Published online Mar 6, 2021. doi: 10.12998/wjcc.v9.i7.1619
Peer-review started: November 10, 2020
First decision: December 8, 2020
Revised: December 13, 2020
Accepted: December 22, 2020
Article in press: December 22, 2020
Published online: March 6, 2021
Processing time: 110 Days and 22.3 Hours
Previous studies have suggested that the costimulatory molecule 4-1BB plays pivotal roles in regulating immunity during chronic viral infection. However, up to now, there are few studies about 4-1BB in chronic hepatitis B (CHB).
To clarify this issue, we report our comprehensive study results on the expression levels of 4-1BB in patients with CHB.
From September 2018 to June 2019, a total of 64 patients with CHB were recruited from the Department of Hepatology, The First Hospital of Jilin University. Peripheral blood samples were collected from 52 treatment-naïve and 12 entecavir-treated patients with CHB as well as 37 healthy donors (including 24 healthy adults and 13 healthy children). The levels of soluble 4-1BB (s4-1BB) in plasma were measured by ELISA. 4-1BB mRNA expression in peripheral blood mononuclear cells was detected by real-time quantitative PCR.
The s4-1BB levels in the plasma of patients with CHB were significantly higher than those in healthy adults (94.390 ± 7.393 ng/mL vs 8.875 ± 0.914 ng/mL, P < 0.001). In addition, the s4-1BB level in plasma was significantly increased in patients with a higher viral load and a disease flare up. However, there were no significant differences between treatment-naïve and entecavir-treated patients. Interestingly, among treatment-naïve patients with CHB, the levels of s4-1BB in plasma had a significant positive correlation with hepatitis B surface antigen, hepatitis B virus DNA, hepatitis B e antigen, and triglyceride levels (r = 0.748, P < 0.001; r = 0.406, P = 0.004; r = 0.356, P = 0.019 and r = -0.469, P = 0.007, respectively). The 4-1BB mRNA expression was higher in the peripheral blood mononuclear cells of patients with CHB than in the peripheral blood mononuclear cells of healthy adults, but the difference was not statistically significant.
These results suggest that the levels of s4-1BB may be associated with pathogenesis of hepatitis B virus and therefore may be a promising biomarker for disease progression.
Core Tip: Over 240 million people all round the world are chronically infected with hepatitis B virus, resulting in > 1 million deaths per year. Previous studies have suggested that the costimulatory molecule 4-1BB plays a pivotal role in regulating immunity during chronic viral infection. However, up to now, there is no study about 4-1BB expression in chronic hepatitis B to clarify the role of 4-1BB in the process of chronic hepatitis B clearly. Here, we report our comprehensive study on the expression levels of soluble 4-1BB in plasma and 4-1BB mRNA in peripheral blood mononuclear cells to further explain it.
- Citation: Zhan MR, Gao XZ, Wang C, Peng F, Wang XM, Xu HQ, Niu JQ. Elevated soluble 4-1BB is associated with serum markers of hepatitis B virus in patients with chronic hepatitis B. World J Clin Cases 2021; 9(7): 1619-1630
- URL: https://www.wjgnet.com/2307-8960/full/v9/i7/1619.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i7.1619
Hepatitis B virus (HBV) infection is a global health problem, and over 240 million people worldwide are chronically infected with HBV, resulting in > 1 million deaths per year from cirrhosis and liver cancer[1]. Infants clear HBV at much lower rates than adults according to a report that infection persists in approximately 90% of infants infected at birth, and adult patients who were infected before age five represent the major global reservoir[2,3]. The interaction between HBV and host immune cells is crucial for the process of HBV persistence and clearance. Immune regulation is one of the most important factors of the antiviral effect, and an HBV functional cure can be achieved by properly orchestrated activation of antiviral immunity[4-6]. It is well known that T cells play an important role in the host immune response to HBV infection[6-8]. T cell activation requires not only antigen receptor signaling but also a second or costimulatory signal to provide contextual information[9].
4-1BB, also named CD137, belonging to the tumor necrosis factor receptor superfamily, is an inducible costimulatory receptor expressed on activated T and natural killer cells[10]. The interaction of 4-1BB with its ligand 4-1BBL, which is constitutively expressed on a fraction of dendritic cells and inducible mainly on activated monocytes, macrophages, B cells, and a small fraction of T cells[11,12], provides a costimulatory signal to enhance the proliferation and cytolytic activity of T cells[13,14] leading to enhanced T cell responses against viral infection in animal models[15-17]. There were reports showing that 4-1BBL or 4-1BB deficiency impairs the primary CD8+ T cell response during acute infection with lymphocytic choriomeningitis virus[16,18], vesicular stomatitis virus[19], and some influenza strains[20]. In addition, the 4-1BB costimulatory pathway affects CD4+ T cell responses to mouse hepatitis virus 68[21] and HSV-1[22], which cause latent infections. Furthermore, studies have reported that activating CD137 signaling and blockade of programmed death-1 increase the responses of intrahepatic HBV-specific T cells and circulating hepatitis C virus-specific T cells[23], and 4-1BB signal enhancement inhibits hepatitis B virus replication in a noncytolytic manner[24]. These results indicate that 4-1BB signaling plays an important role in the process of viral infection and certain inflammatory responses.
Soluble forms of 4-1BB are generated by differential splicing and seem to be secreted by T cells and able to antagonize the activities of mouse 4-1BB (m4-1BB), thereby reducing T cell activation[25-27]. Soluble 4-1BB (s4-1BB) is detectable in patients with inflammatory diseases. Elevated levels of s4-1BB have been detected in the sera of patients with rheumatoid arthritis, systemic sclerosis, multiple sclerosis, systemic lupus erythematosus, and Behcet’s disease[28-31]. Additionally, s4-1BB is increased in patients with acute pancreatitis and is associated with subsequent complications[32].
However, there have been few studies on the expression of 4-1BB in patients with CHB. In this study, the soluble form and mRNA level of 4-1BB in peripheral blood were analyzed to determine whether the costimulatory molecule is aberrantly produced in this disease and to reveal whether 4-1BB is also age-dependent, similar to OX40[33], to further reveal the significance of 4-1BB in CHB.
A total of 64 patients with chronic HBV infection were recruited at the Department of Hepatology, The First Hospital of Jilin University from September 2018 to June 2019. The diagnosis and phase classification of chronic HBV infection were based on the EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. Patients who had a history of HBV infection for > 6 mo and had no treatment with any nucleos(t)ide analogs or immune regulatory agents, including interferon (IFN), at study entry were included [except for the 12 entecavir (ETV)-treated patients]. Patients with alcoholic liver disease, autoimmune disease, malignancy, or serious illness of other systems were excluded. All patients tested negative for human immunodeficiency virus, hepatitis C virus, hepatitis E virus, and hepatitis delta virus. Thirty-seven age- and sex-matched healthy donors (including adults and children) were enrolled as controls (Table 1). Informed consent was obtained from each subject, and the study protocol was approved by the local medical ethics committee of The First Hospital of Jilin University in accordance with the guidelines of the Declaration of Helsinki.
| Feature | CHB, n = 64 | Healthy donors, n = 37 | |||||
| Treatment naïve, n = 52 | ETV treated, n = 12 | P1 value | Healthy adults, n = 24 | Healthy children, n = 13 | P2 value | P3 value | |
| Age in yr | 43.23 ± 11.15 | 42.42 ± 11.27 | 0.821 | 45.58 ± 11.29 | 4.85 ± 3.71 | < 0.001 | 0.397 |
| Sex, male, as n (%) | 37 (71.15) | 7 (58.33) | 0.388 | 15 (62.50) | 9 (69.23) | 0.682 | 0.451 |
| ALT in U/L | 303.61 ± 511.12 | 28.09 ± 12.51 | < 0.001 | 38.77 ± 9.48 | 13.05 ± 4.28 | < 0.001 | < 0.001 |
| AST in U/L | 176.37 ± 269.94 | 27.37 ± 24.17 | < 0.001 | 26.98 ± 7.12 | 32.88 ± 8.63 | 0.057 | < 0.001 |
| GGT in U/L | 136.863 ± 274.897 | 33.700 ± 33.330 | 0.002 | 24.059 ± 24.006 | 9.462 ± 2.218 | 0.303 | < 0.001 |
| ALP in U/L | 91.877 ± 33.706 | 66.320 ± 16.710 | 0.006 | 68.109 ± 21.576 | 249.692 ± 61.245 | 0.003 | 0.002 |
| TBIL in μmol/L | 46.09 ± 88.60 | 16.78 ± 10.85 | 0.294 | 13.45 ± 5.91 | 5.91 ± 3.63 | 0.002 | 0.047 |
| BUN in mmol/L | 5.70 ± 1.84 | - | - | 5.46 ± 1.45 | 3.94 ± 0.68 | < 0.001 | 0.764 |
| SCr in μmol/L | 66.50 ± 19.72 | - | - | 57.36 ± 13.08 | 29.47 ± 11.08 | < 0.001 | 0.042 |
| HBsAg in IU/mL | 10921.33 ± 17128.93 | 4024.78 ± 3602.66 | 0.460 | - | - | - | - |
| HBV DNA as log10 IU/mL | 5.74 ± 1.98 | 1.28 ± 0.68 | < 0.001 | - | - | - | - |
| HBeAg, positive, as n (%) | 29 (55.77) | 5 (41.67) | 0.378 | - | - | - | - |
| TC in mmol/L | 4.055 ± 1.100 | - | - | - | - | - | - |
| TG in mmol/L | 1.40 ± 0.84 | - | - | - | - | - | - |
| HDL in mmol/L | 1.26 ± 0.52 | - | - | - | - | - | - |
| LDL in mmol/L | 2.38 ± 0.79 | - | - | - | - | - | - |
| IFNα in pg/mL | 60.60 ± 39.43 | 75.23 ± 33.69 | 0.115 | - | - | - | - |
| IFNγ in pg/mL | 18.25 ± 19.46 | 15.86 ± 7.56 | 0.389 | - | - | - | - |
| IL-12 in pg/mL | 13.80 ± 11.01 | 15.97 ± 7.01 | 0.247 | - | - | - | - |
| IP-10 in pg/mL | 3523.89 ± 3095.67 | 892.17 ± 4396.24 | 0.001 | - | - | - | - |
| TNFα in pg/mL | 40.68 ± 19.13 | 26.08 ± 11.41 | 0.005 | - | - | - | - |
Peripheral blood mononuclear cells (PBMCs) were freshly isolated from the peripheral blood of patients with CHB and healthy individuals by Ficoll (GE Healthcare, Sweden) density gradient separation and were analyzed by real-time quantitative PCR for 4-1BB mRNA expression. Paired plasma was collected and immediately stored at -80 °C until use.
The levels of hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) were measured using a Roche COBAS 411 Immunoassay System (Roche Diagnostics, Grenzach, Germany). Serum HBV DNA levels were measured using the COBAS AmpliPrep/COBAS TaqMan assay (Roche Molecular Diagnostics, Grenzach, Germany) with a 15 U/mL lowest detection limit. Liver function, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, γ-glutamyl transferase, and alkaline phosphatase, was detected using a Biochemistry Automatic Analyzer (Roche Diagnostics). In addition, the serum was regularly subjected to measurements of kidney function and blood fat tests.
PBMCs were washed by centrifugation in RPMI 1640 medium after 24 h of culture and subsequently resuspended in cold phosphate-buffered saline containing 2.5% fetal bovine serum at a cell density of 1 × 107/mL. CD4+ and CD8+ T cells were prepared from freshly isolated PBMCs by using the magnetic cell sorting separation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of CD4+/CD8+ T cells was 95%-98% as determined by flow cytometry using specific antibodies: CD4-PE (BD Biosciences; Cat 555347) and CD8-APC (BD Biosciences; Cat 555369). Total RNA was isolated from cell pellets using an RNeasy plus Mini Kit (Qiagen, Hilden, Germany). RNA was stored at -80 °C until use.
First-strand cDNA synthesis was performed using a PrimeScript™ RT reagent Kit with gDNA Eraser Kit (Takara, Japan) with random hexamers as the primers. mRNA expression of the gene encoding 4-1BB mRNA was determined by real-time PCR using TB Green™ Premix Ex Taq™ II according to the manufacturer’s protocol (Takara, Japan). The following PCR primer sequences were used: forward: 5’- AGCTGTTACAACATAGTAGCCAC-3’ and reverse: 5’- ATGGTGGTGAAGACG CCAGT-3’ for 4-1BB. Additionally, forward: 5’-GGACAGGGACTGCAAATCTGAT-3’ and reverse: 5’-ATGGTGGTGAAGACGC CAGT-3’ primers for GAPDH were used as an internal control to normalize target mRNA levels. In brief, real-time PCR amplification was carried out in a reaction volume of 25 μL containing 12.5 μL of 2X SYBR Green Premix Ex Taq II, 1 µL of each pair of primers (10 μmol/L) for the target gene or reference gene, 2 µL of template cDNA and RNase-free water up to 25 μL of reaction volume. Thermal conditions were initial denaturation at 95 °C for 3 min, followed by 40 cycles of denaturation at 95 °C for 5 s, primer-specific annealing and an extension step at 60 °C for 30 s. Melting curve analyses starting from 65 °C to 95 °C were performed after each run to confirm the presence of specific PCR products. All reactions were performed in duplicate on an Mx3000P/3005P detection system (Applied Biosystems). The threshold cycle (threshold cycle, Ct = 18) value was measured in accordance with the formula ΔCt = [Ct (target gene)] [Ct (reference gene)] and ΔΔCt = [ΔCt (Study Group)] - [ΔCt (control group)]. The 2-ΔΔCt was calculated to reflect the target gene expression at the study group level in comparison with the control group.
Concentrations of s4-1BB were measured quantitatively in plasma using ELISAs (Jingmei Biotechnology, Jiangsu, China) according to the established protocol. Plasma protein levels of IFNα, IFNγ, IL-12, IP-10, and TNFα were determined by the MILLIPLEX MAP Human Cytokine/Chemokine Magnetic Bead Panel - Immunology Multiplex Assay (Merck-Millipore, United States) according to the manufacturer’s instructions.
Statistical analysis was performed using the IBM SPSS statistic 21 software (Chicago, IL, United States). Clinical demographic data were presented as the mean ± SD. Data for the expression of 4-1BB was presented as the mean ± SE of the mean. For statistical analysis, differences in continuous variables between two independent samples were evaluated by the Mann-Whitney U test or a 2-sided Student’s t test according to the characteristics of the data. Dichotomous variables were compared by the χ2 test. The correlations between s4-1BB and detection markers were assessed by Spearman’s rank correlation test. The statistical software GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, United States) was used for graph creation. A P value < 0.05 was considered statistically significant. P values in the figures are shown as P < 0.05; P < 0.01; and P < 0.001.
A total of 64 patients with CHB and 37 healthy controls were included for analysis. All patients were Chinese. The demographic characteristics, such as age and sex, and clinical parameters, such as liver function tests, HBV-DNA load, inflammatory cytokines and so on, are presented in Table 1. There were no significant differences in age between the treatment-naïve group and ETV-treated group (P = 0.821) or the treatment-naïve group and healthy adult group (P = 0.397), except for the healthy adult group and healthy child group (P < 0.001). Sex had no significant differences according to the above three methods of comparison (P = 0.388, P = 0.451, and P = 0.682, respectively). The levels of ALT, AST, γ-glutamyl transferase, and alkaline phosphatase were higher in the treatment-naïve group than in the ETV-treated group and healthy adult group, as shown in Table 1. The level of HBV DNA was significantly decreased in the ETV-treated group compared to the treatment-naïve group. IP-10 and TNFα concentrations in the treatment-naïve group were significantly higher than those in the ETV-treated group (P = 0.001 and P = 0.005, respectively).
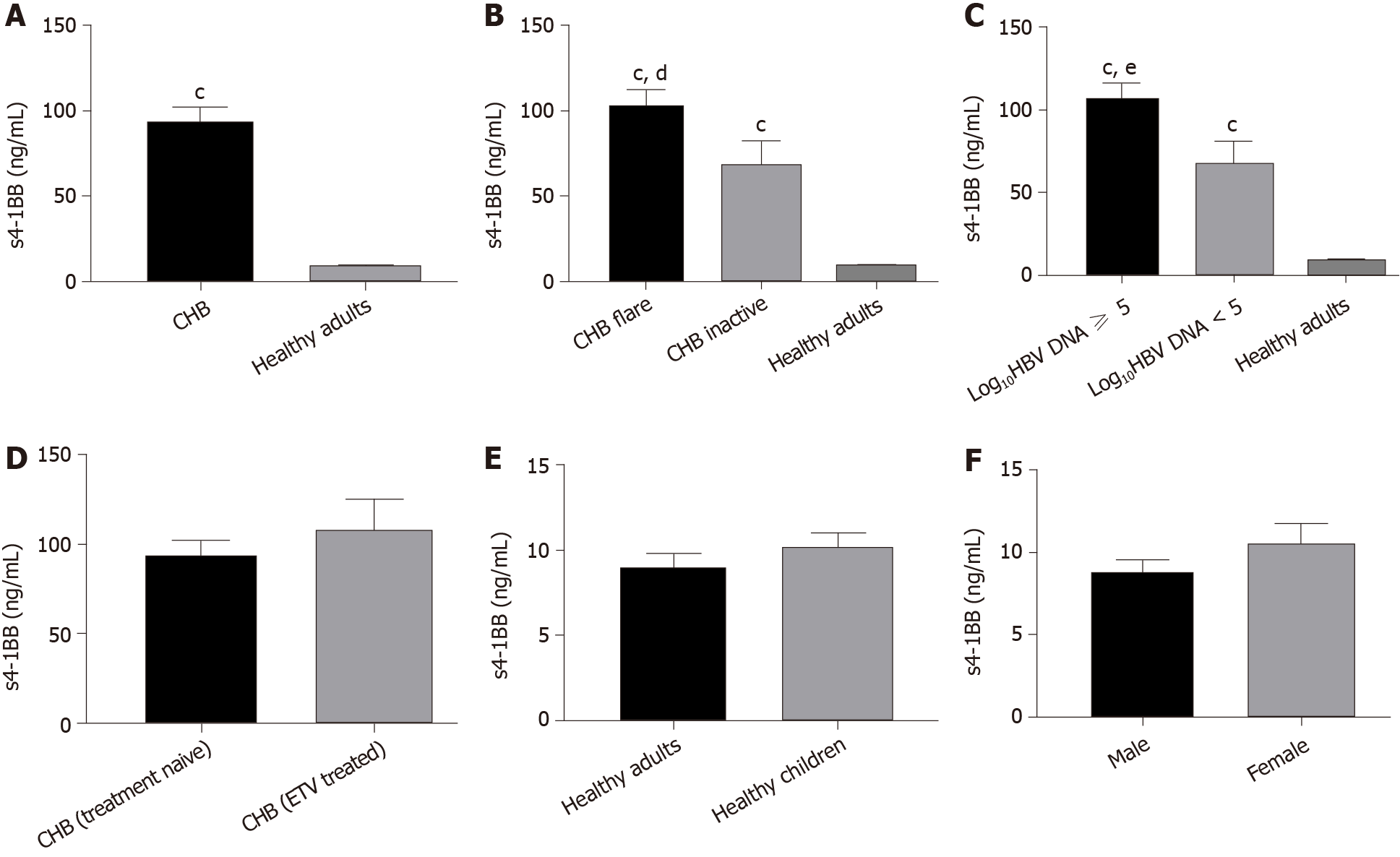
Previous studies have shown that s4-1BB exists in patients with inflammatory diseases. In the present study, plasma levels of s4-1BB were determined by ELISA. As shown in Figure 1A, the concentration of s4-1BB in the plasma of treatment-naïve patients with CHB was significantly higher than that of healthy adults (94.390 ± 7.393 ng/mL vs 8.875 ± 0.914 ng/mL, P < 0.001). Then, these patients with CHB were classified into two stage groups according to either disease flare or inactive and HBV load. The CHB flare group had the highest s4-1BB levels, followed by the CHB inactive group (103.731 ± 8.131 ng/mL vs 67.087 ± 14.640 ng/mL, P = 0.048), and the two groups were both higher than the healthy adult group (P < 0.001, and P < 0.001, respectively) (Figure 1B). Similarly, the high virus load group (log10HBV DNA > 5) had the highest s4-1BB levels, followed by the low virus load group (log10HBV DNA < 5) (107.249 ± 8.463 ng/mL vs 67.319 ± 13.143 ng/mL, P = 0.028), and the two groups were also higher than the healthy adult group (P < 0.001, and P < 0.001, respectively) (Figure 1C). In addition, we compared the s4-1BB levels in plasma between treatment-naïve patients with CHB and ETV-treated patients with CHB and found no significant difference. One study suggested that several costimulated molecules were lower at a young age, which may explain why infants clear HBV at much lower rates than adults[33]. Therefore, we collected plasma from children and measured the plasma levels of s4-1BB and found that there was no significant difference between the healthy adult group and the healthy children group (Figure 1E). Meanwhile, we analyzed the relationship between the s4-1BB concentration and sex and found no difference between the sexes (Figure 1F).
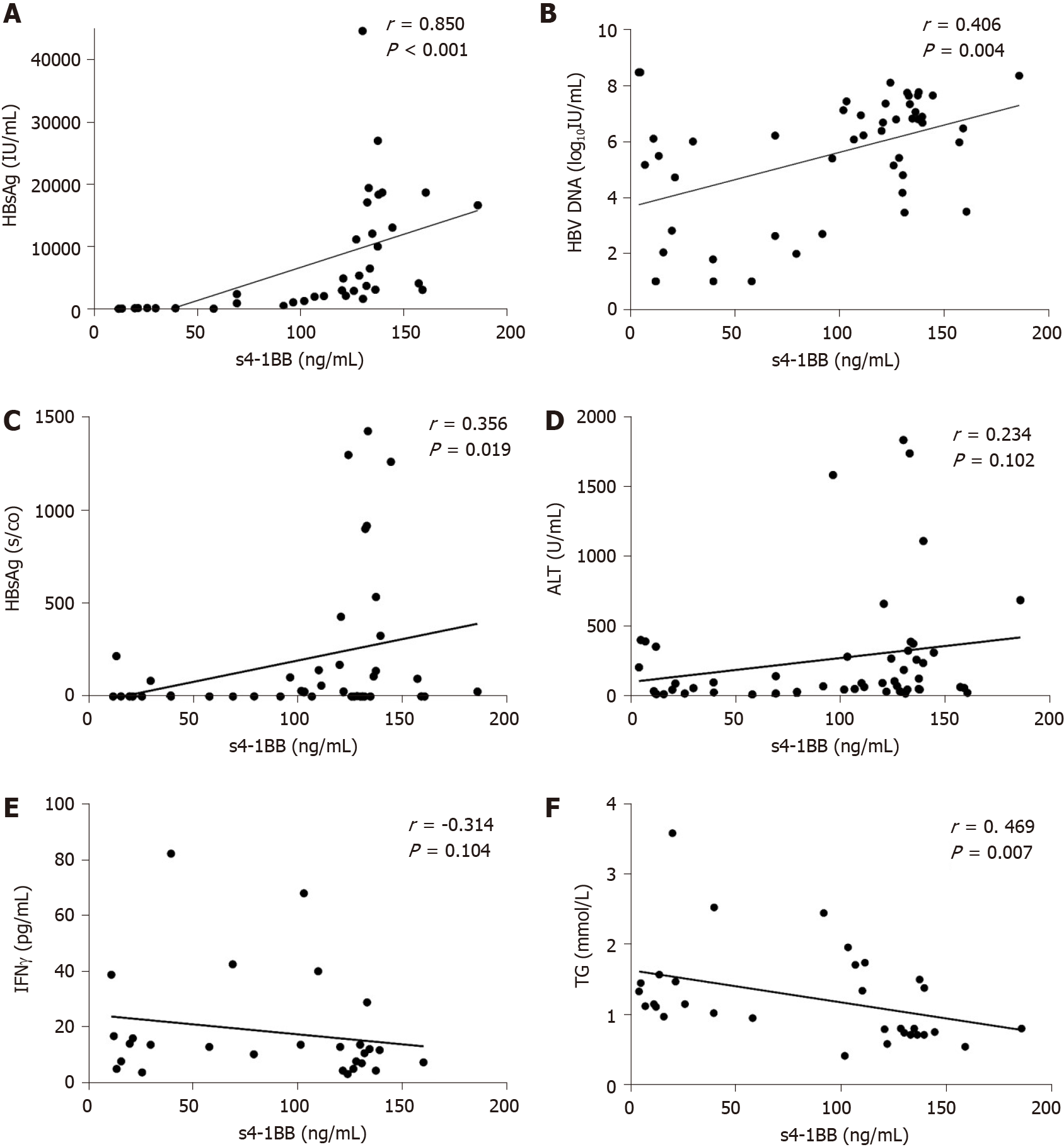
HBV markers in serum are important indicators of viral replication and activity. To explore whether s4-1BB is involved in the development of CHB, we analyzed the relationship between s4-1BB and HBV serological markers in the treatment-naïve patients with CHB (Table 2). We conducted a correlation analysis and found a positive correlation between s4-1BB expression and HBsAg (r = 0.748, P < 0.001), HBV DNA (r = 0.406, P = 0.004), and HBeAg (r = 0.356, P = 0.019). Patients with CHB with higher s4-1BB expression levels had higher HBsAg, HBV DNA, and HBeAg levels (Figure 2A-C). These data suggest that the expression of s4-1BB may correlate with HBV replication in patients with CHB. We evaluated the association between the s4-1BB levels and the inflammatory parameters in patients with CHB and found that there was no correlation between the s4-1BB concentration and inflammatory parameters, such as ALT and AST, and cytokines (IFNα, IFNγ, IL-12, IP-10, and TNFα) in plasma (Figure 2E and 2F). To evaluate whether s4-1BB was correlated with other parameters associated with disease fluctuation, we analyzed the relationship between s4-1BB and those parameters. We found that only triglyceride levels were negatively correlated with the s4-1BB level (r = -0.469, P = 0.007) (Figure 2D).
| s4-1BB | ||
| r | P value | |
| HBsAg in IU/mL | 0.748 | < 0.001 |
| HBV DNA as log10 IU/mL | 0.406 | 0.004 |
| HBeAg in s/co | 0.356 | 0.019 |
| ALT in U/L | 0.234 | 0.102 |
| AST in U/L | 0.165 | 0.246 |
| GGT in U/L | 0.076 | 0.595 |
| ALP in U/L | -0.065 | 0.651 |
| TBIL in µmol/L | 0.063 | 0.662 |
| BUN in mmol/L | -0.075 | 0.623 |
| SCr in µmol/L | -0.226 | 0.135 |
| WBC as × 109/L | -0.171 | 0.229 |
| HGB in g/L | -0.063 | 0.662 |
| PLT as × 109/L | -0.150 | 0.293 |
| INR | 0.344 | 0.014 |
| PTA, % | -0.353 | 0.012 |
| FBG in mmol/L | -0.002 | 0.988 |
| TC in mmol/L | -0.350 | 0.050 |
| TG in mmol/L | -0.469 | 0.007 |
| HDL in mmol/L | 0.148 | 0.481 |
| LDL in mmol/L | -0.360 | 0.077 |
| IFNα in pg/mL | -0.282 | 0.146 |
| IFNγ in pg/mL | -0.314 | 0.104 |
| IL-12 in pg/mL | -0.076 | 0.702 |
| IP-10 in pg/mL | 0.154 | 0.434 |
| TNFα in pg/mL | -0.031 | 0.874 |
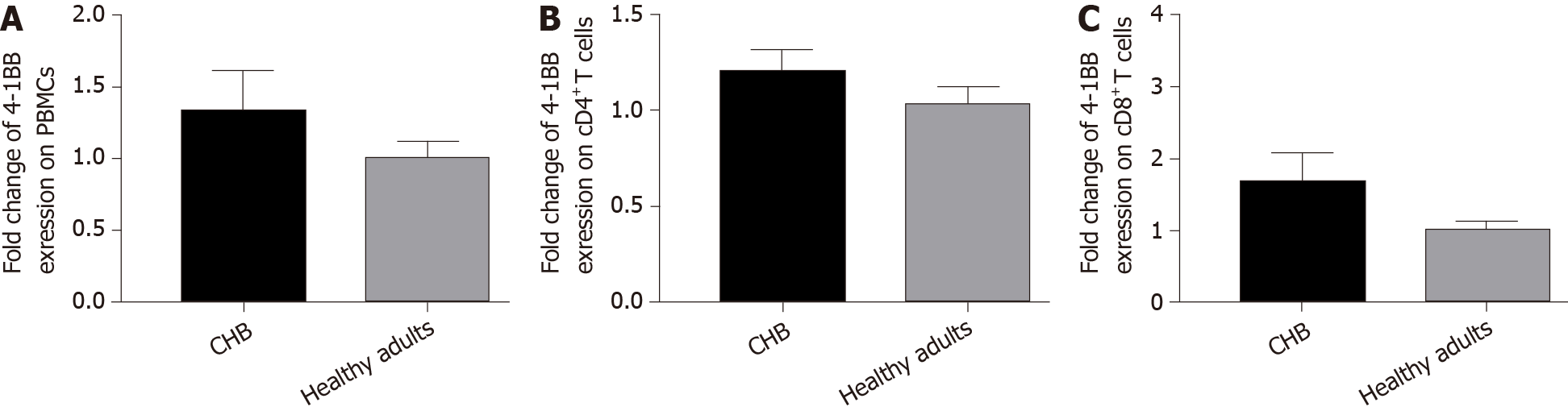
To investigate whether m4-1BB expression is related to CHB disease activity, we evaluated the 4-1BB mRNA levels of PBMCs, CD4+ T cells and CD8+ T cells in peripheral blood by real-time quantitative PCR after magnetic cell sorting and compared them in patients with CHB and healthy adults. We found that the 4-1BB mRNA expression levels in total PBMCs (Figure 3A), CD4+ T cells (Figure 3B), and CD8+ T cells (Figure 3C) of patients with CHB seemed to all be higher than the expression in healthy adults, but there was no statistical significance.
Chronic hepatitis B infection is a global health burden. However, current therapies such as Peg-interferon and nucleos(t)ide analogs cannot achieve a functional cure, and the development of immunological therapeutic strategies is urgently needed. Previous studies showed that HBV infection can be partially or completely cleared by the activating or blocking of immune checkpoints OX40[33,34] and programmed death-1[35,36]. However, the role of costimulatory molecule 4-1BB in patients with CHB is not clear. Here, we detected the expression of the soluble form of 4-1BB and 4-1BB mRNA in PBMCs in patients with CHB and analyzed the correlation with disease activity to determine their clinical significance in CHB for the first time to provide more evidence for 4-1BB-targeted therapies for curing HBV.
Our results showed that s4-1BB was associated with chronic HBV infection, as demonstrated by the significantly higher s4-1BB levels in treatment-naïve patients with CHB than in healthy adults. Previous studies have shown that the activity of immune cells varies with the disease status of CHB[33,34]. When taking HBV DNA into consideration, we found that patients with a high viral load had higher s4-1BB levels. s4-1BB is thought to compete with mCD137 for binding to the CD137 ligand, thereby counteracting the m4-1BB-mediated inhibitory effects on immune cells[25]. It is well known that the persistent exposure of T cells to high viral loads is a key determinant of functional T cell impairment and inhibition, including the tolerogenic effect of the liver environment[4]. Therefore, the elevated s4-1BB level in patients with a high viral load supported this hypothesis. In addition, the s4-1BB levels were higher in patients with CHB with disease flares, indicating that s4-1BB may be an indicator of liver inflammation. However, compared with treatment-naïve patients, ETV-treated patients had a similar level of s4-1BB. It suggested that ETV does not improve the function of T cells although it can effectively inhibit HBV. More evidence is needed to address these issues.
The correlation between serum s4-1BB and other clinical parameters provided additional cues regarding the role of s4-1BB in CHB disease progression. Our data demonstrated that the s4-1BB level positively correlated with HBV markers (HBsAg, HBeAg, and HBV DNA) in treatment-naïve patients with CHB. However, there was no correlation between s4-1BB levels and ALT, AST, total bilirubin, and other liver injury inflammatory values. Combining these results, it appears that s4-1BB may be an important factor related to viral replication. Interestingly, we found that the s4-1BB level was negatively correlated with triglyceride levels. A previous study suggested that stimulation of CD137 by agonistic antibodies lowers liver fat storage and most likely circulating levels of triglycerides[37]. Increased soluble CD137 antagonizes CD137 functions and may eventually contribute to dyslipidemia. The study results could partly explain why the s4-1BB level was negatively correlated with triglyceride levels.
It has been reported that s4-1BB is released by activated lymphocytes and seems to reflect a negative feedback control of inflammation by reducing T cell activation[25,26,31]. Hence, we detected the expression of 4-1BB mRNA by real-time quantitative PCR in the PBMCs of patients with CHB and healthy adults. We found that the 4-1BB mRNA levels in total PBMCs and CD4+/CD8+ T cells all increased in patients with CHB compared with healthy adults. In addition to the increased s4-1BB levels in patients with CHB, we hypothesize that there is a regulatory mechanism by which s4-1BB interacts with 4-1BB in T cells to maintain a balanced immune response. High levels of soluble molecules may be the consequence of two different processes, such as high production or decreased depletion. 4-1BB expression can be induced by Epstein-Barr virus according to previous reports[38,39]. Similarly, in patients with CHB, we speculate that soluble levels increased because HBV infection induces increased 4-1BB mRNA expression in PBMCs and transforms into soluble forms, leading to higher s4-1BB levels in peripheral blood compared with healthy individuals.
The age-associated change in the function of the immune system contributes to the increased susceptibility to infectious diseases[33,40,41]. There was a study indicating that the expression of the costimulatory molecules OX40/OX40L, also belonging to the tumor necrosis factor superfamily, was age-dependent during HBV infection, which can explain why in adult-acquired infection HBV antigens are usually cleared, whereas in infancy-acquired infection they persist[33]. Hence, we detected the s4-1BB protein in the plasma of healthy children but found that there was no difference between healthy children and adults and no correlation between s4-1BB and age in healthy donors. In addition, the s4-1BB levels in healthy female donors were not different from those in males. Perhaps more subjects are needed to determine the relationship between 4-1BB expression and age as well as sex.
In conclusion, this study shows higher levels of s4-1BB in plasma in patients with CHB than in the control groups, especially during a disease flare. The s4-1BB level positively correlated with HBV markers. This reflects that the 4-1BB/4-1BBL pathway may be involved in the disease process. However, further studies of this costimulatory pathway in the HBV infection process and after antiviral treatment are needed to gain better insight into the significance and potential therapeutic implications of our findings.
Chronic hepatitis B infection is a global health burden. However, current therapies cannot achieve a functional cure, and the development of immunological therapeutic strategies is urgently needed. Previous studies have suggested that the costimulatory molecule 4-1BB, a member of the tumor necrosis factor superfamily, plays pivotal roles in regulating immunity during chronic viral infection.
Recently there was a study suggesting that 4-1BB signal enhancement inhibits hepatitis B virus replication in a noncytolytic manner, which indicates that 4-1BB may be a promising target to control hepatitis B virus (HBV) infection. However, there are no studies about 4-1BB in chronic hepatitis B (CHB) up to now.
Our main purpose was to analyze the soluble form and mRNA level of 4-1BB in peripheral blood to determine whether the costimulatory molecule is aberrantly produced in this disease and to provide more evidence for 4-1BB-targeted therapies for curing HBV.
Peripheral blood samples were collected from a total of 64 patients with CHB and 37 healthy controls in this study. The method of ELISA was used to measure the levels of soluble 4-1BB (s4-1BB). 4-1BB mRNA expression in peripheral blood mononuclear cells was detected by real-time quantitative PCR. The cytokines in plasma were assayed using the MILLIPLEX MAP Human Cytokine/Chemokine Magnetic Bead Panel - Immunology Multiplex Assay.
We found a higher level of s4-1BB in the plasma of patients with CHB compared with healthy adults. The s4-1BB level in plasma was significantly increased in patients with a higher viral load and a disease flare up. However, the level of s4-1BB in treatment-naïve patients was not significantly different from that in entecavir-treated patients. Interestingly, among treatment-naïve patients with CHB, the levels of s4-1BB in plasma had a significant positive correlation with hepatitis B surface antigen, HBV DNA, hepatitis B e antigen, and triglyceride levels (r = 0.748, P < 0.001; r = 0.406, P = 0.004; r = 0.356, P = 0.019; and r = -0.469, P = 0.007, respectively). The 4-1BB mRNA expression was higher in the peripheral blood mononuclear cells of patients with CHB than in the peripheral blood mononuclear cells of healthy adults, but the difference was not statistically significant. The number of subjects limited the ability of this study to clarify causality between 4-1BB and CHB.
The levels of s4-1BB may be associated with pathogenesis of HBV and therefore may be a promising biomarker for disease progression and a target for curing CHB.
The mechanism of aberrantly produced 4-1BB needs to be investigated in the future, and whether agitating the target of 4-1BB can cure hepatitis B needs to be also further studied.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Spunde K S-Editor: Zhang L L-Editor: Filipodia P-Editor: Liu JH
| 1. | Revill P, Testoni B, Locarnini S, Zoulim F. Global strategies are required to cure and eliminate HBV infection. Nat Rev Gastroenterol Hepatol. 2016;13:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 2. | Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373:582-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 993] [Article Influence: 62.1] [Reference Citation Analysis (1)] |
| 3. | Nebbia G, Peppa D, Maini MK. Hepatitis B infection: current concepts and future challenges. QJM. 2012;105:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Ferrari C. HBV and the immune response. Liver Int. 2015;35 Suppl 1:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (1)] |
| 5. | Fanning GC, Zoulim F, Hou J, Bertoletti A. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat Rev Drug Discov. 2019;18:827-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 405] [Article Influence: 67.5] [Reference Citation Analysis (1)] |
| 6. | Maini MK, Burton AR. Restoring, releasing or replacing adaptive immunity in chronic hepatitis B. Nat Rev Gastroenterol Hepatol. 2019;16:662-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 7. | Rivino L, Le Bert N, Gill US, Kunasegaran K, Cheng Y, Tan DZ, Becht E, Hansi NK, Foster GR, Su TH, Tseng TC, Lim SG, Kao JH, Newell EW, Kennedy PT, Bertoletti A. Hepatitis B virus-specific T cells associate with viral control upon nucleos(t)ide-analogue therapy discontinuation. J Clin Invest. 2018;128:668-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 8. | Park JJ, Wong DK, Wahed AS, Lee WM, Feld JJ, Terrault N, Khalili M, Sterling RK, Kowdley KV, Bzowej N, Lau DT, Kim WR, Smith C, Carithers RL, Torrey KW, Keith JW, Levine DL, Traum D, Ho S, Valiga ME, Johnson GS, Doo E, Lok AS, Chang KM; Hepatitis B Research Network. Hepatitis B Virus--Specific and Global T-Cell Dysfunction in Chronic Hepatitis B. Gastroenterology 2016; 150: 684-695. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 181] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 9. | Attanasio J, Wherry EJ. Costimulatory and Coinhibitory Receptor Pathways in Infectious Disease. Immunity. 2016;44:1052-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 205] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 10. | Chester C, Sanmamed MF, Wang J, Melero I. Immunotherapy targeting 4-1BB: mechanistic rationale, clinical results, and future strategies. Blood. 2018;131:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 364] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 11. | Goodwin RG, Din WS, Davis-Smith T, Anderson DM, Gimpel SD, Sato TA, Maliszewski CR, Brannan CI, Copeland NG, Jenkins NA. Molecular cloning of a ligand for the inducible T cell gene 4-1BB: a member of an emerging family of cytokines with homology to tumor necrosis factor. Eur J Immunol. 1993;23:2631-2641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 246] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1012] [Cited by in RCA: 1045] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 13. | Myers LM, Vella AT. Interfacing T-cell effector and regulatory function through CD137 (4-1BB) co-stimulation. Trends Immunol. 2005;26:440-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Shao Z, Schwarz H. CD137 ligand, a member of the tumor necrosis factor family, regulates immune responses via reverse signal transduction. J Leukoc Biol. 2011;89:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 15. | Halstead ES, Mueller YM, Altman JD, Katsikis PD. In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nat Immunol. 2002;3:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 142] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Tan JT, Whitmire JK, Ahmed R, Pearson TC, Larsen CP. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J Immunol. 1999;163:4859-4868. [PubMed] |
| 17. | Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev. 2009;229:192-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 240] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 18. | Clouthier DL, Watts TH. TNFRs and Control of Chronic LCMV Infection: Implications for Therapy. Trends Immunol. 2015;36:697-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Kwon BS, Hurtado JC, Lee ZH, Kwack KB, Seo SK, Choi BK, Koller BH, Wolisi G, Broxmeyer HE, Vinay DS. Immune responses in 4-1BB (CD137)-deficient mice. J Immunol. 2002;168:5483-5490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 169] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Lin GH, Sedgmen BJ, Moraes TJ, Snell LM, Topham DJ, Watts TH. Endogenous 4-1BB ligand plays a critical role in protection from influenza-induced disease. J Immunol. 2009;182:934-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Fuse S, Bellfy S, Yagita H, Usherwood EJ. CD8+ T cell dysfunction and increase in murine gammaherpesvirus latent viral burden in the absence of 4-1BB ligand. J Immunol. 2007;178:5227-5236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Seo SK, Park HY, Choi JH, Kim WY, Kim YH, Jung HW, Kwon B, Lee HW, Kwon BS. Blocking 4-1BB/4-1BB ligand interactions prevents herpetic stromal keratitis. J Immunol. 2003;171:576-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Fisicaro P, Valdatta C, Massari M, Loggi E, Ravanetti L, Urbani S, Giuberti T, Cavalli A, Vandelli C, Andreone P, Missale G, Ferrari C. Combined blockade of programmed death-1 and activation of CD137 increase responses of human liver T cells against HBV, but not HCV. Gastroenterology 2012; 143: 1576-1585. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Wang L, Yi Y, Jiang W, Yin D, Fan J, Ye W, Zhao W. Immune active cells with 4-1BB signal enhancement inhibit hepatitis B virus replication in noncytolytic manner. Cell Immunol. 2018;328:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Luu K, Shao Z, Schwarz H. The relevance of soluble CD137 in the regulation of immune responses and for immunotherapeutic intervention. J Leukoc Biol. 2020;107:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Shao Z, Sun F, Koh DR, Schwarz H. Characterisation of soluble murine CD137 and its association with systemic lupus. Mol Immunol. 2008;45:3990-3999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Michel J, Schwarz H. Expression of soluble CD137 correlates with activation-induced cell death of lymphocytes. Cytokine. 2000;12:742-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Jung HW, Choi SW, Choi JI, Kwon BS. Serum concentrations of soluble 4-1BB and 4-1BB ligand correlated with the disease severity in rheumatoid arthritis. Exp Mol Med. 2004;36:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Hamaguchi Y, Hasegawa M, Matsushita T, Komura K, Takehara K, Fujimoto M. Clinical association of serum CD137 (4-1BB) levels in patients with systemic sclerosis. J Dermatol Sci. 2009;53:159-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Sharief MK. Heightened intrathecal release of soluble CD137 in patients with multiple sclerosis. Eur J Neurol. 2002;9:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Michel J, Langstein J, Hofstädter F, Schwarz H. A soluble form of CD137 (ILA/4-1BB), a member of the TNF receptor family, is released by activated lymphocytes and is detectable in sera of patients with rheumatoid arthritis. Eur J Immunol. 1998;28:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 32. | Shao Z, Schäffler A, Hamer O, Dickopf J, Goetz A, Landfried K, Voelk M, Kopp A, Herfarth H, Karrasch T, Brünnler T, Koh LK, Buechler C, Schwarz H. Admission levels of soluble CD137 are increased in patients with acute pancreatitis and are associated with subsequent complications. Exp Mol Pathol. 2012;92:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Publicover J, Gaggar A, Jespersen JM, Halac U, Johnson AJ, Goodsell A, Avanesyan L, Nishimura SL, Holdorf M, Mansfield KG, Judge JB, Koshti A, Croft M, Wakil AE, Rosenthal P, Pai E, Cooper S, Baron JL. An OX40/OX40L interaction directs successful immunity to hepatitis B virus. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Martinet J, Dufeu-Duchesne T, Bruder Costa J, Larrat S, Marlu A, Leroy V, Plumas J, Aspord C. Altered functions of plasmacytoid dendritic cells and reduced cytolytic activity of natural killer cells in patients with chronic HBV infection. Gastroenterology 2012; 143: 1586-1596. e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 35. | Gane E, Verdon DJ, Brooks AE, Gaggar A, Nguyen AH, Subramanian GM, Schwabe C, Dunbar PR. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study. J Hepatol. 2019;71:900-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 246] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 36. | Liu J, Zhang E, Ma Z, Wu W, Kosinska A, Zhang X, Möller I, Seiz P, Glebe D, Wang B, Yang D, Lu M, Roggendorf M. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS Pathog. 2014;10:e1003856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 216] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 37. | Kim CS, Tu TH, Kawada T, Kim BS, Yu R. The immune signaling molecule 4-1BB stimulation reduces adiposity, insulin resistance, and hepatosteatosis in obese mice. Endocrinology. 2010;151:4725-4735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Yoshimori M, Imadome K, Komatsu H, Wang L, Saitoh Y, Yamaoka S, Fukuda T, Kurata M, Koyama T, Shimizu N, Fujiwara S, Miura O, Arai A. CD137 expression is induced by Epstein-Barr virus infection through LMP1 in T or NK cells and mediates survival promoting signals. PLoS One. 2014;9:e112564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Aravinth SP, Rajendran S, Li Y, Wu M, Yi Wong AH, Schwarz H. Epstein-Barr virus-encoded LMP1 induces ectopic CD137 expression on Hodgkin and Reed-Sternberg cells via the PI3K-AKT-mTOR pathway. Leuk Lymphoma. 2019;60:2697-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200:1613-1622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 41. | Jones SC, Brahmakshatriya V, Huston G, Dibble J, Swain SL. TLR-activated dendritic cells enhance the response of aged naive CD4 T cells via an IL-6-dependent mechanism. J Immunol. 2010;185:6783-6794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |