Published online Mar 6, 2021. doi: 10.12998/wjcc.v9.i7.1592
Peer-review started: October 26, 2020
First decision: December 8, 2020
Revised: December 22, 2020
Accepted: December 28, 2020
Article in press: December 28, 2020
Published online: March 6, 2021
Processing time: 125 Days and 14.5 Hours
Early hepatic artery thrombosis (E-HAT) is a serious complication after liver transplantation (LT), which often results in graft failure and can lead to patient deaths. Treatments such as re-transplantation and re-anastomosis are conventional therapeutic methods which are restricted by the shortage of donors and the patient’s postoperative intolerance to re-laparotomy. Due to the advances in interventional techniques and thrombolytics, endovascular treatments are increasingly being selected by more and more centers. This study reviews and reports our single-center experience with intra-arterial thrombolysis as the first choice therapy for E-HAT after deceased donor LT.
To evaluate the feasibility and reasonability of intra-arterial thrombolysis for E-HAT after deceased donor LT.
A total of 147 patients who underwent deceased donor LT were retrospectively reviewed in our hospital between September 2011 and December 2016. Four patients were diagnosed with E-HAT. All of these patients underwent intra-arterial thrombolysis with alteplase as the first choice therapy after LT. The method of arterial anastomosis and details of the diagnosis and treatment of E-HAT were collated. The long-term prognosis of E-HAT patients was also recorded. The median follow-up period was 26 mo (range: 23 to 30 mo).
The incidence of E-HAT was 2.7% (4/147). E-HAT was considered when Doppler ultrasonography showed no blood flow signals and a definite diagnosis was confirmed by immediate hepatic arterial angiography when complete occlusion of the hepatic artery was observed. The patients were given temporary thrombolytics (mainly alteplase) via a 5-Fr catheter which was placed in the proximal part of the thrombosed hepatic artery followed by continuous alteplase using an infusion pump. Alteplase dose was adjusted according to activated clotting time. The recanalization rate of intra-arterial thrombolysis in our study was 100% (4/4) and no thrombolysis-related mortality was observed. During the follow-up period, patient survival rate was 75% (3/4), and biliary complications were present in 50% of patients (2/4).
Intra-arterial thrombolysis can be considered first-line treatment for E-HAT after deceased donor LT. Early diagnosis of E-HAT is important and follow-up is necessary even if recanalization is successful.
Core Tip: This retrospective study evaluated the feasibility and reasonability of intra-arterial thrombolysis for early hepatic artery thrombosis (E-HAT) after deceased donor liver transplantation. The incidence of E-HAT was 2.7% (4/147). The patients were given temporary thrombolytics followed by continuous alteplase using an infusion pump. Alteplase dose was adjusted according to activated clotting time. The recanalization rate of intra-arterial thrombolysis in our study was 100% and no thrombolysis-related mortality was observed. Intra-arterial thrombolysis can be considered first-line treatment for E-HAT after deceased donor liver transplantation. Early diagnosis of E-HAT is important and follow-up is necessary even if recanalization is successful.
- Citation: Li T, Sun XD, Yu Y, Lv GY. Intra-arterial thrombolysis for early hepatic artery thrombosis after liver transplantation. World J Clin Cases 2021; 9(7): 1592-1599
- URL: https://www.wjgnet.com/2307-8960/full/v9/i7/1592.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i7.1592
Hepatic artery thrombosis (HAT) is a devastating complication after liver transplantation (LT) with an overall incidence varying from 2% to 9%[1,2]. Early hepatic artery thrombosis (E-HAT) is diagnosed within the first 21 d after transplantation[3]. Without effective revascularization, E-HAT usually results in graft failure and can lead to patient deaths. Although, re-transplantation is still the gold standard treatment for HAT, most E-HAT cases are managed with re-transplantation (74%), whereas early revascularization is carried out in only 13% with a 75% success rate[4]. Furthermore, re-transplantation in all cases is restricted by the shortage of donors and the patient’s postoperative condition as some patients are unable to tolerate re-laparotomy. In such cases, other treatment options such as surgical revascularization, percutaneous thrombolysis, percutaneous angioplasty, and a conservative approach are then considered. However, none of these treatments have been shown to be superior.
Although many patients have been reported to have undergone successful endovascular treatment for HA complications[5-7], less invasive therapeutic options have been chosen by many centers for E-HAT, especially in the first few days after LT. However, the efficacy of intra-arterial thrombolysis has not yet been demonstrated.
In the present study, we retrospectively reviewed E-HAT patients in our hospital who underwent intra-arterial thrombolysis with alteplase as the first choice therapy after LT. The aim of this study was to evaluate the feasibility and reasonability of intra-arterial thrombolysis for E-HAT after deceased donor LT.
The medical records of 147 adult patients who underwent deceased donor LT at The First Hospital of Jilin University from September 2011 to December 2016 were retrospectively reviewed. All patients received liver grafts from deceased donors. The arterial anastomosis method and details of the diagnosis and treatment of HAT were collated. The long-term outcomes of these HAT patients were also recorded. The median follow-up period was 26 mo (range: 23 to 30 mo). This study was approved by the Ethics Committee of the First Hospital of Jilin University.
The method used for arterial anastomosis depended on the graft and recipient vascular anatomy. Anastomosis of the HA was microscopically performed with continuous 7-0 or 8-0 prolene sutures at different sites between the graft and donor arterial systems. The principle of HA anastomosis was to identify the optimal artery to minimize the size mismatch. The length must be adequate to allow a tension-free anastomosis and at the same time to avoid kinks and rotations. The most common graft artery was the artery bifurcation of the common HA (CHA) and the splenic artery (SA), while the most common recipient artery was the artery bifurcation of the CHA and the gastroduodenal artery (GDA). Artery reconstructions were performed if any accessory HAs existed. Accessory HAs were anastomosed end-to-end to the donor SA or GDA branch.
Routine surveillance of HA anastomoses with Doppler ultrasonography was performed periodically after surgery: Once per day for the first week, twice per week until discharge, and then once per week for three months. If sonography revealed abnormal findings, hepatic arterial angiography was immediately performed. HAT was defined as complete occlusion of the HA on hepatic arterial angiography.
Intra-arterial thrombolysis was our first-choice therapy for E-HAT. Selective catheterization of the celiac trunk using the right femoral artery access was performed. A 5-Fr catheter was placed over the proximal part of the thrombosed HA, and thrombolytics were injected temporarily or continuously. With the temporary injections of thrombolytics, partial recanalization was observed as partial resolution of the thrombus. In addition, delineation of the intrahepatic branches, localized post-stenotic dilatation and filling defects were observed. After continuous injection of alteplase, complete recanalization of the HA was observed on hepatic arterial angiography, without obvious filling defects in the hepatic trunk and intrahepatic branches.
After intra-arterial thrombolysis, anticoagulant therapy with enoxaparin sodium at a dose of 4000 Axa IU, twice a day for approximately 5 to 7 d was administered, followed by aspirin at a dose of 100 mg/d or clopidogrel at a dose of 75 mg/d for at least six months.
The demographic data of the recipients and surgical details are shown in Table 1. The incidence of E-HAT was 2.7% (4 of 147). The median age of these patients was 50 years (range: 36 to 67 years). The patient population consisted of two men and two women. The underlying liver diseases in these patients were hepatocellular carcinoma in two cases, liver cirrhosis in one (unknown cause), and acute hepatic failure in one (unknown cause). One hepatocellular carcinoma case received trans-catheter arterial chemoembolization seven months before transplantation. None of the patients had a history of epigastric surgery. All patients received liver grafts from deceased donors. One of the patients underwent ABO-incompatible transplantation due to acute hepatic failure (hepatic encephalopathy grade 4). Warm ischemia time ranged from 2 min 10 s to 4 min 10 s, while cold ischemia time ranged from 237 min to 441 min. Artery bifurcation of the CHA and the SA to artery bifurcation of the CHA and the GDA was used for HA anastomosis in three patients, and the trunk of the CHA to the trunk of the CHA in the remaining patient. No accessory HAs were observed. Continuous anastomosis was performed with 7-0 prolene sutures in three patients and 8-0 prolene sutures in one patient.
| Recipients and details of operation | |
| Period | September, 2011-December, 2016 |
| Number of cases | 2.7% (4 of 147 cases) |
| Gender | Male: 2; Female: 2 |
| Age (yr) | 50 (36-67) |
| History of TACE | 1 case |
| History of epigastric operation | None |
| Original disease | HCC: 2; Liver cirrhosis (unknown cause): 1; Acute hepatic failure (unknown cause): 1 |
| ABO-compatibility | Identical: 2; Compatible: 1; Incompatible: 1 |
| Anastomotic site | Donor: Artery bifurcation of the CHA and the SA: 3; The trunk of CHA:1; Recipient: artery bifurcation of the CHA and the GDA: 3; The trunk of CHA: 1 |
| Operative suture | 7-0 prolene: 3; 8-0 prolene: 1 |
| Multiple anastomoses | None |
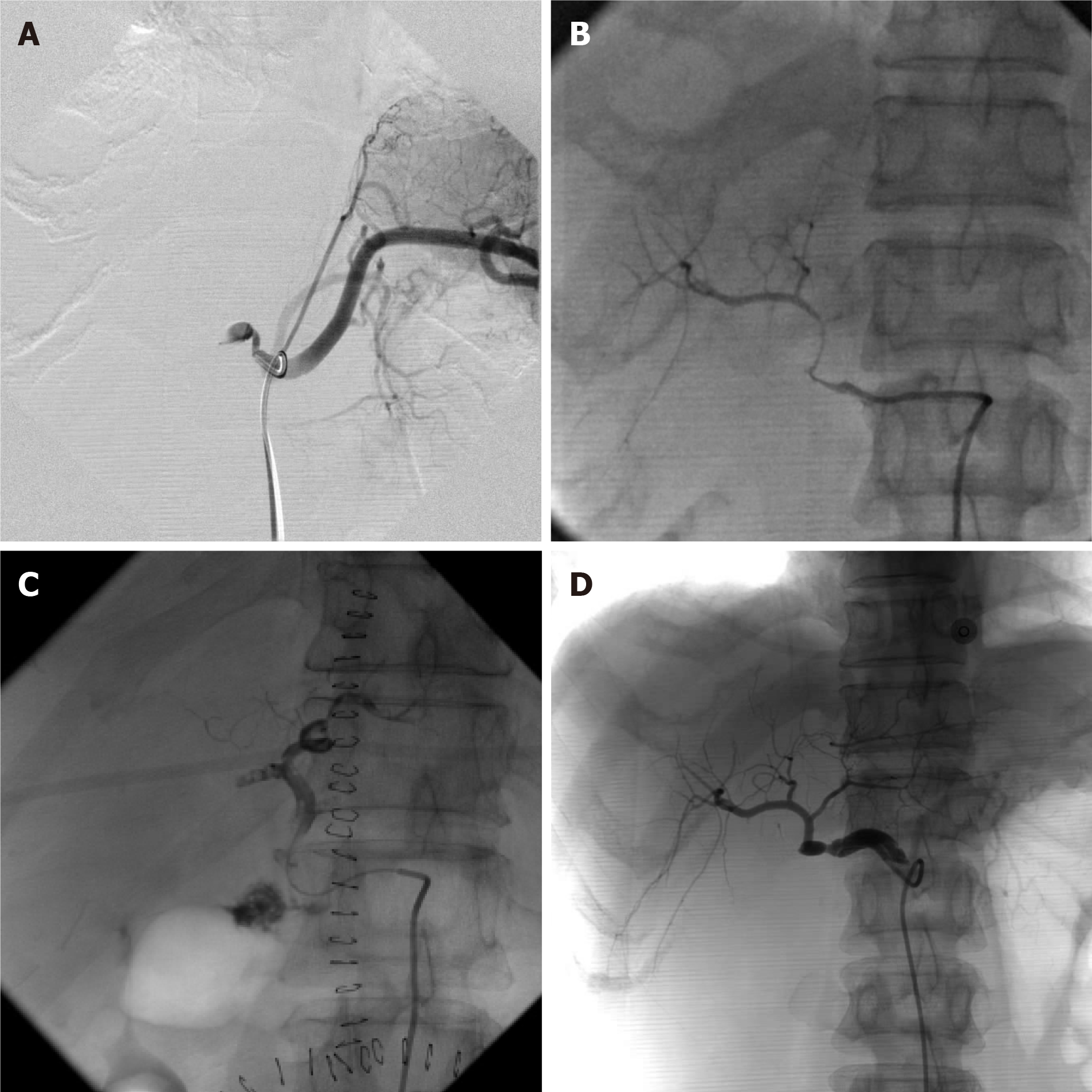
Details of the patients’ diagnosis and treatment are summarized in Table 2. With the routine surveillance mentioned above, E-HAT was considered in four patients when Doppler ultrasonography showed no blood flow signals 2 to 14 d after LT. A definite diagnosis was immediately confirmed by hepatic arterial angiography which showed complete occlusion of the HA (Figure 1A).
| Case 1 | Case 2 | Case 3 | Case 4 | |||
| Diagnostic time (POD) | 8 | 14 | 2 | 3 | ||
| Diagnostic method | Suspected | Doppler ultrasonography | Doppler ultrasonography | Doppler ultrasonography | Doppler ultrasonography | |
| Confirmed | Hepatic arterial angiography | Hepatic arterial angiography | Hepatic arterial angiography | Hepatic arterial angiography | ||
| Thrombolytic drugs | Temporarily | Drugs | Urokinase and Fibrinogenase | Alteplase | Alteplase | Alteplase |
| Approach | Through catheter | Through catheter | Through catheter | Through catheter | ||
| Continuously | Drugs | Alteplase | Alteplase | Alteplase | Alteplase | |
| Approach | Peripheral vein | Through catheter | Through catheter | Through catheter | ||
| Thrombolysis time (h) | 14 | 26 | 8.5 | 15 | ||
| Average ACT (s) | 177.8 | 144.6 | 146.3 | 160.0 | ||
| Complications related to intra-arterial thrombolysis | Local bleeding puncture site hematoma | None | Intraperitoneal hemorrhage | Intraperitoneal hemorrhage | ||
All four patients with E-HAT were treated with intra-arterial thrombolysis. After selective catheterization, the patients were given temporary thrombolytics [one was treated with urokinase (120000 IU) and fibrinogenase (10 mg) yet arterial anastomotic leakage was observed, so the others received alteplase (12.5-50 mg) instead] via a 5-Fr catheter which was placed in the proximal part of the thrombosed HA and partial recanalization was observed (Figure 1B). All patients were given alteplase continuously using an infusion pump. Continuous alteplase was administered via a 5-Fr catheter in all patients except one who showed arterial anastomotic leakage during intraoperative thrombolysis (Figure 1C), and was given alteplase via a peripheral vein.
Alteplase dose was adjusted according to activated clotting time (ACT), which was maintained between 140 and 180 s. Overall technical success due to endovascular treatment was achieved in 100% of patients. After 8.5-26 h continuous thrombolysis, hepatic arterial arteriography was performed again. Complete recanalization of the HA system was observed in all patients (Figure 1D).
Complications related to intra-arterial thrombolysis were observed in three patients (local puncture site hematoma in one and intraperitoneal hemorrhage in two). During conservative treatment, only one patient with intraperitoneal hemorrhage required laparoscopic hemostasis. All four patients recovered and were discharged with normal liver function.
The follow-up period ranged from 23 to 30 mo, with the exception of one patient who died of severe thrombocytopenia 5 mo after ABO-incompatible LT. All other patients are still alive. Among the surviving patients, one is in a healthy condition without any complications, while the other two experienced delayed biliary complications. Biliary complications were found 4 mo and 20 mo after LT, which manifested as bile leakage and biliary stricture. These patients underwent external biliary drainage and endoscopic retrograde cholangiography, and stent implantation, respectively.
E-HAT is a serious complication after LT, which often results in patient death and is the second leading cause of early graft failure after primary non-function[8]. Potential risk factors for E-HAT may be surgical or nonsurgical. Careful selection of paired arteries with microsurgical techniques should minimize the possibility of arterial complications. Artery bifurcation is usually used in our unit to expand the anastomotic opening to some extent. While, nonsurgical factors such as the patient’s blood coagulation state, lack of ABO compatibility, preoperative trans-catheter arterial chemoembolization, and multiple anastomoses affect the occurrence of E-HAT[3,9-11]. These patients are considered to be at high-risk, and more intensive monitoring and a reasonable anticoagulant scheme should be adopted after LT. To reduce the incidence of graft failure and life-threatening vascular occlusion events, some LT centers even use perioperative anticoagulation practices for pediatric LT[12]. Early diagnosis and treatment of E-HAT are most important for graft salvage and patient survival. In our unit, routine surveillance of HA anastomoses with Doppler ultrasonography is undertaken periodically after surgery, which leads to early detection of E-HAT and creates the best opportunity for treatment.
In the current study, the incidence of E-HAT after deceased donor LT was 2.7% (4 of 147), which was similar to the results in previous reports of 2% to 9%[1,2]. E-HAT is diagnosed within the first 21 d after transplantation. In the current study, the median interval from deceased donor LT to the occurrence of E-HAT was 7 d (range: 2 to 14 d), and all cases were classified as E-HAT. Early HAT after liver transplantation has been associated with a high mortality rate of 34.3% in adults[13]. As the hepatic parenchyma and biliary duct rely on the plexus of blood vessels derived from only the anastomosed graft artery, E-HAT may cause fulminant hepatic necrosis and bile duct necrosis, and frequent uncontrollable sepsis in immunosuppressed recipients, leading to early graft failure[14]. However, early detection and management of E-HAT are very important in preventing early graft failure.
In general, there are three different treatment modalities for E-HAT: Re-transplantation, surgical revascularization, and endovascular revascularization. Although re-transplantation is traditionally the gold standard, in areas with organ shortage such as Asia, timely re-transplantation may not be feasible. In a systematic review of E-HAT after LT, the success rate of a surgical revascularization attempt was 50.9% in adult recipients and re-transplantation was required in 32.3% of patients after an attempt at revascularization[13]. Taking into account the advances in interventional techniques and thrombolytics, we utilized intra-arterial thrombolysis as our first choice therapy for E-HAT after deceased donor LT.
There is no consensus on the optimal thrombolytics as they have been successfully used in both temporary and continuous infusions for E-HAT. Some studies indicated the preferred use of urokinase, streptokinase and tissue plasminogen activator[5,15]. Alteplase is the only approved thrombolytic agent for acute ischemic stroke[16]. Our earliest use of alteplase was the result of an unsatisfactory thrombolysis effect with urokinase and fibrinolysis, which achieved complete recanalization of the HA system after 14 h continuous infusion. Three further attempts also obtained great results. In this study, the E-HAT-associated mortality rate was 0%, suggesting that our treatment strategy using intra-arterial thrombolysis is an acceptable option for E-HAT after deceased donor LT.
Hemorrhage is the most commonly reported complication of thrombolysis and is seen in approximately 20% of patients[6]. There is no standard monitoring method to assess the degree of thrombolysis. ACT was used in our unit which was controlled between 140 and 180 s, in an attempt to maintain the thrombolysis effect and reduce complications. However, one local puncture site hematoma and two intraperitoneal hemorrhages were observed, and we assume that ACT may be able to control a lower complication rate.
The HA is the only blood supply to the bile duct system. Decreased HA perfusion can lead to biliary complications such as necrosis, the formation of bilomas, bile leaks, and the development of non-anastomotic strictures[17]. For this reason, preparations for biliary complications should be carried out in patients with E-HAT, even if recanalization is successful. Two patients experienced delayed biliary complications 4 mo and 20 mo after LT in this study, which manifested as bile leakage and biliary stricture. With active treatment, the patients were in good condition at the last follow-up.
E-HAT is a serious complication after LT, which often results in graft failure and can lead to patient deaths. An early diagnosis of E-HAT is important and follow-up is necessary after meticulous surgery. Doppler ultrasonography is the gold standard for screening protocols. We believe routine surveillance can screen out most cases of E-HAT very early after LT, allowing treatment to avoid graft loss. Unlike the kinks, pseudo-aneurysms or other arterial complications seen following orthotopic LT, E-HAT involves thrombogenesis within hepatic arteries in the normal anatomical location. Given the high recanalization rate of intra-arterial thrombolysis and no thrombolysis-related mortality in our study, intra-arterial thrombolysis could be considered a first-line treatment for E-HAT after deceased donor LT. Even when intra-arterial thrombolysis has failed, re-anastomosis and re-transplantation are still possible. Due to the high incidence of biliary complications after E-HAT, close follow-up and active treatment are necessary to avoid long-term graft loss. Further research is required to understand and reduce the incidence of E-HAT and subsequent biliary complications following the conservative management of these patients. However, it should be noted that this study had some limitations due to the small number of patients. Given the low incidence rate of E-HAT and its high mortality, in addition to the restricted donors for re-transplantation in Asian countries, we hope that our experience offers an alternative treatment option for E-HAT and leads to better prognosis for such patients.
Early hepatic artery thrombosis (E-HAT) is a serious complication after liver transplantation (LT), which often results in graft failure and can lead to patient deaths. Besides re-transplantation and re-anastomosis, endovascular treatments have increasingly been selected in recent years, yet the efficacy of intra-arterial thrombolysis has not yet been demonstrated.
This study reviews and reports our single-center experience with intra-arterial thrombolysis as the first choice therapy for E-HAT after deceased donor LT.
To evaluate the feasibility and reasonability of intra-arterial thrombolysis for E-HAT after LT.
A total of 147 patients who underwent LT were retrospectively reviewed. The patients who were diagnosed with E-HAT underwent intra-arterial thrombolysis with alteplase as the first choice therapy. The method of arterial anastomosis and details of the diagnosis and treatment of E-HAT were collated. The long-term prognosis of E-HAT patients was also recorded.
The incidence of E-HAT was 2.7% (4/147). E-HAT was suspected following Doppler ultrasonography and confirmed by hepatic arterial angiography. The patients were given temporary thrombolytics (mainly alteplase) followed by continuous alteplase using an infusion pump. Alteplase dose was adjusted according to activated clotting time. The recanalization rate of intra-arterial thrombolysis in our study was 100% (4/4) and no thrombolysis-related mortality was observed. During the follow-up period, patient survival rate was 75% (3/4), and biliary complications were present in 50% of patients (2/4).
Intra-arterial thrombolysis can be considered first-line treatment for E-HAT after deceased donor LT. Early diagnosis of E-HAT is important and follow-up is necessary even if recanalization is successful.
Further research is required to understand and reduce the incidence of E-HAT and subsequent biliary complications following the conservative management of these patients.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Moretti R, Raissi D S-Editor: Zhang L L-Editor: Webster JR P-Editor: Li JH
| 1. | Proposito D, Loinaz Segurola C, Garcia Garcìa I, Jimènez C, Gonzalez Pinto I, Gomez Sanz R, De La Cruz J, Moreno Gonzàlez E. [Assessment of risk factors in the incidence of hepatic artery thrombosis in a consecutive series of 687 Liver transplantations]. Ann Ital Chir. 2001;72:187-205. [PubMed] |
| 2. | Abou Ella KA, Al Sebayel MI, Ramirez CB, Rabea HM. Hepatic artery thrombosis after orthotopic liver transplantation. Saudi Med J. 2001;22:211-214. [PubMed] |
| 3. | Mourad MM, Liossis C, Gunson BK, Mergental H, Isaac J, Muiesan P, Mirza DF, Perera MT, Bramhall SR. Etiology and management of hepatic artery thrombosis after adult liver transplantation. Liver Transpl. 2014;20:713-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 4. | Stange B, Settmacher U, Glanemann M, Nüssler NC, Bechstein WO, Neuhaus P. Hepatic artery thrombosis after orthotopic liver transplantation. Transplant Proc. 2001;33:1408-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Abdelaziz O, Hosny K, Amin A, Emadeldin S, Uemoto S, Mostafa M. Endovascular management of early hepatic artery thrombosis after living donor liver transplantation. Transpl Int. 2012;25:847-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Sommacale D, Aoyagi T, Dondero F, Sibert A, Bruno O, Fteriche S, Francoz C, Durand F, Belghiti J. Repeat endovascular treatment of recurring hepatic artery stenoses in orthotopic liver transplantation. Transpl Int. 2013;26:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Singhal A, Stokes K, Sebastian A, Wright HI, Kohli V. Endovascular treatment of hepatic artery thrombosis following liver transplantation. Transpl Int. 2010;23:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Pareja E, Cortes M, Navarro R, Sanjuan F, López R, Mir J. Vascular complications after orthotopic liver transplantation: hepatic artery thrombosis. Transplant Proc. 2010;42:2970-2972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Pomposelli JJ. Hepatic Artery Thrombosis After Liver Transplant: Not A Surgical Problem? Transplantation. 2016;100:2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Yang Y, Zhao JC, Yan LN, Ma YK, Huang B, Yuan D, Li B, Wen TF, Wang WT, Xu MQ, Yang JY. Risk factors associated with early and late HAT after adult liver transplantation. World J Gastroenterol. 2014;20:10545-10552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 11. | Gilbo N, Van Praet L, Jochmans I, Sainz-Barriga M, Verslype C, Maleux G, Laleman W, van der Merwe S, Cassiman D, Nevens F, Monbaliu D, Pirenne J. Pre-operative trans-catheter arterial chemo-embolization increases hepatic artery thrombosis after liver transplantation - a retrospective study. Transpl Int. 2018;31:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Voulgarelis S, Vitola B, Lerret SM, Hong JC, Scott JP. Perioperative anticoagulation practices for pediatric liver transplantation. Pediatr Transplant. 2018;22:e13193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Bekker J, Ploem S, de Jong KP. Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant. 2009;9:746-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 376] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 14. | Tzakis AG, Gordon RD, Shaw BW Jr, Iwatsuki S, Starzl TE. Clinical presentation of hepatic artery thrombosis after liver transplantation in the cyclosporine era. Transplantation. 1985;40:667-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 317] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Kogut MJ, Shin DS, Padia SA, Johnson GE, Hippe DS, Valji K. Intra-Arterial Thrombolysis for Hepatic Artery Thrombosis following Liver Transplantation. J Vasc Interv Radiol. 2015;26:1317-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Logallo N, Kvistad CE, Nacu A, Naess H, Waje-Andreassen U, Asmuss J, Aamodt AH, Lund C, Kurz MW, Rønning OM, Salvesen R, Idicula TT, Thomassen L. The Norwegian tenecteplase stroke trial (NOR-TEST): randomised controlled trial of tenecteplase vs. alteplase in acute ischaemic stroke. BMC Neurol. 2014;14:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Frongillo F, Grossi U, Avolio AW, Sganga G, Nure E, Pepe G, Bianco G, Lirosi MC, Agnes S. Factors predicting ischemic-type biliary lesions (ITBLs) after liver transplantation. Transplant Proc. 2012;44:2002-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |