Published online Mar 6, 2021. doi: 10.12998/wjcc.v9.i7.1631
Peer-review started: July 26, 2020
First decision: August 7, 2020
Revised: August 21, 2020
Accepted: January 5, 2021
Article in press: January 5, 2021
Published online: March 6, 2021
Processing time: 211 Days and 14 Hours
Most small intestinal lipomas are treated surgically, and some require repeated surgeries for multiple lipomas. However, application of endoscopic submucosal dissection (ESD) technology in the deep small intestine is rarely reported owing to the special anatomical structure of the small intestine, medical equipment limitations, and the lack of relevant experience among endoscopists.
Two patients with small intestinal lipomas treated at the Air Force Medical Center from November 2015 to September 2019 were selected to undergo balloon-assisted ESD to treat the lipomas and explore the technical feasibility and safety of ESD for treating small intestinal lipomas. The two patients successfully underwent balloon-assisted ESD to treat four small intestinal lipomas, with a complete resection rate of 100% (4/4), without intraoperative or postoperative bleeding, perforation, or other complications. After 3-6 mo of postoperative follow-up, the clinical symptoms caused by the lipomas were significantly relieved or disappeared after treatment.
Balloon-assisted ESD is a safe and reliable new method for treating deep intestinal lipomas and shows good clinical feasibility.
Core Tip: Endoscopic submucosal dissection (ESD) technology plays an increasingly important role in the treatment of early gastrointestinal cancer and benign gastrointestinal tumors, but its application in the deep small intestine is rarely reported. In this study, two patients with small intestinal lipoma were successfully treated by ESD with balloon-assisted endoscopy, without intraoperative or postoperative bleeding, perforation, or other complications. The clinical symptoms caused by the lipomas were relieved or disappeared in the postoperative follow-up. We believe that balloon-assisted ESD for the treatment of small intestinal lipoma is safe and reliable, with good clinical feasibility, and can be used as a new method for the treatment of deep intestinal lipomas.
- Citation: Chen HY, Ning SB, Yin X, Li BR, Zhang J, Jin XW, Sun T, Xia ZB, Zhang XP. Balloon-assisted endoscopic submucosal dissection for treating small intestinal lipomas: Report of two cases. World J Clin Cases 2021; 9(7): 1631-1638
- URL: https://www.wjgnet.com/2307-8960/full/v9/i7/1631.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i7.1631
Small intestinal lipomas are benign tumors that usually do not cause clinical symptoms. However, large intestinal lipomas can occasionally cause repeated intussusception, intestinal obstruction, and gastrointestinal bleeding, leading to more serious clinical consequences[1]. Most small intestinal lipomas are treated surgically, but there are also some researchers who try to use endoscopic resection (Table 1). Endoscopic submucosal dissection (ESD) is a newer endoscopic treatment technology. As ESD technology develops, it plays an increasingly important role in treating early cancers and benign tumors of the digestive tract[2]. Some researchers have used gastroscopy and colonoscopy to perform ESD to treat duodenal and ileal lipomas[3,4]. However, application of ESD technology in the deep small intestine is rarely reported owing to the special anatomical structure of the small intestine, medical equipment limitations, and the lack of relevant experience among endoscopists. This study explored the clinical feasibility of ESD treatment for deep intestinal lipomas under single-balloon enteroscopy (SBE). The methods and cases are reported herein.
| Ref. | Age | Sex | Location | Tumor size | Treatment |
| Baskaran et al[18] | 55 | M | Ileum | N/A | Surgical resection |
| Baskaran et al[18] | 34 | M | Ileum | N/A | Surgical resection |
| Pezzoli et al[19] | 64 | M | Jejunum | N/A | Surgical resection |
| Pezzoli et al[19] | 44 | M | Ileum | N/A | Surgical resection |
| Chou et al[20] | 57 | M | Ileum | 3 cm × 1.5 cm × 1.5 cm | Surgical resection |
| Morimoto et al[3] | 62 | M | Ileum | > 3 cm | ESD with colonoscopy |
| Toya et al[21] | 79 | M | Jejunum | 3.5 cm × 1.7 cm | EMR with DBE |
Inclusion criteria: The inclusion criteria were: (1) Patients with small intestinal lipoma(s) admitted to the department of gastroenterology, Air Force Characteristic Medical Center of the PLA from November 2015 to September 2019; (2) Patients who had clinical symptoms such as repeated abdominal pain, abdominal distension, obstruction, and hemorrhage as well as patients in remission; (3) Lipoma base diameter was ≥ 1.5 cm, and the risks due to endoscopic mucosal resection (EMR) were expected to be high and (4) The patients and their families agreed to ESD treatment and provided informed consent.
Exclusion criteria: The exclusion criteria were: (1) Patients with severe basic diseases who could not tolerate anesthesia or endoscopic examination; (2) Complete intestinal obstruction made intestinal preparation impossible; (3) Presence of lipoma hemorrhage with hemorrhagic shock or extraluminal lipomas; (4) No consent to ESD treatment was provided; and (5) Diameter of the lipoma base was < 1.5 cm, and the risks due to EMR were expected to be low (could be treated by EMR and regular follow-up).
Equipment information: The following equipment was used: SBE (SIF-0260, Olympus), outer thimble (ST-SB1, Olympus), IT knife (KD-612U, Olympus), dual knife (KD-650U, Olympus), transparent cap (D-201-11804, Olympus), endoscopic injector (WS-2423PN, Wilson), argon plasma coagulation (APC2, ERBE), high-frequency electric knife (VID 300S, ERBE), hemostatic forceps (FD-411UR, Olympus), disposable metal clip (ROCC-D-26-230, Micro-Tech; Nanjing Co.), and instrument channel adaptor (MAJ-1606, Olympus; used to connect to the biopsy channel to temporarily replace the auxiliary water supply function of the balloon-assisted endoscopy as it does not have a special channel for the water supply).
Steps and key techniques of ESD with SBE for treating intestinal lipomas: Preoperative assessment: The general condition of the patients should be comprehensively evaluated, and the coagulation function should be corrected before treatment in patients with coagulation disorders.
As with routine SBE, once the SBE reaches the lesion, the outer thimble should be moved to the top and then pulled back with the SBE after inflating the balloon so that the lens can freely approach the lesion. After completing these steps, the endoscopist can begin the ESD as the assistant fixes the outer thimble.
Lesion marking: Marking is unnecessary if the lesion is prominent, and the basal area has a clear boundary; however, boundaries that are unclear or undetermined post injection should be marked accordingly.
Submucosal injections are performed with 1:4 diluted sodium hyaluronate. After sufficient submucosal injection, the mucosa is incised after being well elevated.
Mucosal incision and lipoma dissection: The mucosa is cut in an arc as close to the tumor edge as possible, with the gravity-side mucosa being incised first to enable more easily exposing the yellowish mass under the mucosa. During dissection, a clear field of vision must be maintained as far as possible to pretreat the large blood vessels as far as possible to avoid perioperative bleeding. Multiple submucosal injections can ensure sufficient separation of the lesion and muscle layer. Dissection should not be performed too quickly.
Postoperative wound management: After completely removing the tumor, the wound surface should be carefully examined. For visible bleeding or tiny blood vessels, coagulation treatments, such as argon plasma coagulation and thermal tweezers, can be used. If the muscle layer is damaged or perforated during the operation, the wound surface must be closed with metal clips. Postoperative perforation or bleeding is difficult to correct endoscopically because of the anatomy of the small intestine; therefore, the wound surface should be sutured as much as possible.
Antibiotics were routinely administered within 48 postoperative hours to prevent wound infection. Patients were given intravenous nutrition for 3-5 d, then the diet was gradually restored according to recovery status.
Case 1: A 44-year-old male patient was admitted to our hospital because of repeated right lower abdominal pain for 5 years.
Case 2: A 62-year-old male patient was admitted to our hospital because of multiple masses in his small intestine and one of them caused intussusception.
Case 1: The patient underwent colonoscopy due to abdominal pain and showed a 3 cm × 4 cm yellowish mass in the terminal ileum 20 cm from the ileocecal valve, with a smooth surface. The mass could slide under the mucosa and occupied nearly the entire intestinal cavity.
Case 2: The patient underwent emergency surgical treatment in August 2017 because of small bowel intussusception. In addition to small intestinal intussusception, preoperative abdominal computed tomography (CT) examination revealed multiple fat-density mass shadows in his small intestine. Postoperative pathology showed that the intussusception was caused by a 5.2 cm × 4.5 cm × 3.1 cm intestinal submucosal lipoma. The patient continued to experience repeated abdominal distention and abdominal pain post surgery.
Case 1: The patient had no gastrointestinal solid tumor before.
Case 2: Abdominal CT showed multiple pancreatic lipomas.
Case 1: The patient had a flat and soft abdomen without tenderness, rebound pain, or muscle tension.
Case 2: Longitudinal scar about 10 cm long was seen in the center of the abdomen, with no other positive abdominal signs.
Case 1: The results showed that no significant abnormality was found in tumor markers or other routine examinations.
Case 2: The results showed that no significant abnormality was found in tumor markers or other routine examinations.
Case 1: CT imaging showed a 2.3 cm × 1.3 cm fat density, with clear boundaries in the terminal ileum, causing the intestinal cavity to narrow. Contrast-enhanced scanning showed no enhancement.
Case 2: Abdominal CT in November 2018 showed multiple fat-density masses remaining in the small intestine with intestinal cavity dilatation, with the largest mass being approximately 5 cm in diameter.
Pathology confirmed that the small intestinal tumors were submucosal lipomas.
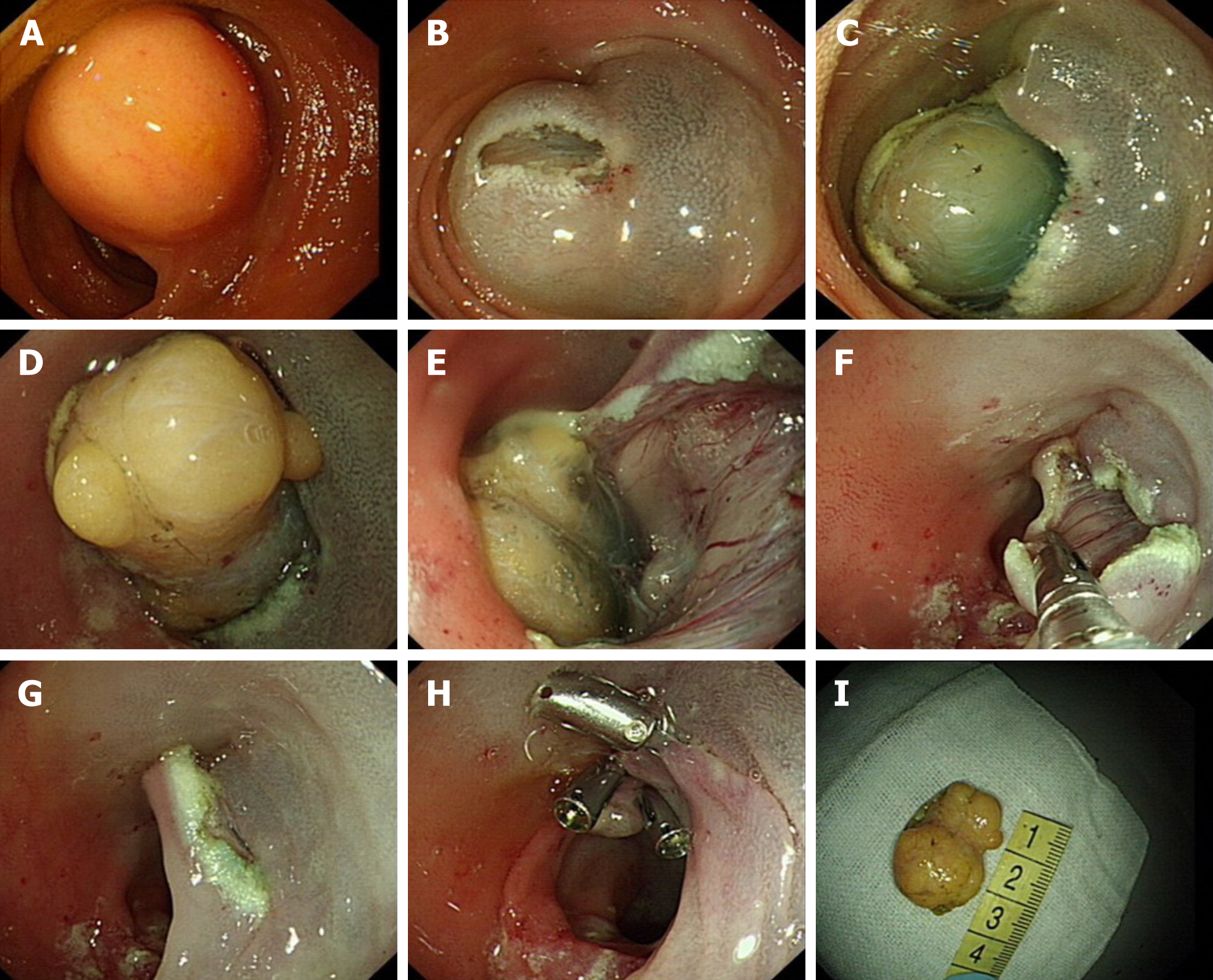
Case 1: After communicating with the patient and his family, who expressed their willingness to try endoscopic treatment, we performed the ESD with SBE transanally in November 2015. The tumor was dissected successfully with no bleeding or muscle layer damage and the wound surface was sutured with three metal clips (Figure 1).
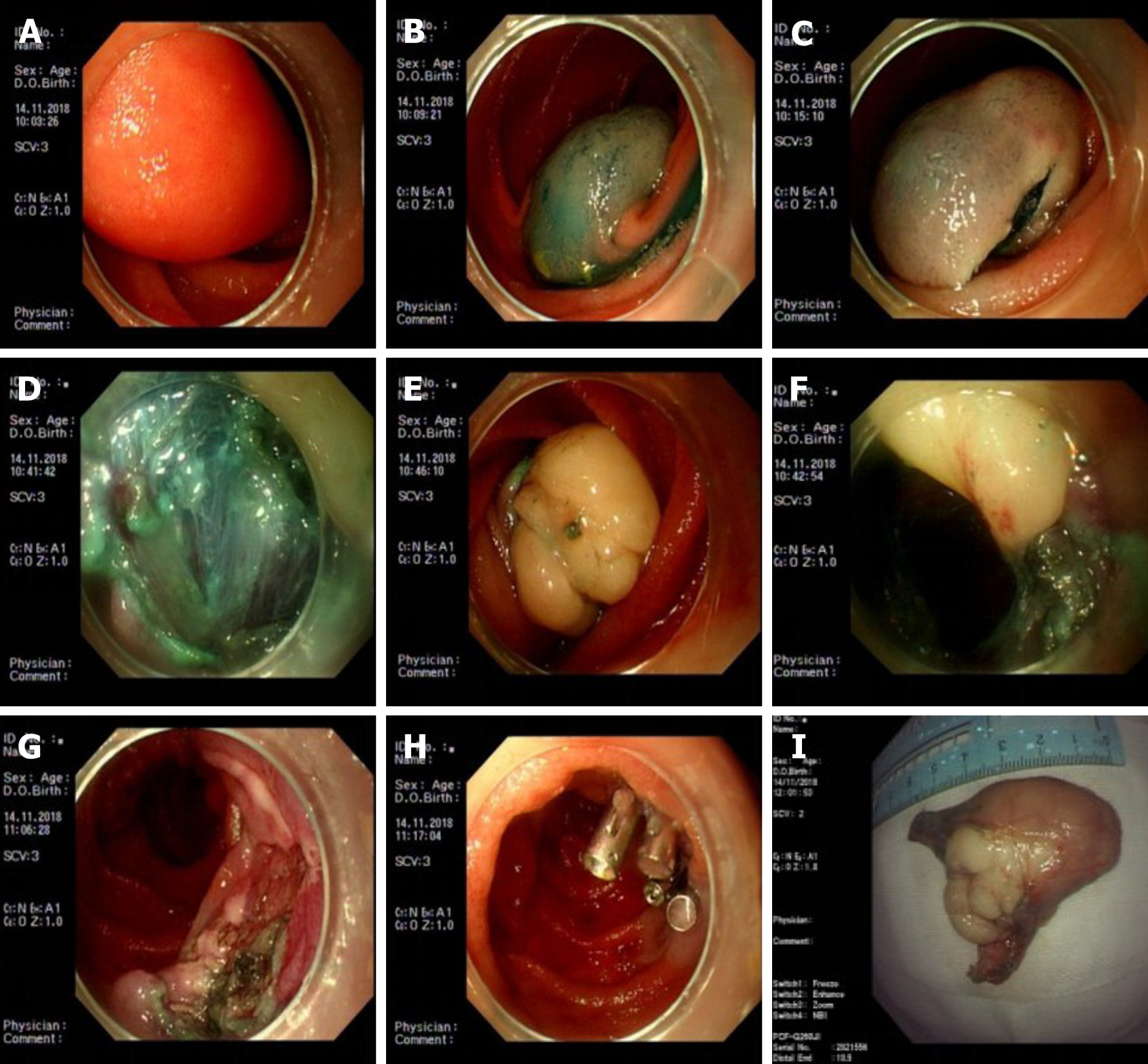
Case 2: Three SBEs were performed in our hospital. In November 2018, the first SBE showed a 3.0 cm × 6.0 cm yellowish mass in the jejunum 80 cm from the pylorus, with a smooth mucosal surface, accounting for 2/3 of the intestinal cavity. we communicated with the family and resected the tumor by ESD under SBE. The patient’s abdominal distension and pain were significantly relieved after the ESD. After a month of recovery and communication with family members, we continued the endoscopic treatment of the remaining lipoma. To ensure the effectiveness and safety of the treatment, we only treated one site at a time. In December 2018, the second SBE showed a 3.0 cm × 3.0 cm yellowish mass with a smooth mucosal surface 140 cm from the pylorus and we performed ESD again. After two treatments, the patient’s clinical symptoms were mostly relieved, but a slightly large intestinal lipoma remained. The patient chose clinical observation, but the abdominal pain reoccurred 9 mo later. Thus, we conducted the third SBE examination in September 2019. An anastomosis was observed in the jejunum 180 cm from the pylorus. The 3 cm × 3 cm lipoma was 320 cm from the pylorus and had the same smooth mucosal surface, accounting for approximately 1/2 of the intestinal cavity. No bleeding or muscle layer damage occurred, and no postoperative bleeding, delayed perforation, or other complications occurred during three ESD treatments (Figure 2).
The two patients underwent four ESDs with SBE; one patient underwent 3 ESDs for multiple small intestinal lipomas, all of which were completely removed, with no intraoperative or postoperative bleeding, perforation, stenosis, or other complications. Neither of the two patients had obvious postoperative discomfort, and the previous abdominal discomfort disappeared. The patients were followed for 3–6 mo postoperatively. CT and/or SBE showed good local mucosal recovery and no tumor recurrence (Table 2).
| Patient | Date of treatment | Site of lipoma | Size of lipoma (cm) | Treatm ent method | Clinical symptoms after treatment | Complications | Follow-up |
| No. 1 | November 2015 | Ileum 10 cm from the ileocecal valve | 3.0 cm × 2.0 cm × 2.0 cm | ESD | Abdominal pain disappeared | None | No recurrence or long-term complications |
| No. 2 | August 2017 | Jejunum 180 cm from the pylorus | 5.2 cm × 4.5 cm × 3.1 cm | Surgery | Repeated abdominal distention and abdominal pain | ||
| November 2018 | Jejunum 80 cm from the pylorus | 4.8 cm × 2.7 cm × 0.7 cm | ESD | Symptoms significantly relieved | |||
| December 2018 | Jejunum 140 cm from the pylorus | 2.4 cm × 1.7 cm × 1.7 cm | ESD | Symptoms basically relieved | |||
| September 2019 | Jejunum 320 cm from the pylorus | 2.0 cm × 2.0 cm × 1.0 cm | ESD | Symptoms disappeared |
Small intestinal lipomas are rare benign tumors of the digestive tract, originating from submucosal or subserosal adipose tissue. Lipomas have clear boundaries and can be divided into intraluminal, extraluminal, intramural, and mixed types according to lesion location and growth mode. Intraluminal type is the most common and has a good prognosis[5]. The cause of the disease is unclear and may be related to systemic fat metabolism disorders, Whipple disease, intestinal malnutrition, and inflammatory stimulation[6]. Small intestinal lipomas are mostly asymptomatic and can be found accidentally during an endoscopic examination or surgery and generally do not require special treatment. Large lipomas (≥ 2 cm) often have complications such as intussusception and intestinal obstruction and can cause related clinical symptoms. Their clinical manifestations depend on the tumor size, location, and morphology and include abdominal distension, abdominal masses, and gastrointestinal bleeding. Surgery, including laparotomy and laparoscopic-assisted resection, is the first-choice treatment for symptomatic tumors[7-9]. Gotoda, a Japanese scholar, reported the first case of successful resection of a gastrointestinal mass > 2 cm in diameter via ESD in 1999[10]. As ESD technology gradually improves, it is being widely used in clinical practice for its advantages of less surgical trauma, fast postoperative recovery, and low cost. ESD is a reliable and minimally invasive method for treating early and benign tumors in the esophagus, stomach, and colon, thus preventing many unnecessary surgeries.
Endoscopic treatment in the deep intestine is technically difficult because the small intestinal wall is thin, the mucosal blood supply is abundant, the intestinal tract is long and curved, and the intestinal cavity space is narrow. The duodenum is one of the most difficult areas to implement ESD[11]. The clinical application of balloon-assisted endoscopy (including double-balloon and single-balloon colonoscopies) has enabled endoscopically treating small intestinal diseases[11-14]. The authors’ institution is exploring several new technologies for early diagnosing and treating small intestinal diseases by balloon-assisted endoscopy, such as resection of large polyps, metal stent placement, benign stricture dissection, and endoscopic hemostasis of hemangiomas and other hemorrhagic diseases. Our institution has accumulated experience in piecemeal resection and en bloc resection via EMR of large small intestinal polyps in patients with Peutz–Jeghers syndrome[15,16].
Symptomatic small intestinal lipomas requiring treatment are usually large. Whether minimally invasive treatment can be performed under endoscopy requires clinical verification. Small intestinal lipomas are usually located in the submucosa and differ from small intestinal polyps derived from the mucosal layer. After submucosal injection, the degree of the polypectomy snare is difficult to control; if the snare is too shallow, it may not resect the whole tumor, and if it is too deep, it may cause intraoperative or postoperative perforation. ESD may be more effective because EMR is difficult and carries a high risk. A case report of ESD for a small intestinal lipoma in the terminal ileum via colonoscopy preliminarily demonstrated the feasibility of ESD in the small intestine[3]. Because the endoscopy did not destroy the small intestinal mucosa circumferentially, and the tumor surface covering the mucosa was retained, postoperative small intestinal stricture was theoretically unlikely to occur. Recent accumulating literature has demonstrated the feasibility of ESD in the difficult sections of the colon under balloon-assisted endoscopy, such as ESD in the liver region of the colon, ascending colon, cecum, and ileocecal valve[17]. Our institution has performed ESD in the difficult portions of the colon via balloon-assisted endoscopy and preliminarily verified its feasibility and safety. However, ESD for benign tumors of the deep intestine is rarely reported. Because both double-balloon endoscopy and SBE have no auxiliary water supply function, and because the long body of the small intestine is usually tortuous near the lesion, the treatment is more difficult. Therefore, the operator must retain superior endoscopic control ability.
ESD under balloon-assisted endoscopy has a high block resection rate for large small intestinal lipomas with no uncontrolled serious complications, which thus has high clinical application feasibility and safety. However, ESD treatment of small intestinal lipomas via balloon-assisted endoscopy confers technical difficulties; therefore, the endoscopist must master the operative skills needed for balloon-assisted endoscopy and be experienced in performing ESD. We recommend that highly experienced departments apply and promote this technology to provide a new alternative method for patients with small intestinal lipomas.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kawabata H, Sugimoto M S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Wilson JM, Melvin DB, Gray G, Thorbjarnarson B. Benign small bowel tumor. Ann Surg. 1975;181:247-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Zheng X, Zuo N, Lin H, Zheng L, Ni M, Wu G, Chen J, Zhuo S. Margin diagnosis for endoscopic submucosal dissection of early gastric cancer using multiphoton microscopy. Surg Endosc. 2020;34:408-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Morimoto T, Fu KI, Konuma H, Izumi Y, Matsuyama S, Ogura K, Miyazaki A, Watanabe S. Peeling a giant ileal lipoma with endoscopic unroofing and submucosal dissection. World J Gastroenterol. 2010;16:1676-1679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Huang WH, Peng CY, Yu CJ, Chou JW, Feng CL. Endoloop-assisted unroofing for the treatment of symptomatic duodenal lipomas. Gastrointest Endosc. 2008;68:1234-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Tahlan RN, Garg P, Bishnoi PK, Singla SL. Mesenteric lipoma: an unusual cause of small intestinal volvulus. Indian J Gastroenterol. 1997;16:159. [PubMed] |
| 6. | Nincheri Kunz M, Evaristi L, Spadoni R, Cozzani R, Valle O, Bacigalupo B. [Lipoma of the small intestine as a rare cause of intestinal occlusion]. Minerva Chir. 1994;49:859-865. [PubMed] |
| 7. | Yatagai N, Ueyama H, Shibuya T, Haga K, Takahashi M, Nomura O, Sakamoto N, Osada T, Yao T, Watanabe S. Obscure gastrointestinal bleeding caused by small intestinal lipoma: a case report. J Med Case Rep. 2016;10:226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Oda I, Gotoda T, Hamanaka H, EguchIi T, Saito Y, Matsuda T, Bhandari P, Emura F, Saito D, Ono H. Endoscopic submucosal dissection for early gastric cancer: Technical feasibility, operation time and complications from a large consecutive series. Dig Endosc. 17:54-58. [RCA] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 333] [Article Influence: 16.7] [Reference Citation Analysis (2)] |
| 9. | Gotoda T. Endoscopic resection of early gastric cancer: the Japanese perspective. Curr Opin Gastroenterol. 2006;22:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Gaspar JP, Stelow EB, Wang AY. Approach to the endoscopic resection of duodenal lesions. World J Gastroenterol. 2016;22:600-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 11. | Yamamoto H, Sugano K. A new method of enteroscopy--the double-balloon method. Can J Gastroenterol. 2003;17:273-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Yamamoto H, Kita H, Sunada K, Hayashi Y, Sato H, Yano T, Iwamoto M, Sekine Y, Miyata T, Kuno A, Ajibe H, Ido K, Sugano K. Clinical outcomes of double-balloon endoscopy for the diagnosis and treatment of small-intestinal diseases. Clin Gastroenterol Hepatol. 2004;2:1010-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 427] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 13. | May A, Nachbar L, Ell C. Double-balloon enteroscopy (push-and-pull enteroscopy) of the small bowel: feasibility and diagnostic and therapeutic yield in patients with suspected small bowel disease. Gastrointest Endosc. 2005;62:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 259] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 14. | Tsujikawa T, Saitoh Y, Andoh A, Imaeda H, Hata K, Minematsu H, Senoh K, Hayafuji K, Ogawa A, Nakahara T, Sasaki M, Fujiyama Y. Novel single-balloon enteroscopy for diagnosis and treatment of the small intestine: preliminary experiences. Endoscopy. 2008;40:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 209] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 15. | Zhang YF, Ning SB, Li BR, Zhang J, Li J, Tang J, Zhu M, Jin XW, Zhao Q, Mao GP. Combined use of single-balloon enteroscope and colonoscope for self-expandable metal stent placement in patients with malignant small intestinal obstruction: a single-center comparative clinical observation. J Huazhong Univ Sci Technolog Med Sci. 2017;37:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Ning S, Zhang Y, Zu Z, Mao X, Mao G. Enteroscopic sclerotherapy in blue rubber bleb nevus syndrome. Pak J Med Sci. 2015;31:226-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Yamashina T, Hayashi Y, Sakamoto H, Yano T, Miura Y, Shinozaki S, Sunada K, Kawarai Lefor A, Yamamoto H. Balloon-assisted endoscopy facilitates endoscopic submucosal dissection of difficult superficial proximal colon tumors. Endoscopy. 2018;50:800-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Baskaran V, Patnaik PK, Seth AK, Dogra R, Chaudhry R. Intestinal lipoma: a rare cause of lower gastrointestinal haemorrhage. Trop Gastroenterol. 2003;24:208-210. [PubMed] |
| 19. | Pezzoli A, Pennazio M, Fusetti N, Simone L, Zelante A, Cifalà V, Sprujevnik T, Carella A, Gullini S. Occult intestinal haemorrhage due to lipoma of the small bowel detected with the combined use of the new endoscopic techniques. A report of two cases. Dig Liver Dis. 2008;40:306-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Chou JW, Feng CL, Lai HC, Tsai CC, Chen SH, Hsu CH, Cheng KS, Peng CY, Chung PK. Obscure gastrointestinal bleeding caused by small bowel lipoma. Intern Med. 2008;47:1601-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Toya Y, Endo M, Orikasa S, Sugai T, Matsumoto T. Lipoma of the small intestine treated with endoscopic resection. Clin J Gastroenterol. 2014;7:502-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |