Published online Mar 6, 2021. doi: 10.12998/wjcc.v9.i7.1676
Peer-review started: September 23, 2020
First decision: December 14, 2020
Revised: December 26, 2020
Accepted: January 6, 2021
Article in press: January 6, 2021
Published online: March 6, 2021
Processing time: 158 Days and 13.8 Hours
Thymic-enteric adenocarcinoma with positive expression of CDX2 and CK20 is rare in adults, with only 16 reported cases. However, standard treatment options for this type of thymic adenocarcinoma has not yet been established. Therefore, we report a case of stage IV thymic-enteric adenocarcinoma treated with radiotherapy, chemotherapy, and anti-angiogenesis therapy.
We report a case of thymic-enteric adenocarcinoma occurring in a 44-year-old woman. The tumor was considered unresectable owing to its invasiveness. The patient was treated with six cycles of oxaliplatin (130 mg/m2, day 1) and capecitabine (1000 mg/m2 BID, days 1-14). During the first three cycles of chemotherapy, concurrent radiotherapy (60 Gy/30 fractions) and anti-angiogenic therapy using apatinib were recommended. The primary tumor achieved partial remission based on the Response Evaluation Criteria in Solid Tumors. During follow-up, there was no evidence of disease relapse, except a high serum CA19-9 level. The patient is alive and regularly followed. Based on the previous literature and the present case, we believe that early diagnosis of thymic-enteric adenocarcinoma is important.
XELOX (capecitabine plus oxaliplatin) combined with radiotherapy is an optional therapy for inoperable thymic-enteric adenocarcinoma.
Core Tip: This report introduces the diagnosis and treatment of a metastatic thymic-enteric adenocarcinoma with positive expression of CDX2 and CK20. For the first time, radiotherapy and chemotherapy combined with anti-angiogenesis therapy were used. The tumor was partially remitted, and there was no sign of recurrence. XELOX (capecitabine plus oxaliplatin) combined with radiotherapy is an alternative treatment for inoperable metastatic thymic-enteric adenocarcinoma.
- Citation: Li M, Pu XY, Dong LH, Chang PY. Metastatic thymic-enteric adenocarcinoma responding to chemoradiation plus anti-angiogenic therapy: A case report. World J Clin Cases 2021; 9(7): 1676-1681
- URL: https://www.wjgnet.com/2307-8960/full/v9/i7/1676.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i7.1676
Thymic cancer is rare, accounting for only 0.06% of all thymic neoplasms[1]. It may be asymptomatic or associated with an intermittent cough, chest pain, or dyspnea. According to the 2015 World Health Organization thymic cancer classification, its histological types include squamous cell carcinoma, lymphoid epithelioid carcinoma, or basal-like carcinoma. However, the enteric type was first identified in 2003[2]. To date, a total of 16 cases of thymic adenocarcinoma with positive expression of CDX2 and CK20 have been reported (Table 1). However, standard treatment options for this type of thymic adenocarcinoma have not yet been established. We report a case of stage IV thymic-enteric adenocarcinoma treated with radiotherapy, chemotherapy, and anti-angiogenesis therapy. Our primary result demonstrated the effectiveness of a comprehensive approach.
| Case No. | Age | Gender | Ki-67 | Treatment | Outcome | Ref. |
| 1 | 70 | Male | 20%-30% | Surgery | AWD, 7 mo | [2] |
| 2 | 59 | Female | NA | Surgery + chemoradiotherapy + radiotherapy | Alive with disease, 11 mo (bone and lung metastasis) | [8] |
| 3 | 41 | Female | 90% | Surgery | AWD, 18 mo | [9] |
| 4 | 39 | Female | NA | Surgery | AWD, 159 mo (recurrence +) | [9] |
| 5 | 28 | Female | NA | Surgery + chemotherapy (GEMOX) + radiotherapy | AWD, 30 mo (2 times recurrence) | [10] |
| 6 | 55 | Male | NA | Surgery + radiotherapy | AWD, 14 mo | [11] |
| 7 | 36 | Female | NA | Surgery + chemotherapy (Taxol/CDDP) + radiotherapy | DOD, 15 mo | [12] |
| 8 | 66 | Female | NA | Surgery | AWD, 5 yr | [13] |
| 9 | NA | NA | NA | Surgery | NA | [14] |
| 10 | 52 | Female | NA | Surgery + chemotherapy (cisplatin + etoposide/carboplatin + paclitaxel) + radiotherapy | Alive with disease, 11 mo (lung and lymph node metastasis) | [15] |
| 11 | 38 | Male | NA | Surgery + radiotherapy + chemotherapy (carboplatin + docetaxel) | DOD, 12 mo (bone metastasis) | [15] |
| 12 | 55 | Male | NA | Surgery + chemotherapy (carboplatin + docetaxel/paclitaxel) | DOD, 24 mo (bone, liver, lung, adrenal gland metastases) | [15] |
| 13 | 41 | Male | NA | Surgery | AWD, 43 mo (lung metastasis+) | [16] |
| 14 | 34 | Male | NA | Surgery + chemotherapy (carboplatin, Adriamycin, cyclophosphamide, and vincristine) + radiotherapy | DOD, 20 mo | [17] |
| 15 | 15 | Male | NA | Surgery + radiotherapy | DOD, 26 mo | [18] |
| 16 | 29 | Female | NA | Surgery | AWD, 8 mo | [19] |
| Present case | 44 | Female | 70% | Concurrent chemoradiotherapy + antiangiogenic therapy | AWD |
A 44-year-old woman was admitted to our hospital for dyspnea with chest pain in April 2018.
The patient had no history of present illness.
The patient had no history of past illness.
The patient had no personal and family history.
No obvious abnormalities were found on physical examination.
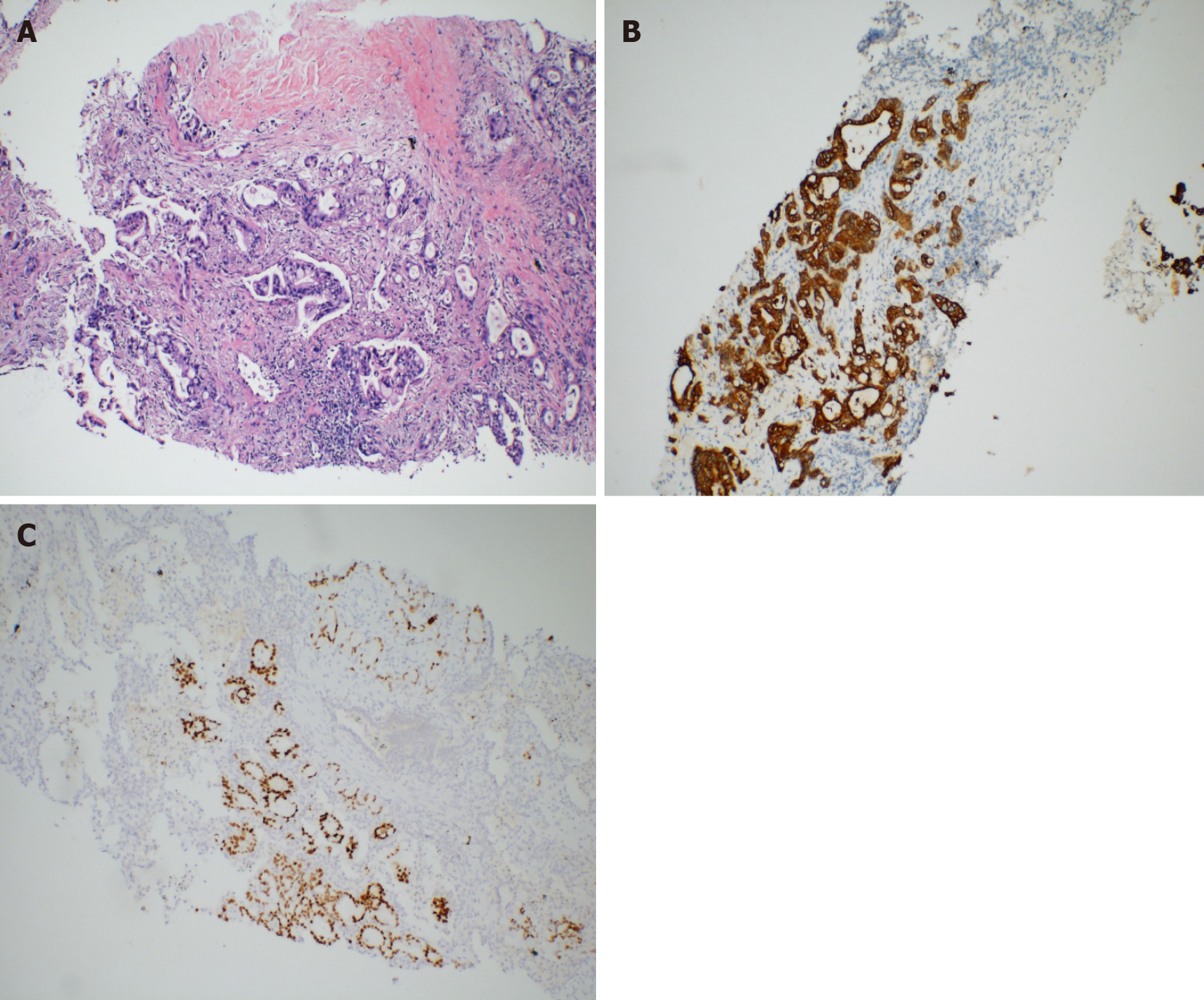
Laboratory tests showed elevated levels of several serum tumor markers (CA19-9, 483.98 U/mL; CA125, 111.44 IU/mL; CA242, 138.50 IU/mL; cytokeratin-19 fragment, 5.34 ng/mL; and carcinoembryonic antigen, 20.07 ng/mL). Liver and kidney function tests were normal. The pericardial effusion was bloody, and tumor cells were detected. Mediastinal mass biopsy showed pathological adenocarcinoma infiltration (Figure 1A). Immunohistochemical staining showed that the tumor cells were positive for CK20 (Figure 1B), CDX2 (Figure 1C), villin, and EGFR; partially positive for CA15-3; and negative for lung cancer markers, including CK7, TTF-1, and Napsin A. The Ki-67 index was 70%. Moreover, wild types of KRAS, NRAS, and BRAF were detected. The pathological results suggested intestinal metastatic adenocarcinoma. However, no other primary tumor was found on systemic examination.
Whole-body positron emission computed tomography (CT) (Figure 2A) showed an anterior calcified mediastinal mass measuring approximately 43 mm × 38 mm with an increased edge radioactivity uptake [maximum standardized uptake value (SUV) of 6.4; CT value of 41.8 HU]; increased pericardial radioactive uptake and a fluid density shadow; and increased sternal spot-like radioactivity uptake (maximum SUV value of 3.4).
After consultation with histopathologists in our institution, the tumor was diagnosed as a thymic adenocarcinoma (enteric type T4N0M1b stage IVb) with pericardial and sternal metastases according to the American Joint Committee on Cancer Staging Manual Eight Edition (2017).
A comprehensive therapeutic regimen was administered. First, the patient underwent six cycles of oxaliplatin (130 mg/m2, day 1) and capecitabine (1000 mg/m2, BID, days 1-14) therapy. During the first three cycles of chemotherapy, concurrent radiotherapy (60 Gy/30 fractions) targeting the thymic mass was planned. Meanwhile, anti-angiogenic therapy using apatinib, a VEGFR2 inhibitor (Jiangsu Hengrui Pharmaceutical Company Limited, Jiangsu Province, China), was omitted. This elicited grade II myelosuppression and grade II radioactive esophagitis during the concurrent chemoradiotherapy.
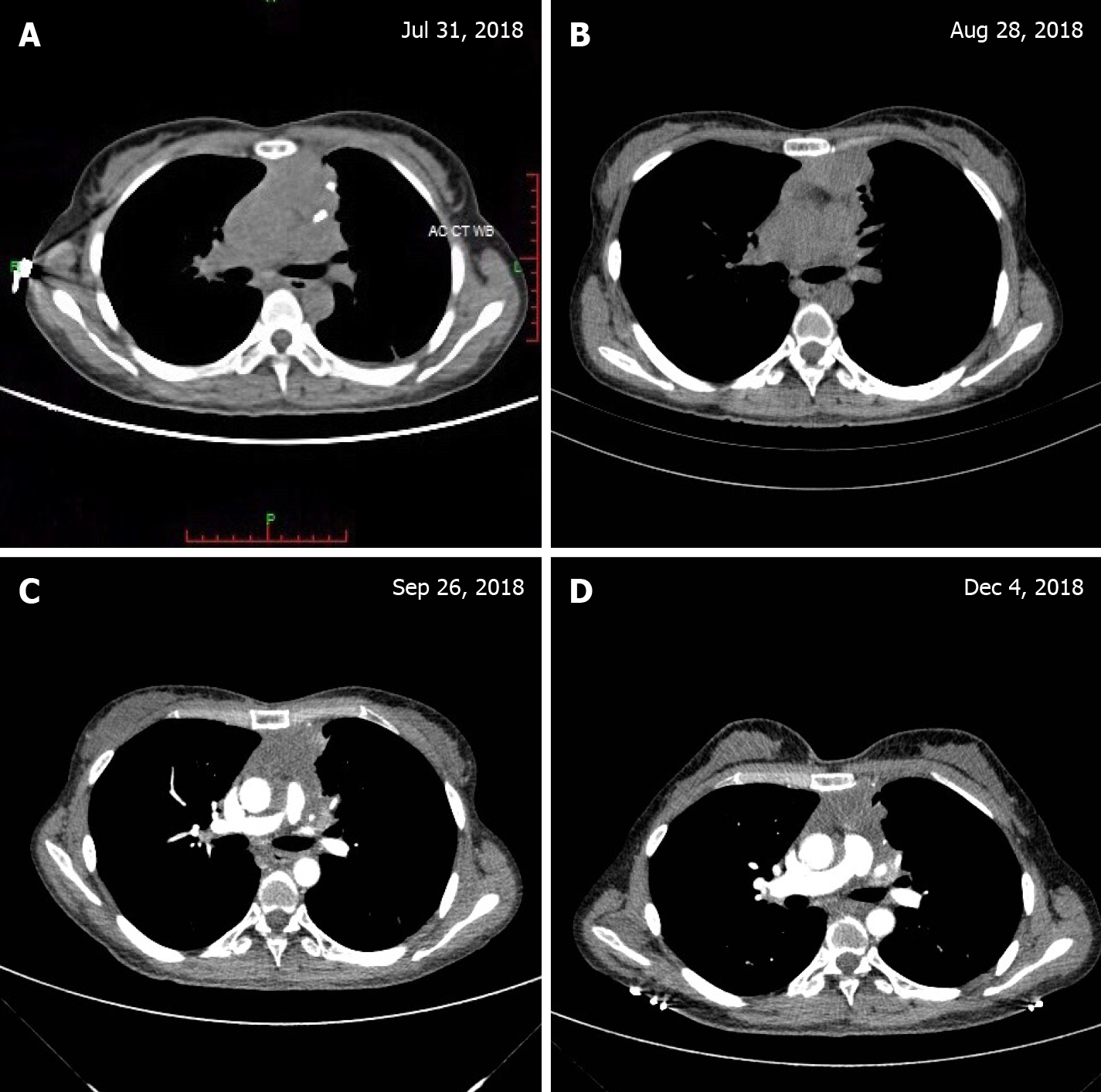
The patient’s chest pain and dyspnea were significantly relieved after chemo-radiotherapy. The thymus mass size was reduced after radiotherapy (30 Gy/15 fractions), but the patient occasionally experienced tachycardia. To reduce heart toxicity, we narrowed the irradiation field. During treatment, the tumor continued to shrink, as shown in Figure 2B and C. After six cycles of XELOX, chest CT showed that the tumor was approximately 37 mm × 20 mm (Figure 2D). Laboratory tests showed that only one serum tumor marker (CA19-9, 60.70 U/mL) remained elevated. The patient refused physical examination during her long-term follow-up; however, she was alive without recurrence for 16 mo when this paper was written.
During embryogenesis, the thymus and gut originate from the same arch endoderm. Intriguingly, tuft cells were found to be present in the adult thymus[3]. In fact, tuft cells are functional intestinal epithelial cells[3]. Moreover, mutations in tuft cells elicit gut carcinogenesis in humans. In this regard, thymic tuft cells' role as potential sources for thymic carcinogenesis should be investigated.
To the best of our knowledge, there is no standard of care for the thymic-enteric adenocarcinoma. Previously, patients were mainly treated surgically because the disease was localized. In this case, tumor resection was challenging because of pericardial involvement. Initially, the Ki-67 index was 70%, suggesting that the tumor cells expanded in number. Proliferative cells are sensitive to ionizing irradiation[4]. Therefore, we chose radiotherapy for controlling the primary tumor, thereby alleviating the patient’s symptoms. Meanwhile, systematic chemotherapy plus anti-angiogenic therapy was used as the main treatment. Based on the similarities between thymic-enteric adenocarcinoma and colorectal cancer in histologic phenotype, the XELOX regimen was selected. Capecitabine inhibits DNA synthesis[5], while oxaliplatin induces immunogenic cell death. Moreover, angiogenic factors such as VEGF were detected in the malignant effusion[6]. Secreted by tumor cells, VEGF is a potent inducer of angiogenesis[6]. In this process, VEGF can significantly increase vascular permeability. Inhibition of angiogenesis in tumors limits pericardial effusion[7]. Thus, apatinib was selected for this patient.
In this case, the primary tumor achieved partial remission according to the Response Evaluation Criteria in Solid Tumors, and there was no evidence of relapse during follow-up, except for high serum CA19-9 levels. In addition, treatment-related toxicity was manageable. Only grade II myelosuppression and grade II radioactive esophagitis occurred during treatment, thus demonstrating that our treatment was effective in this patient.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mohamed SY S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Greene MA, Malias MA. Aggressive multimodality treatment of invasive thymic carcinoma. J Thorac Cardiovasc Surg. 2003;125:434-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Choi WW, Lui YH, Lau WH, Crowley P, Khan A, Chan JK. Adenocarcinoma of the thymus: report of two cases, including a previously undescribed mucinous subtype. Am J Surg Pathol. 2003;27:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Miller CN, Proekt I, von Moltke J, Wells KL, Rajpurkar AR, Wang H, Rattay K, Khan IS, Metzger TC, Pollack JL, Fries AC, Lwin WW, Wigton EJ, Parent AV, Kyewski B, Erle DJ, Hogquist KA, Steinmetz LM, Locksley RM, Anderson MS. Thymic tuft cells promote an IL-4-enriched medulla and shape thymocyte development. Nature. 2018;559:627-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 223] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 4. | Shi X, Yuan X, Tao D, Gong J, Hu G. Analysis of DNA ploidy, cell cycle and Ki67 antigen in nasopharyngeal carcinoma by flow cytometry. J Huazhong Univ Sci Technolog Med Sci. 2005;25:198-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Comella P. A review of the role of capecitabine in the treatment of colorectal cancer. Ther Clin Risk Manag. 2007;3:421-431. [PubMed] |
| 6. | Zebrowski BK, Yano S, Liu W, Shaheen RM, Hicklin DJ, Putnam JB Jr, Ellis LM. Vascular endothelial growth factor levels and induction of permeability in malignant pleural effusions. Clin Cancer Res. 1999;5:3364-3368. [PubMed] |
| 7. | Roviello G, Ravelli A, Polom K, Petrioli R, Marano L, Marrelli D, Roviello F, Generali D. Apatinib: A novel receptor tyrosine kinase inhibitor for the treatment of gastric cancer. Cancer Lett. 2016;372:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 8. | Wang L, Wang D, Qian K, Lu D, Chen L, Zhao L, Teng L. Thymic adenocarcinoma associated with thymic cyst: a case report and review of literature. Int J Clin Exp Pathol. 2015;8:5890-5895. [PubMed] |
| 9. | Jung HY, Cho H, Chung JH, Bae SB, Lee JH, Lee HJ, Jang SH, Oh MH. A Rare Case of Primary Tubular Adenocarcinoma of the Thymus, Enteric Immunophenotype: A Case Study and Review of the Literature. J Pathol Transl Med. 2015;49:331-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Moser B, Schiefer AI, Janik S, Marx A, Prosch H, Pohl W, Neudert B, Scharrer A, Klepetko W, Müllauer L. Adenocarcinoma of the thymus, enteric type: report of 2 cases, and proposal for a novel subtype of thymic carcinoma. Am J Surg Pathol. 2015;39:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Maghbool M, Ramzi M, Nagel I, Bejarano P, Siebert R, Saeedzadeh A, Daneshbod Y. Primary adenocarcinoma of the thymus: an immunohistochemical and molecular study with review of the literature. BMC Clin Pathol. 2013;13:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Teramoto K, Kawaguchi Y, Hori T, Ishida M, Hashimoto M, Kitamura S, Motoishi M, Hanaoka J, Tezuka N, Okabe H. Thymic papillo-tubular adenocarcinoma containing a cyst: report of a case. Surg Today. 2012;42:988-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Abdul-Ghafar J, Yong SJ, Kwon W, Park IH, Jung SH. Primary thymic mucinous adenocarcinoma: a case report. Korean J Pathol. 2012;46:377-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Seon HJ, Kim KH, Choi YD, Song SY, Yoon HJ, Kim YH, Jeong MH, Park JC. Angina pectoris caused by the extrinsic compression of coronary artery by primary thymic mucinous adenocarcinoma. Int J Cardiol. 2012;156:e13-e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Weissferdt A, Moran CA. Thymic carcinoma, part 1: a clinicopathologic and immunohistochemical study of 65 cases. Am J Clin Pathol. 2012;138:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Maeda D, Ota S, Ikeda S, Kawano R, Hata E, Nakajima J, Mori M, Fukayama M. Mucinous adenocarcinoma of the thymus: a distinct variant of thymic carcinoma. Lung Cancer. 2009;64:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Yin YG, Lu HZ. Primary Mucinous Adenocarcinoma of the Thymus: A Case Report and Literature Review. Chin Med Sci J. 2017;32:201-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Sawai T, Inoue Y, Doi S, Ikuta Y, Kimino K, Nakashima M, Soda H, Kohno S. Tubular adenocarcinoma of the thymus: case report and review of the literature. Int J Surg Pathol. 2006;14:243-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Tamai M, Ishida M, Ebisu Y, Okamoto H, Miyasaka C, Ohe C, Uemura Y, Saito T, Murakawa T, Tsuta K. Thymic enteric type adenocarcinoma: A case report with cytological features. Diagn Cytopathol. 2018;46:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |