Published online Mar 6, 2021. doi: 10.12998/wjcc.v9.i7.1646
Peer-review started: August 4, 2020
First decision: November 8, 2020
Revised: November 26, 2020
Accepted: December 10, 2020
Article in press: December 10, 2020
Published online: March 6, 2021
Processing time: 208 Days and 18.2 Hours
Toxic epidermal necrolysis (TEN) is often associated with skin wounds affecting large areas. Healing of this type of wound is difficult because of pressure, infection and other factors. It can increase the length of hospital stay and result in wound sepsis and even death.
A 49-year-old woman developed a skin lesion covering 80% of the total body surface area after using a kind of Chinese medicinal ointment on a burn wound on her back; she developed life-threatening wound sepsis and septic shock. Methicillin-resistant Staphylococcus aureus, carbapenem-resistant Acinetobacter baumannii, carbapenem-resistant Pseudomonas aeruginosa and other bacteria were cultured from wound tissue, deep venous catheter and blood samples. Imipenem cilastatin sodium, tigecycline and teicoplanin were used for anti-infection therapy. Finally, the patient was transferred to the burn department because of severe wound sepsis. In the burn intensive care unit, pain-free dressing changes and autologous scalp skin grafting were performed to heal the wound in addition to reasonable and effective antibacterial treatment according to microbial susceptibility test results. After three operations within 2 wk, the wound healed and sepsis resolved.
TEN patients with large areas of skin injury may develop wound infection and life-threatening wound sepsis. Autologous scalp skin grafting may be beneficial for rapid wound healing and reducing the risk of sepsis in TEN patients, and it leaves no scar at the donor site.
Core Tip: Toxic epidermal necrolysis is often associated with large skin wound areas. Healing the wound is difficult because of pressure, infection and other factors. Autologous scalp skin grafting may be a good option to promote rapid wound healing and reduce the risk of wound sepsis.
- Citation: Xue DD, Zhou L, Yang Y, Ma SY. Autologous scalp skin grafting to treat toxic epidermal necrolysis in a patient with a large skin injury: A case report. World J Clin Cases 2021; 9(7): 1646-1653
- URL: https://www.wjgnet.com/2307-8960/full/v9/i7/1646.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i7.1646
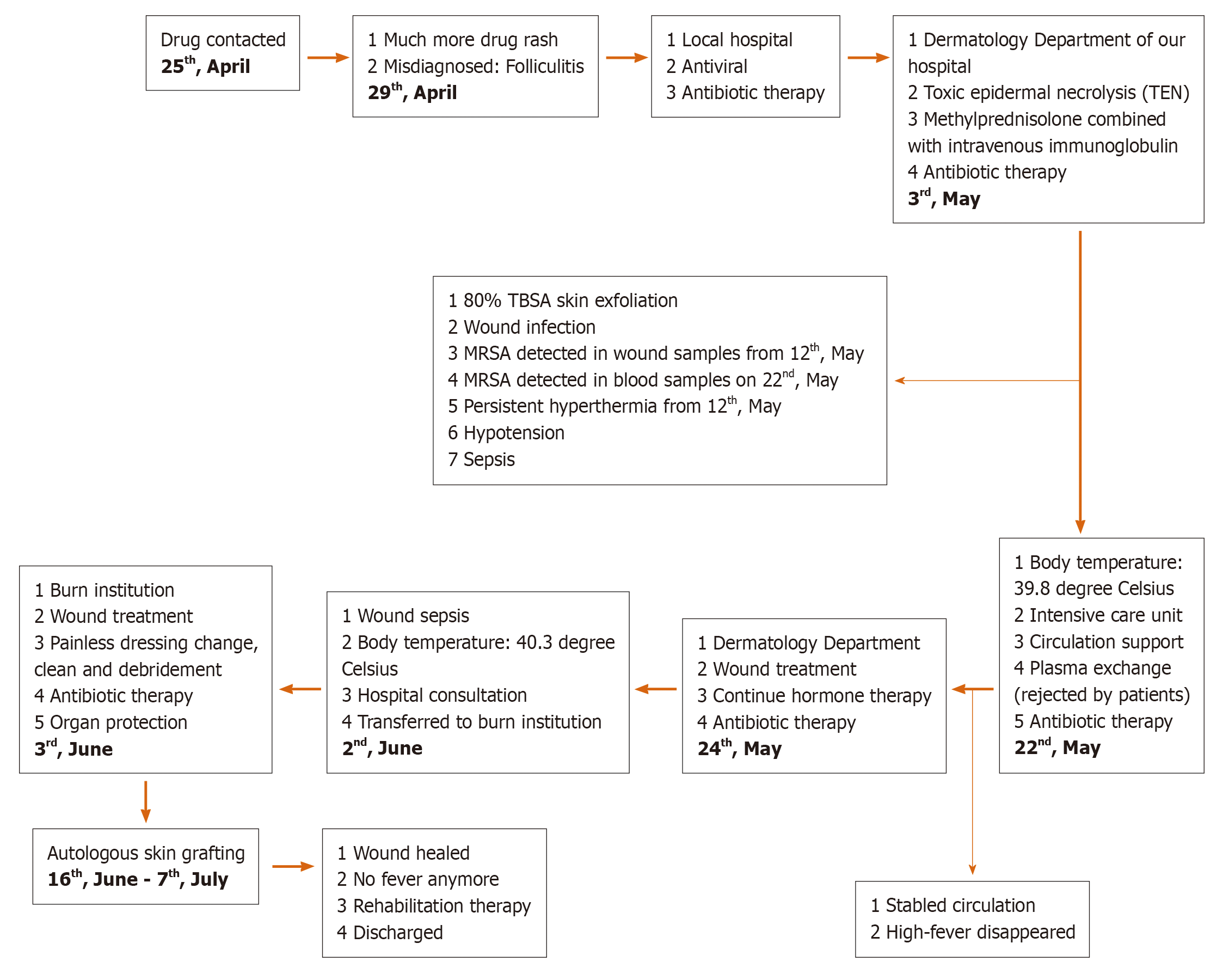
Toxic epidermal necrolysis (TEN) is considered a delayed allergic drug reaction that often manifests as fever and painful local erythema at onset, spreads quickly and causes slack bullae or epidermal peeling. Nikolsky’s sign can be observed in patients, and extensive skin and mucous membrane erosion [skin injury area > 30% of total body surface area (TBSA) involvement] generally occurs within 24-72 h but can develop as long as 4-28 d after drug exposure[1,2]. Almost any drug can cause a drug rash[3]. When the skin injury area is large, at more than 50% TBSA, the condition is extremely serious and is considered a dermatological emergency, as it is associated with high risks of death and disability[4-6]. The risk of mortality increases with increasing surface area involvement, and a meta-analysis of the literature showed the mortality risk to be between 16% and 55%[7]. It becomes difficult to heal the wound because of pressure, infection and other factors[8-10]. It eventually increases the length of hospital stay and can result in wound sepsis and even death. Thus, in such patients, guidance on how to promote quickly wound healing and reduce the occurrence of wound infection and systemic infection would be beneficial for designing treatment regimens. Dry exposed therapy, dressing changes, topical antibiotics and new-type dressings are often used to prevent secondary infections[11]. In this report, the case of a patient with severe TEN with an 80% TBSA skin injury and wound sepsis treated by autologous scalp skin grafting is presented (Figure 1). The use of the patient’s personal information and medical data in our manuscript has been approved by the Ethics Committee of the First Affiliated Hospital of Army Medical University.
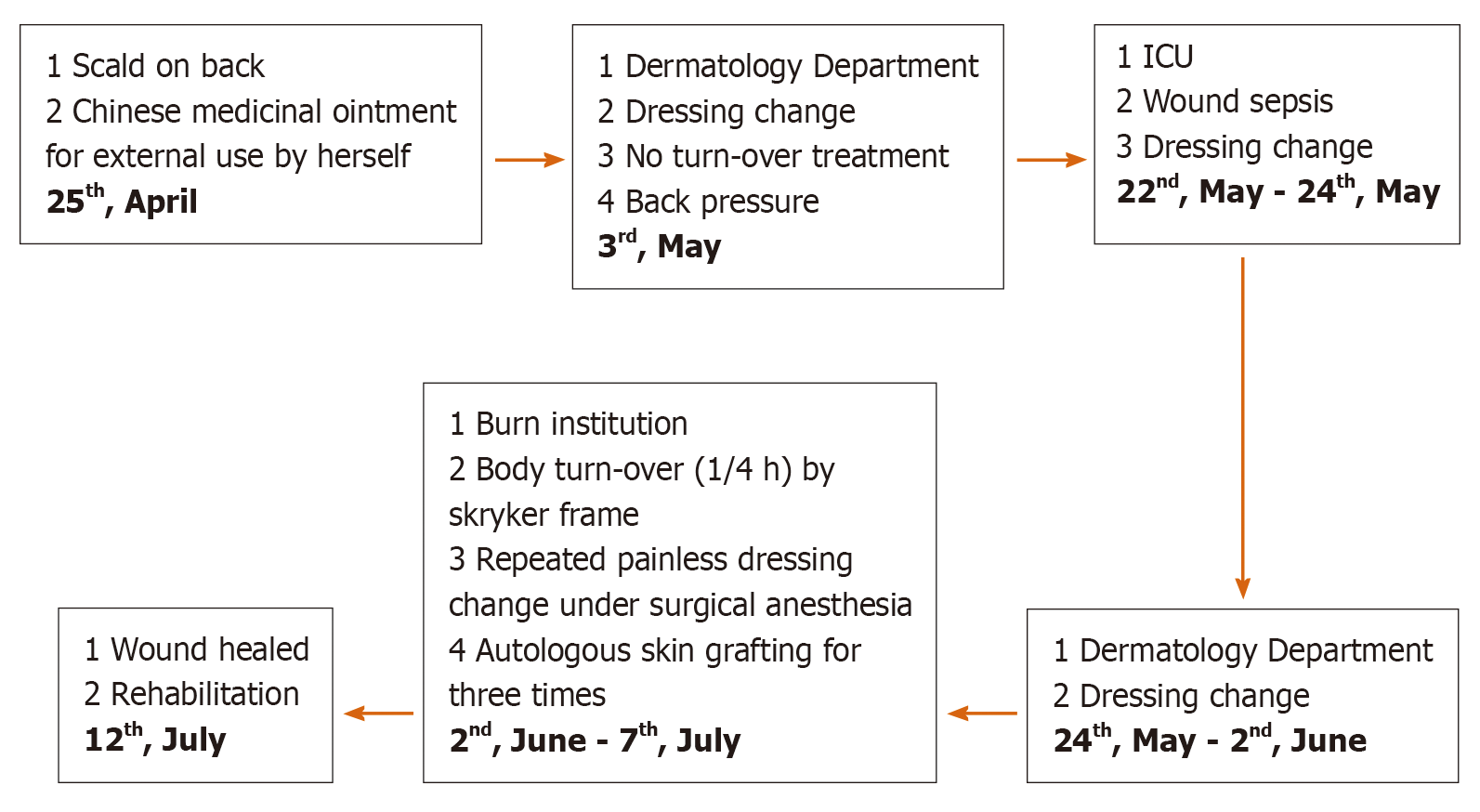
A woman aged 49 years who suffered from a superficial partial-thickness steam burn (1% TBSA) was admitted to the hospital for a red rash that appeared locally on the back and elsewhere, such as the face and neck, after using a kind of Chinese medicinal ointment on the burn wound on her back.
Initially, the local hospital diagnosed the patient’s condition as folliculitis, shingles or chickenpox and treated the patient with various drugs, including erythromycin and acyclovir. Her condition gradually worsened, with systemic rash development; blister formation; wide-spread peeling of the skin, mucosa and other epithelial tissue; dermis exposure and a high fever within 96 h. On the 8th d after onset, the patient was urgently transferred to the Dermatology Department at our hospital.
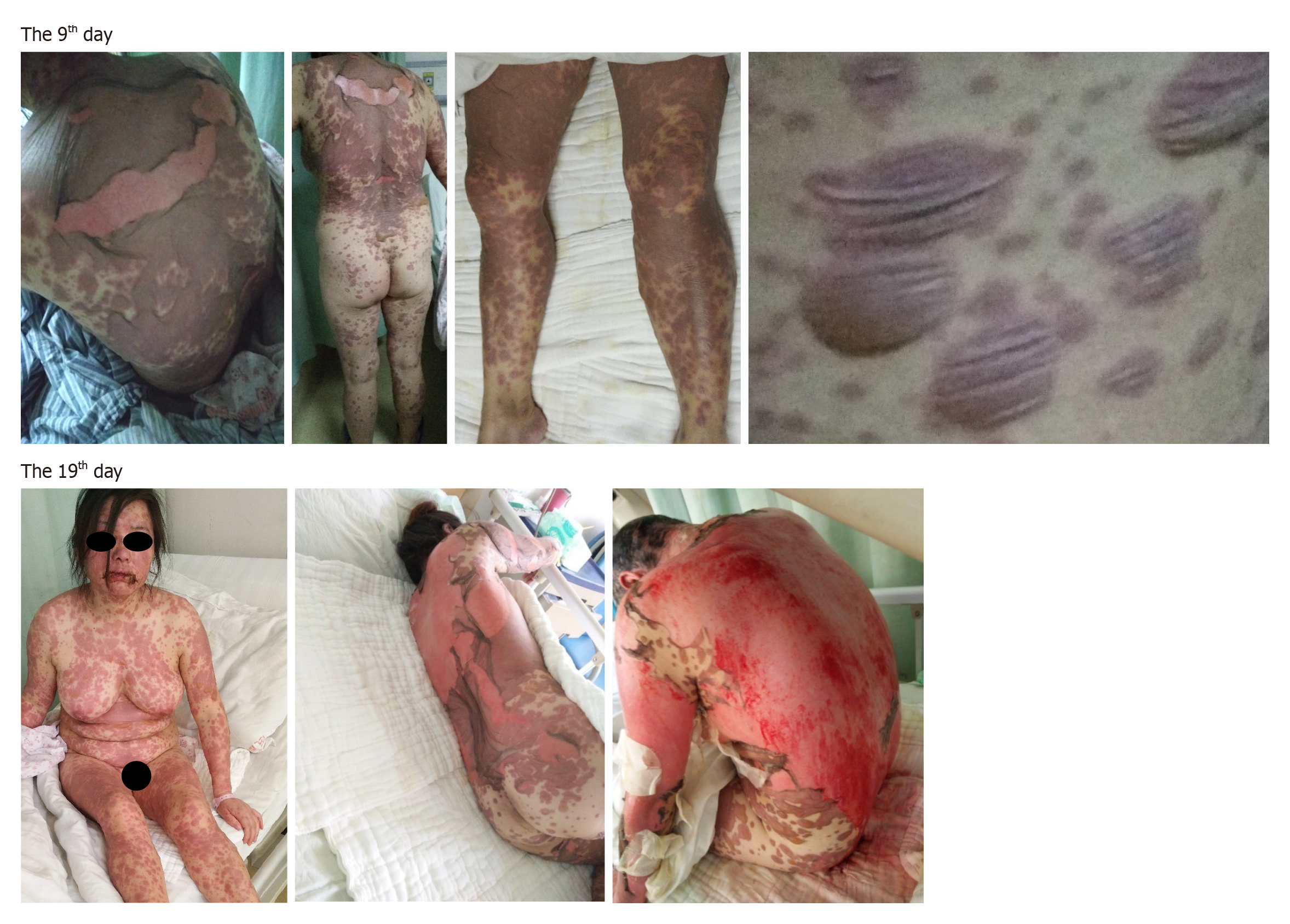
The patient presented with typical rash and lesion morphology, suggestive of a delayed drug allergic reaction (fever, painful local erythema at onset, slack bullae or epidermal peeling), Nikolsky’s sign, extensive skin and mucous membrane erosion and skin injury covering approximately 80% of the TBSA (Figure 2).
The diagnosis was confirmed to be TEN according to typical rash and lesion morphologies and the patient’s medical history.
After prednisone pulse therapy, immunoglobulin fortification and anti-infection therapy, the rash was controlled, but damaged skin still covered approximately 80% of the TBSA. Wound healing was a challenge due to pressure and infection; subsequently, wound sepsis and systemic infection developed.
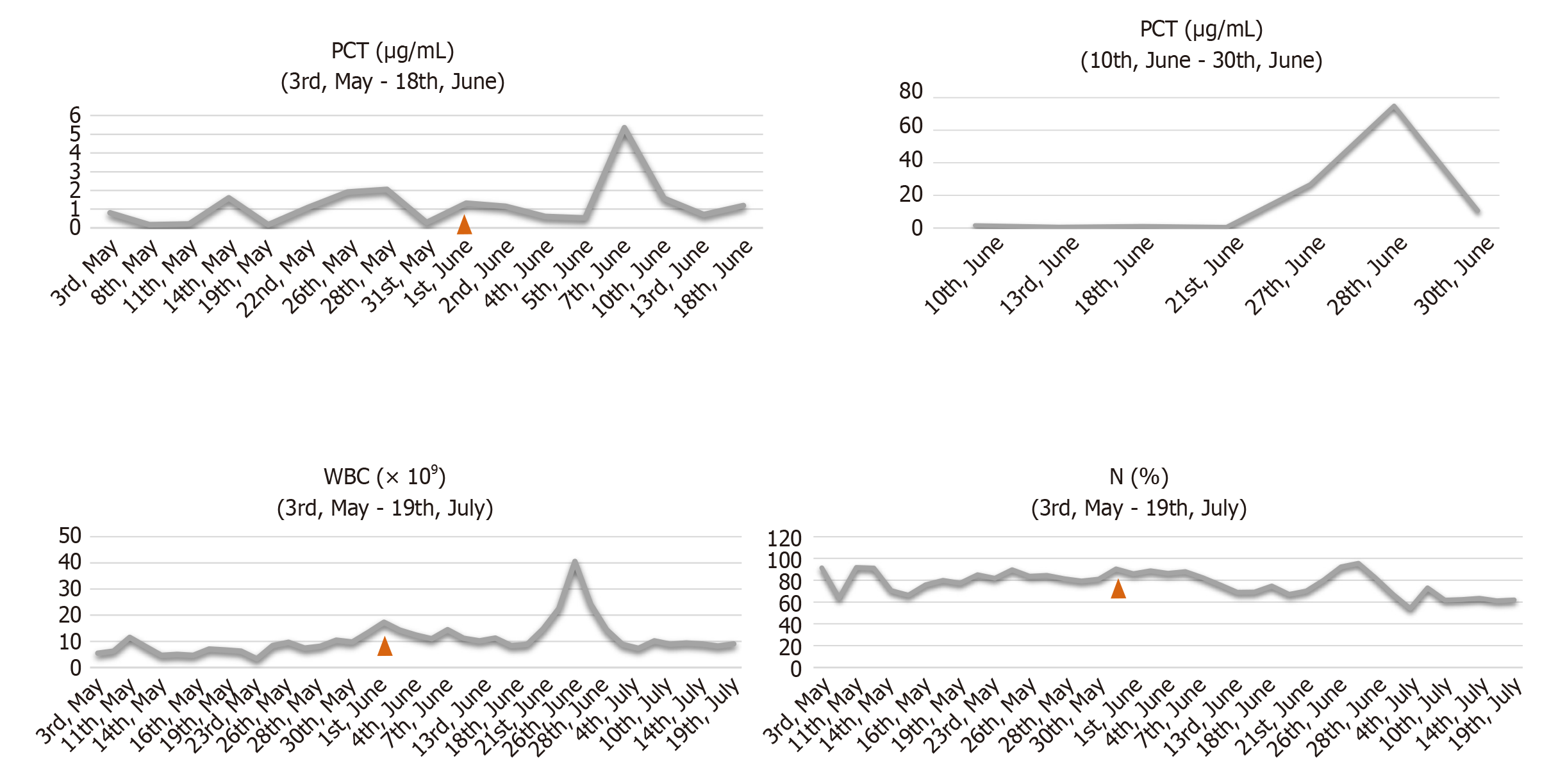
Methicillin-resistant Staphylococcus aureus, carbapenem-resistant Acinetobacter baumannii, carbapenem-resistant Pseudomonas aeruginosa and other bacteria were cultured from wound tissue, deep venous catheter and blood samples. The intensive care unit of our hospital recommended plasma exchange treatment, but the patient refused it. After fluid therapy, norepinephrine for circulation maintenance, a combined antibiotic regimen for anti-infection therapy [imipenem and cilastatin sodium (1000 mg every 8 h) combined with tigecycline (500 mg every 12 h)], organ function protection therapies and water and electrolyte balance correction, the woman’s vital signs stabilized. After a hospital-wide discussion, the patient was finally transferred to the burn intensive care unit (BICU) because of the size of the wound and severe wound sepsis. In the BICU, we gradually reduced the steroid dose, performed additional microbiological investigations and strengthened anti-infection therapy. We particularly emphasized wound treatment. A Stryker frame was used to turn the body every 4 h (1/4 h) to reduce pressure and moisture on the back wound. Painless dressing changes under surgical anesthesia were conducted for thorough wound cleaning, including wound necrotic tissue removal. Mupirocin and epidermal growth factor were used to improve the wound bed. We also strengthened the management of various catheters, including the central venous catheter, tracheal tube, urinary catheter and nutrition support tube and provided psychological counseling and rehabilitation training (Figure 1).
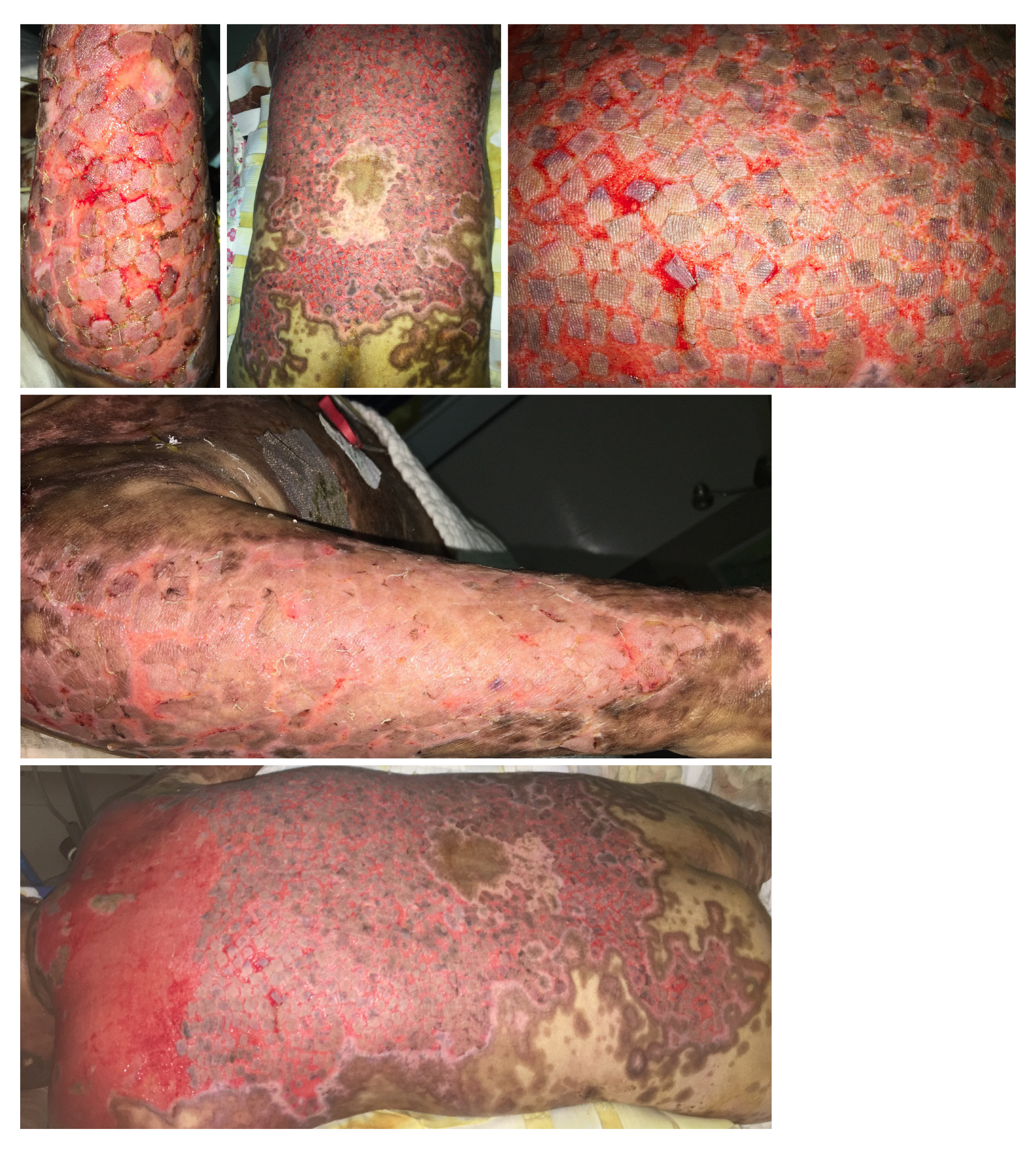
When the general condition of the patient met the requirement for skin grafting, wound debridement and skin grafting were performed (Figures 3 and 4). Split-thickness scalp skin was used as an autologous skin source. The skin transplantation procedure was divided into two parts that were performed simultaneously. In one part, edematous granulation tissue and necrotic tissue were scraped off with a scalpel and repeatedly rinsed with hydrogen peroxide (0.1%) and normal saline three times. Gauze soaked with 1:20000 epinephrine solution was used to stop bleeding for 5 min. In the other part, normal saline containing 1:200000 epinephrine was injected into the scalp to promote skin swelling, resulting in hemostasis. A razor-thin slice of skin was cut with an air-driven dermatome, and the donor area of the head was covered with Vaseline and gauze; the multi-layer sterile dressing was applied under pressure. After cleaning with sterile normal saline, the split-thickness skin was cut into stamp-like pieces sized 0.5 cm × 0.5 cm; finally, the stamp-like skin from the scalp was transplanted onto the wound and bandaged with gauze.
After autologous scalp-skin grafting, all the wounds healed within 2 wk, and the infection indicators and vital signs returned to normal (Figure 5). The patient was discharged, with no recurrent wound infection or skin injury.
Treatment of skin and mucosal wounds is important in TEN patients with large skin lesions over 50% of the TBSA. Studies have shown that when large areas of the skin are damaged, impaired skin barrier function can lead to electrolyte disturbance, hypermetabolism, excessive fluid exudation and increased risk of infection[12]. However, many factors, such as pressure, infection, nutrition and immune status, may lead to deepening of the wound surface, resulting in healing difficulty or even no healing[13,14]. How to prevent and treat wound deepening is important. Wound cleaning, debridement and drainage, frequent changes in body position to prevent wound pressure and moisture, regular dressing changes and the selection of appropriate new functional dressings are useful treatment strategies to promote wound healing[15,16]. New antibacterial dressings containing silver ions, biological materials and allogeneic skin dressings have been demonstrated to be beneficial in wound healing[17-19].
In the case presented here, more than 80% of the patient’s skin wound was exposed and infected, making healing difficult. Moreover, the patient eventually developed wound sepsis. For this patient, how to heal the wound quickly and effectively became the priority. Autologous skin grafting was performed as an alternative surgical treatment for rapid wound healing. Autologous skin grafting is a method of surgically harvesting the patient’s own skin with skin cutting tools and then transplanting it onto a wound to promote rapid healing of the wound. Because of its strong regenerative ability, the scalp can heal with no scar after split-thickness skin is harvested. Skin harvesting from other donor body sites will produce scars, which will affect the appearance, feeling and function of the skin. Moreover, the scalp, as the donor site, can repeatedly supply split-thickness skin grafts up to 10 times. Autologous scalp-skin grafting provides a good alternative to surgical repair of large-area skin wound. The scalp generally produces no scarring at the donor site after healing. Additionally, combined with reasonable skin expansion techniques, such as postage stamp skin grafting (the skin expansion ratio is about 1:2 to 1:4) and Meek grafting (the skin expansion ratio is about 1:9)[20], the coverage area of the wound was enlarged.
In TEN patients, when the wound has difficulty healing due to pressure, infection or other factors, autologous scalp skin grafting may be helpful in shortening hospitalization durations, reducing complications and preventing mortality.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Morozov S S-Editor: Chen XF L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Carrasquillo OY, Santiago-Vazquez M, Cardona R, Cruz-Manzano M, Figueroa LD. Stevens-Johnson syndrome and toxic epidermal necrolysis: a retrospective descriptive study. Int J Dermatol. 2019;58:1293-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Bequignon E, Duong TA, Sbidian E, Valeyrie-Allanore L, Ingen-Housz-Oro S, Chatelin V, Coste A, Wolkenstein P, Chosidow O, Papon JF. Stevens-Johnson syndrome and toxic epidermal necrolysis: ear, nose, and throat description at acute stage and after remission. JAMA Dermatol. 2015;151:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens-Johnson syndrome: a review. Crit Care Med. 2011;39:1521-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Nizamoglu M, Ward JA, Frew Q, Gerrish H, Martin N, Shaw A, Barnes D, Shelly O, Philp B, El-Muttardi N, Dziewulski P. Improving mortality outcomes of Stevens Johnson syndrome/toxic epidermal necrolysis: A regional burns centre experience. Burns. 2018;44:603-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | McCullough M, Burg M, Lin E, Peng D, Garner W. Steven Johnson Syndrome and Toxic Epidermal Necrolysis in a burn unit: A 15-year experience. Burns. 2017;43:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Antoon JW, Goldman JL, Lee B, Schwartz A. Incidence, outcomes, and resource use in children with Stevens-Johnson syndrome and toxic epidermal necrolysis. Pediatr Dermatol. 2018;35:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Abela C, Hartmann CE, De Leo A, de Sica Chapman A, Shah H, Jawad M, Bunker CB, Williams GJ, Leon-Villapalos J. Toxic epidermal necrolysis (TEN): the Chelsea and Westminster Hospital wound management algorithm. J Plast Reconstr Aesthet Surg. 2014;67:1026-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Li J, Mao M, Tang N, Zhai R, Zhu W, Yi M, Chen M. [Clinical characteristics and prognosis for 126 patients with severe drug eruption]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2017;42:953-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Papp A, Sikora S, Evans M, Song D, Kirchhof M, Miliszewski M, Dutz J. Treatment of toxic epidermal necrolysis by a multidisciplinary team. A review of literature and treatment results. Burns. 2018;44:807-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, Harr T. Current Perspectives on Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Clin Rev Allergy Immunol. 2018;54:147-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 200] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 11. | Mahar PD, Wasiak J, Hii B, Cleland H, Watters DA, Gin D, Spinks AB. A systematic review of the management and outcome of toxic epidermal necrolysis treated in burns centres. Burns. 2014;40:1245-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Rowan MP, Cancio LC, Elster EA, Burmeister DM, Rose LF, Natesan S, Chan RK, Christy RJ, Chung KK. Burn wound healing and treatment: review and advancements. Crit Care. 2015;19:243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 417] [Cited by in RCA: 547] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 13. | Han G, Ceilley R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv Ther. 2017;34:599-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 763] [Cited by in RCA: 1200] [Article Influence: 150.0] [Reference Citation Analysis (0)] |
| 14. | Sekiguchi A, Motegi SI, Uchiyama A, Uehara A, Fujiwara C, Yamazaki S, Perera B, Nakamura H, Ogino S, Yokoyama Y, Akai R, Iwawaki T, Ishikawa O. Botulinum toxin B suppresses the pressure ulcer formation in cutaneous ischemia-reperfusion injury mouse model: Possible regulation of oxidative and endoplasmic reticulum stress. J Dermatol Sci. 2018;90:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Obagi Z, Damiani G, Grada A, Falanga V. Principles of Wound Dressings: A Review. Surg Technol Int. 2019;35:50-57. [PubMed] |
| 16. | Childs DR, Murthy AS. Overview of Wound Healing and Management. Surg Clin North Am. 2017;97:189-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 17. | Young JB, Gondek SP, Troche M, Summitt JB, Rae L, Thayer WP, Kahn SA. The use of porcine xenografts in patients with toxic epidermal necrolysis. Burns. 2016;42:1728-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Paggiaro AO, E Silva Filho ML, de Carvalho VF, Isaac C, Gemperli R. The Role of Biological Skin Substitutes in Stevens-Johnson Syndrome: Systematic Review. Plast Surg Nurs. 2018;38:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Lee HY. Wound management strategies in Stevens-Johnson syndrome/toxic epidermal necrolysis: An unmet need. J Am Acad Dermatol. 2018;79:e87-e88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Quintero EC, Machado JFE, Robles RAD. Meek micrografting history, indications, technique, physiology and experience: a review article. J Wound Care. 2018;27:S12-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |