seeding in intraductal papillary mucinous neoplasm of the pancreas patients who underwent endoscopic ultrasound-guided fine-needle aspiration: the PIPE Study. Endoscopy. 2014;46:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |