Published online Mar 6, 2021. doi: 10.12998/wjcc.v9.i7.1563
Peer-review started: August 16, 2020
First decision: November 3, 2020
Revised: November 10, 2020
Accepted: December 16, 2020
Article in press: December 16, 2020
Published online: March 6, 2021
Processing time: 197 Days and 0.2 Hours
Nomograms for prognosis prediction in colorectal cancer patients are few, and prognostic indicators differ with age.
To construct a new nomogram survival prediction tool for middle-aged and elderly patients with stage III rectal adenocarcinoma.
A total of 2773 eligible patients were divided into the training cohort (70%) and the validation cohort (30%). Optimal cutoff values were calculated using the X-tile software for continuous variables. Univariate and multivariate Cox proportional hazards regression analyses were used to determine overall survival (OS) and cancer-specific survival (CSS)-related prognostic factors. Two nomograms were successfully constructed. The discriminant and predictive ability and clinical usefulness of the model were also assessed by multiple methods of analysis.
The 95%CI in the training group was 0.719 (0.690-0.749) and 0.733 (0.702-0.74), while that in the validation group was 0.739 (0.696-0.782) and 0.750 (0.701-0.800) for the OS and CSS nomogram prediction models, respectively. In the validation group, the AUC of the three-year survival rate was 0.762 and 0.770, while the AUC of the five-year survival rate was 0.722 and 0.744 for the OS and CSS nomograms, respectively. The nomogram distinguishes all-cause mortality from cancer-specific mortality in patients with different risk grades. The time-dependent AUC and decision curve analysis showed that the nomogram had good clinical predictive ability and decision efficacy and was significantly better than the tumor-node-metastases staging system.
The survival prediction model constructed in this study is helpful in evaluating the prognosis of patients and can aid physicians in clinical diagnosis and treatment.
Core Tip: This investigation was based on a large-scale population study of middle-aged and elderly patients with stage III rectal adenocarcinoma. In this study, we analyzed the clinical data of thousands of patients with stage III rectal adenocarcinoma aged 45 years or older and determined the relevant prognostic factors and the degree of impact. New cutoff values were identified and used to construct nomograms. The nomograms showed excellent clinical predictive ability and decision power. The nomograms constructed in this study have clinical utility.
- Citation: Liu H, Li Y, Qu YD, Zhao JJ, Zheng ZW, Jiao XL, Zhang J. Construction of a clinical survival prognostic model for middle-aged and elderly patients with stage III rectal adenocarcinoma . World J Clin Cases 2021; 9(7): 1563-1579
- URL: https://www.wjgnet.com/2307-8960/full/v9/i7/1563.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i7.1563
Colorectal cancer (CRC) is the second most common malignant tumor worldwide, and its incidence is increasing[1]. Rectal adenocarcinoma is a common type of rectal cancer[2]. Seventy percent of patients with rectal cancer have no evidence of distant metastasis at the time of diagnosis[3]. Middle-aged and elderly patients are often susceptible to rectal adenocarcinoma, and stage III rectal adenocarcinoma is the most prevalent type in this population[4]. Therefore, providing this population with the tools necessary for prognosis can help physicians make correct clinical decisions regarding relevant treatment, benefiting physicians and patients.
A statistical model based on the Cox proportional hazards model for individualized prediction analysis of clinical events, can comprehensively include multiple prognostic indicators to construct a survival prediction model and visualize the risk of prognostic factors and outcomes. A nomogram is an effective tool for visual assessment and quantifies individual risk by using important prognostic factors. In the diagnosis and treatment of a variety of cancers, nomograms currently show good predictive performance, such as for pancreatic cancer[5] and liver cancer[6]. Nomograms can improve the accuracy of survival prediction and provide physicians with more accurate diagnosis and treatment.
In previous studies related to clinical survival, the sample sizes were small, and the evaluations were incomplete. Simultaneously, changes and advances in medical technology also affect the prognosis of patients. However, it is still necessary to study the survival prognostic factors in middle-aged and elderly patients with stage III rectal adenocarcinoma. Presently, there are few prognostic models for the population. The nomogram helps to assess the clinical risks and benefits for the patient and assists the physician in making clinical decisions[7]. Therefore, we assessed the five-year overall survival (OS) and cancer-specific survival (CSS) of these patients. We also constructed a nomogram based on multiple prognostic indicators to predict survival.
Data were obtained from the Surveillance, Epidemiology, and End Results Program (SEER) database (https://seer.cancer.gov/data/). The data used in this study have been licensed from the SEER database (accession number: 12285-Nov2019). This study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (ethical approval number: QYFY WZLL 25780) The SEER database has a large clinical multicenter data sample that provides baseline patient data and a variety of clinical indicators. This provided a strong basis for us to analyze the prognostic survival factors of rectal cancer patients from various aspects and construct nomogram models[8].
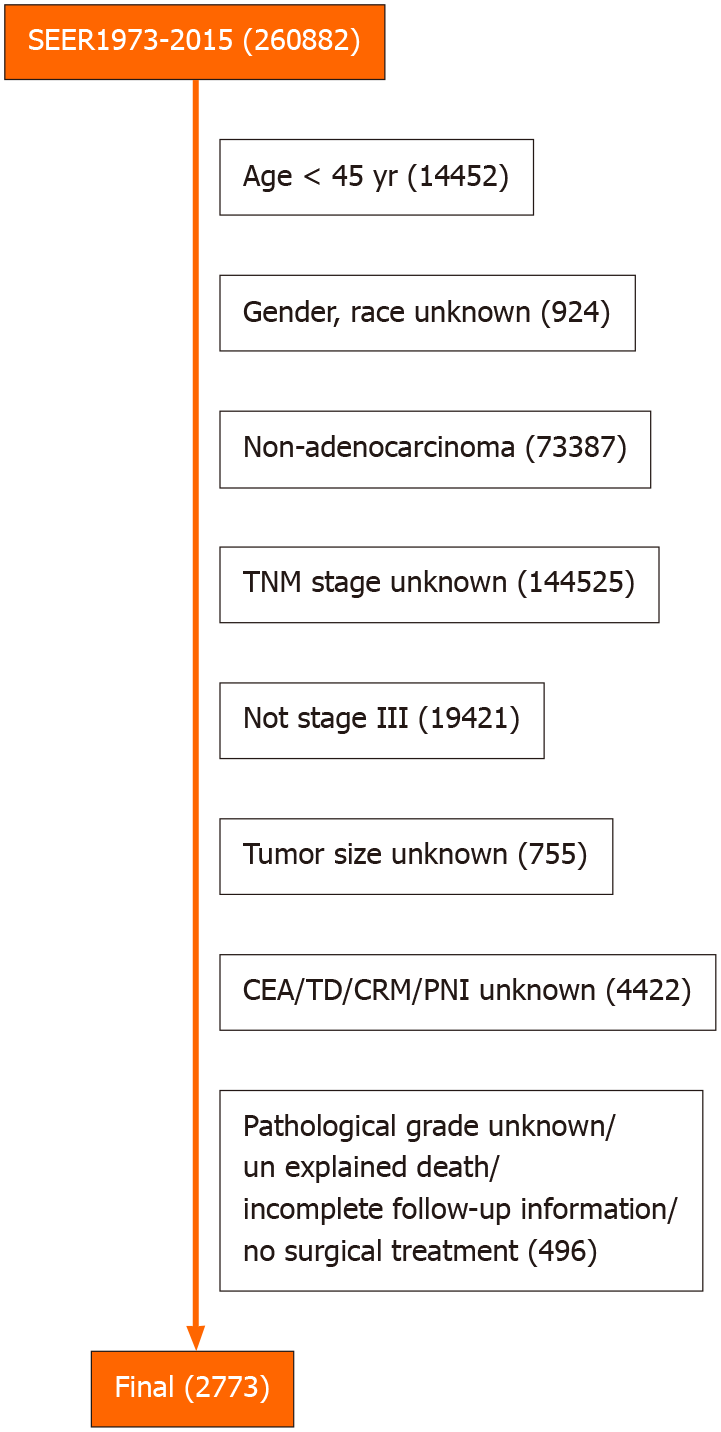
The inclusion criteria were: (1) Patients registered during 2010-2015; (2) Surgical treatment; (3) The pathological diagnosis was rectal adenocarcinoma; (4) Clinical stage III disease and (5) Relevant follow-up data were complete. The exclusion criteria were: (1) Pathological diagnosis and grading were unclear; (2) Ethnicity unknown; (3) Unknown tumor-node-metastases (TNM) stage; (4) Carcinoembryonic antigen (CEA), tumor engraftment (TD), perineural invasion (PNI), and circumferential resection margin (CRM) data were unknown; (5) Tumor size was unknown; and (6) The number of positive lymph nodes (pLN), number of examined lymph nodes (DLNs), and lymph node-positive rate (LNR) were unknown.
We collected the following demographic information: patient age, ethnicity, and sex. We also collected the following clinical information: year of diagnosis, tumor location, CEA, TD, CRM, PNI, histological grade, clinical stage, tumor size, pLN, DLNs, LNR, metastasis status, histopathological type (malignant behavior based on International Classification of Diseases for Oncology, 3rd Edition), survival time, survival status, and cause of death. Clinical staging was based on the American Joint Committee on Cancer (AJCC) 8th edition staging system. The highest CEA value in the preoperative tests was considered for evaluation.
The clinical data of 260833 patients were obtained from the SEER database. After exclusion, 2773 eligible patients were enrolled in the study (Figure 1). Eligible patients were randomly divided into the training and validation cohorts at 70% and 30%, respectively, using R software. The training cohort was used to construct a nomogram for the clinical survival prediction model, and the validation cohort was used for internal validation.
OS and CSS were study endpoints. At the same time, survival outcomes at three and five years were assessed. According to the C-index, receiver operating characteristic (ROC) curve, and calibration curve, the effectiveness, accuracy, and predictive ability of the nomogram were evaluated. The Kaplan-Meier curves were used to assess the discriminatory ability of the nomograms for patients with different risk grades. The area under the curve (AUC) of the time-dependent curve and decision curve helped us to judge the predictive performance and clinical application of the model. OS was defined as the time from the first day of last follow-up or diagnosis to death[9]. CSS was defined as the time from diagnosis to death due to rectal adenocarcinoma and last follow-up[10].
Based on the relationship between the predictor and survival outcome, X-tile software creates the optimal segmentation point for this variable and helps visualize the image[11]. Statistical analysis was performed using SPSS software (SPSS 24.0, Chicago, IL, United States) and R software (R software, version 4.0). R packages “rms,” “foreign,” and “caret” were used for randomization, while "rms," "foreign," and "survival" were used to perform Cox regression analysis, calculate risk score and C-index, and draw the calibration curve, decision curve and survival curve of the prediction model. The R packages “regplot” and “timeROC” were used to draw the nomogram plot, the ROC and the time-dependent AUC, respectively. All statistical tests were two-way.
Optimal cutoff values for OS were assessed using the X-tile software for the continuous variables including age, tumor size, pLN, DLNs, and LNR.
Frequency and percentage were used to evaluate the baseline data of the patients in both groups. Differences between the two groups were analyzed using the chi-square test. Univariate vs multivariate Cox analysis was used to screen for independent prognostic factors, which were then used to construct the nomograms. Hazard ratios (HR) and 95%CI were also calculated. The predictive ability of the model was assessed by C-index and AUC. The similarity between the predicted and actual results was compared by a calibration curve. P values < 0.05 were considered significant.
The "prediction" function of the R software was used to calculate a risk score for each patient. The optimal cutoff value for the risk score in the training cohort was clarified by the X-tile software. Through the optimal cutoff value, patients were divided into three groups: high risk group, medium risk group, and low risk group. The log-rank test was used to assess survival differences between patients with different degrees of risk and to plot survival curves based on risk scores.
The AUC values of the prediction model at different time points can be visualized by time-dependent AUC[12]. The predictive ability of TNM stage was compared with the nomogram using this method.
Clinical utility and net benefit were assessed by decision curve analysis[13]. The clinical efficacy of the nomogram was tested and compared with the TNM stage using the decision curve.
The differences in baseline patient characteristics between the two groups are shown in Table 1 and Supplementary Figure 1. The randomization was satisfactory. In the univariate OS analysis, except for race, gender, and tumor location, other factors were significant. In the univariate CSS analysis, except for race, sex, tumor location, and DLNs, other factors were significant (Table 2). The variables with significant differences were included in the multivariate analysis, which showed that there were significant differences in age, stage, CEA, CRM, TD, PNI, LNR, and tumor size in OS and CSS (Table 3).
| Training cohort (1944) | Validation cohort (829) | Overall (2773) | |||||
| Variable | Quantity | SCALE | Quantity | SCALE | Quantity | SCALE | P value |
| Age (yr) | 0.596 | ||||||
| 45-62 | 1107 | 56.94% | 456 | 55.01% | 1563 | 56.36% | |
| 62-75 | 588 | 30.25% | 258 | 31.12% | 846 | 30.51% | |
| > 75 | 249 | 12.81% | 115 | 13.87% | 364 | 13.13% | |
| Race | 0.425 | ||||||
| Other | 146 | 7.51% | 66 | 7.96% | 212 | 7.65% | |
| White | 1511 | 77.73% | 656 | 79.13% | 2167 | 78.15% | |
| Black | 287 | 14.76% | 107 | 12.91% | 394 | 14.21% | |
| Sex | 0.051 | ||||||
| Female | 794 | 40.84% | 305 | 36.79% | 1099 | 39.63% | |
| Male | 1150 | 59.16% | 524 | 63.21% | 1674 | 60.37% | |
| Grade | 0.859 | ||||||
| I/II | 1642 | 84.47% | 698 | 84.20% | 2340 | 84.39% | |
| III/IV | 302 | 15.53% | 131 | 15.80% | 433 | 15.61% | |
| Site | 0.692 | ||||||
| Rectosigmoid Junction | 660 | 33.95% | 275 | 33.17% | 935 | 33.72% | |
| Rectum | 1284 | 66.05% | 554 | 66.83% | 1838 | 66.28% | |
| Stage | 0.138 | ||||||
| III A | 199 | 10.24% | 90 | 10.86% | 289 | 10.42% | |
| III B | 1394 | 71.71% | 615 | 74.19% | 2009 | 72.45% | |
| III C | 351 | 18.06% | 124 | 14.96% | 475 | 17.13% | |
| Tumor size | 0.204 | ||||||
| ≤ 68 | 1626 | 83.64% | 677 | 81.66% | 2303 | 83.05% | |
| > 68 | 318 | 16.36% | 152 | 18.34% | 470 | 16.95% | |
| CEA | 0.558 | ||||||
| Low | 1074 | 55.25% | 468 | 56.45% | 1542 | 55.61% | |
| High | 870 | 44.75% | 361 | 43.55% | 1231 | 44.39% | |
| TD | 0.473 | ||||||
| Neg | 1481 | 76.18% | 621 | 74.91% | 2102 | 75.80% | |
| Pos | 463 | 23.82% | 208 | 25.09% | 671 | 24.20% | |
| CRM | 0.409 | ||||||
| Pos | 354 | 18.21% | 162 | 19.54% | 516 | 18.61% | |
| Neg | 1590 | 81.79% | 667 | 80.46% | 2257 | 81.39% | |
| PNI | 0.197 | ||||||
| Neg | 1551 | 79.78% | 679 | 81.91% | 2230 | 80.42% | |
| Pos | 393 | 20.22% | 150 | 18.09% | 543 | 19.58% | |
| pLN | 0.152 | ||||||
| 0 | 508 | 26.13% | 204 | 24.61% | 712 | 25.68% | |
| 1-6 | 1212 | 62.35% | 546 | 65.86% | 1758 | 63.40% | |
| > 6 | 224 | 11.52% | 79 | 9.53% | 303 | 10.93% | |
| DLNs | 0.241 | ||||||
| ≤ 26 | 1675 | 86.16% | 728 | 87.82% | 2403 | 86.66% | |
| > 26 | 269 | 13.84% | 101 | 12.18% | 370 | 13.34% | |
| LNR | 0.246 | ||||||
| 0-0.12 | 1146 | 58.95% | 477 | 57.54% | 1623 | 58.53% | |
| < 0.31 | 463 | 23.82% | 221 | 26.66% | 684 | 24.67% | |
| > 0.31 | 335 | 17.23% | 131 | 15.80% | 466 | 16.80% | |
| Variable | OS | CSS | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age (yr) | < 0.001 | < 0.001 | ||||
| 45-62 | ||||||
| 62-75 | 1.684 | 1.32-2.148 | < 0.001 | 1.649 | 1.649-1.255 | < 0.001 |
| > 75 | 3.102 | 2.372-4.057 | < 0.001 | 2.687 | 2.687-1.964 | < 0.001 |
| Race | 0.3 | 0.4 | ||||
| Other | ||||||
| White | 0.752 | 0.519-1.091 | 0.134 | 0.7399 | 0.739-0.485 | 0.163 |
| Black | 0.682 | 0.432-1.076 | 0.009 | 0.7474 | 0.747-0.449 | 0.262 |
| Sex | 0.08 | 0.3 | ||||
| Female | ||||||
| Male | 1.209 | 0.9731-1.502 | 0.087 | 1.139 | 1.139-0.8914 | 0.297 |
| Grade | < 0.001 | < 0.001 | ||||
| I/II | ||||||
| III/IV | 1.214 | 2.02-1.214 | < 0.001 | 1.788 | 1.788-1.352 | < 0.001 |
| Site | 0.3 | 0.4 | ||||
| Rectosigmoid Junction | ||||||
| Rectum | 0.894 | 0.718-1.114 | 0.319 | 0.889 | 0.889-0.693 | 0.356 |
| Stage | < 0.001 | < 0.001 | ||||
| III A | ||||||
| III B | 2.399 | 1.446-3.979 | < 0.001 | 3.225 | 3.225-1.651 | < 0.001 |
| III C | 3.941 | 2.316-6.706 | < 0.001 | 6.037 | 6.037-3.027 | < 0.001 |
| Tumor size size | < 0.001 | < 0.001 | ||||
| ≤ 68 | ||||||
| > 68 | 1.651 | 1.279-2.131 | < 0.001 | 1.663 | 1.663-1.245 | < 0.001 |
| CEA | < 0.001 | < 0.001 | ||||
| Low | ||||||
| High | 1.724 | 1.395-2.131 | < 0.001 | 1.576 | 1.576-1.239 | < 0.001 |
| TD | < 0.001 | < 0.001 | ||||
| Neg | ||||||
| Pos | 2.009 | 1.612-2.504 | < 0.001 | 2.072 | 2.072-1.614 | < 0.001 |
| CRM | < 0.001 | < 0.001 | ||||
| Neg | ||||||
| Pos | 2.205 | 1.756-2.77 | < 0.001 | 2.551 | 2.551-1.981 | < 0.001 |
| PNI | < 0.001 | < 0.001 | ||||
| Neg | ||||||
| Pos | 2.092 | 1.665-2.629 | < 0.001 | 2.222 | 2.222-1.718 | <0.001 |
| pLN | < 0.001 | <0.001 | ||||
| 0 | ||||||
| 1-6 | 1.379 | 1.039-1.831 | 0.026 | 1.711 | 1.711-1.21 | 0.002 |
| > 6 | 2.27 | 1.594-3.233 | < 0.001 | 2.924 | 2.924-1.927 | < 0.001 |
| DLNs | 0.02 | 0.07 | ||||
| ≤ 26 | ||||||
| > 26 | 1.412 | 1.072-1.858 | 0.014 | 1.348 | 1.348-0.981 | 0.066 |
| LNR | < 0.001 | < 0.001 | ||||
| 0-0.12 | ||||||
| < 0.31 | 1.405 | 1.088-1.815 | 0.009 | 1.64 | 1.64-1.228 | < 0.001 |
| > 0.31 | 2.261 | 1.755-2.914 | < 0.001 | 2.594 | 2.594-1.944 | < 0.001 |
| Variable | OS | CSS | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age (yr) | ||||||
| 45-62 | ||||||
| 62-75 | 1.773 | 1.384-2.272 | < 0.001 | 1.731 | 1.311-2.286 | < 0.001 |
| > 75 | 3.427 | 2.601-4.516 | < 0.001 | 2.976 | 2.157-4.106 | < 0.001 |
| Grade | ||||||
| I/II | ||||||
| III/IV | 1.188 | 0.914-1.545 | 0.198 | 1.281 | 0.96-1.709 | 0.092 |
| Stage | ||||||
| III A | ||||||
| III B | 1.680 | 1.005-2.809 | 0.047 | 2.369 | 1.204-4.662 | 0.013 |
| III C | 1.770 | 0.968-3.24 | 0.064 | 2.960 | 1.391-6.297 | 0.005 |
| Tumor size | ||||||
| ≤ 68 | ||||||
| > 68 | 1.669 | 1.277-2.18 | < 0.001 | 1.638 | 1.209-2.219 | 0.001 |
| CEA | ||||||
| Low | ||||||
| High | 1.426 | 1.148-1.772 | 0.001 | 1.261 | 0.986-1.612 | 0.064 |
| TD | ||||||
| Neg | ||||||
| Pos | 1.525 | 1.204-1.933 | < 0.001 | 1.520 | 1.164-1.985 | 0.002 |
| CRM | ||||||
| Neg | ||||||
| Pos | 1.538 | 1.204-1.963 | < 0.001 | 1.773 | 1.351-2.328 | < 0.001 |
| PNI | ||||||
| Neg | ||||||
| Pos | 1.550 | 1.204-1.996 | < 0.001 | 1.520 | 1.147-2.015 | 0.004 |
| pLN | ||||||
| 0 | ||||||
| 1-6 | 1.096 | 0.784-1.532 | 0.592 | 1.378 | 0.925-2.053 | 0.115 |
| > 6 | 0.895 | 0.497-1.612 | 0.712 | 1.076 | 0.57-2.031 | 0.821 |
| DLNs | ||||||
| ≤ 26 | ||||||
| > 26 | 1.254 | 0.928-1.694 | 0.141 | |||
| LNR | ||||||
| 0-0.12 | ||||||
| < 0.31 | 1.279 | 0.953-1.717 | 0.101 | 1.330 | 0.963-1.839 | 0.084 |
| > 0.31 | 1.871 | 1.298-2.697 | < 0.001 | 1.809 | 1.22-2.684 | 0.003 |
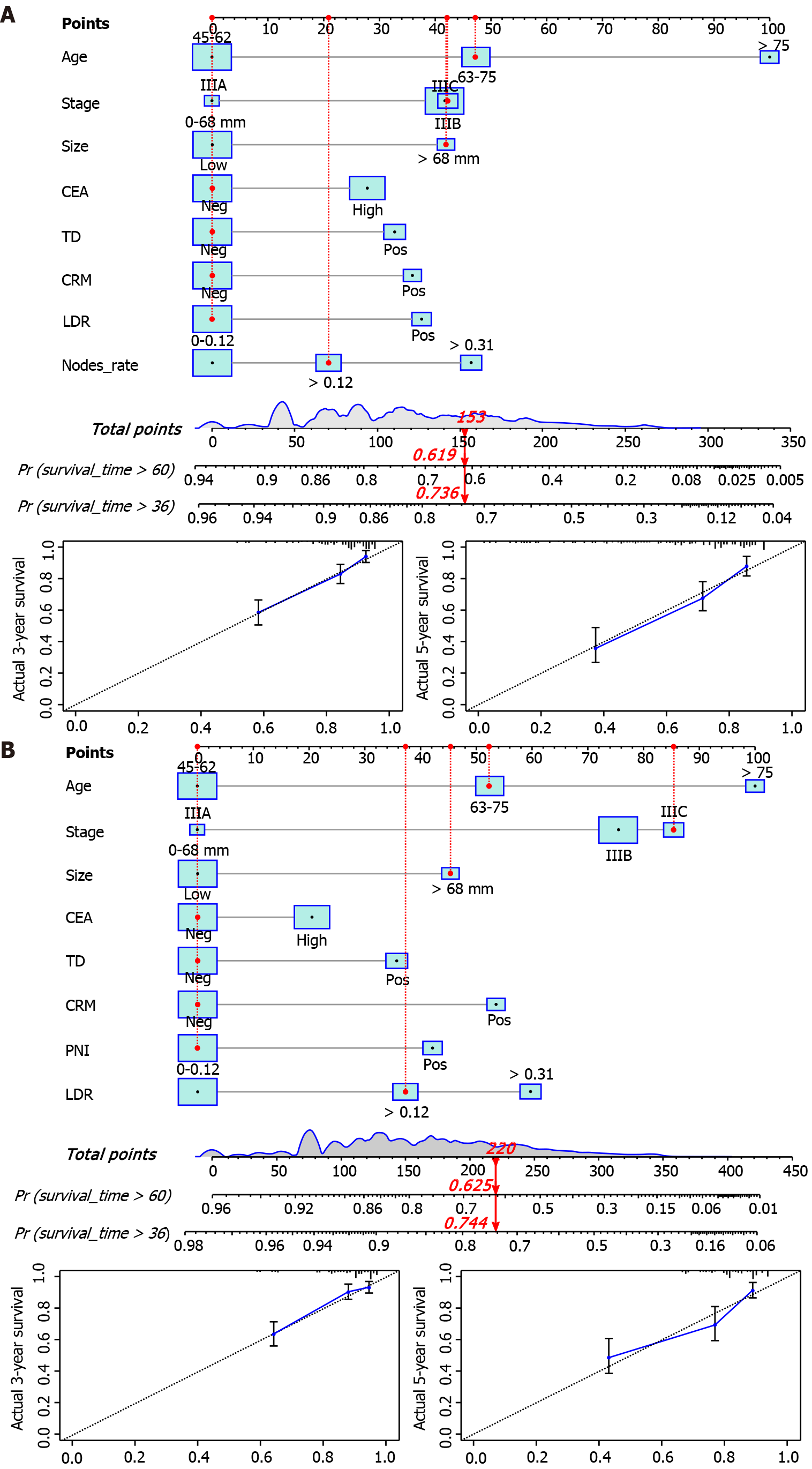
Factors with minor effects were excluded based on the results of Cox regression analysis. We used age, stage, tumor size, CEA, TD, CRM, PNI, and LNR as predictor variables of OS and CSS. Two nomogram models for survival prediction were constructed and calibration curves were plotted for the validation cohort (Figure 2). From the calibration curve, we can see that the predicted results are very similar to the actual results. The 3- and 5-year OS/CSS probabilities of the target population were predicted by calculating the total score of the predictors in the nomogram (Supplementary Table 1).
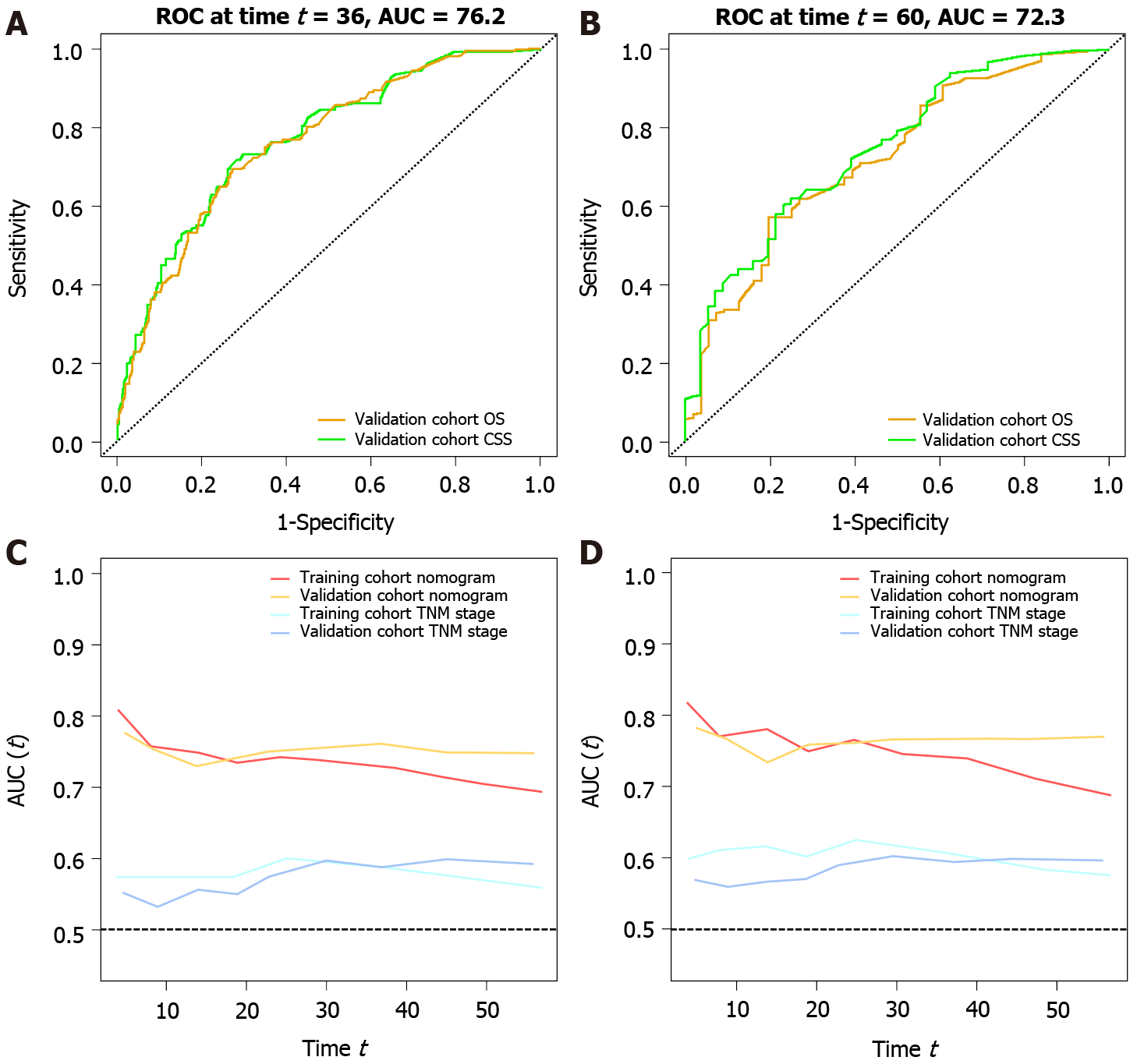
The 95%CI of the OS and CSS prediction models for the training cohort were 0.719 (0.690-0.749) and 0.733 (0.702-0.746), respectively. The 95%CI of the OS and CSS prediction models for the validation cohort was 0.739 (0.696-0.782) and 0.750 (0.701-0.800), respectively. In the validation group, the AUC of the three-year survival rate was 0.762 and 0.770, and the AUC of the five-year survival rate was 0.722 and 0.744 for the OS and CSS nomograms, respectively. The prediction model showed excellent predictive ability (Figure 3A and B).
Time-dependent AUCs are not only useful for evaluating the predictive ability of nomograms, but they can also be compared with the predictive abilities of different clinical stages. Using this curve, we found that the AUC values of the predictive models for OS and CSS were higher than the TNM stage between 0 and 60 mo. We can consider the predictive power of this model to be superior to TNM stage (Figure 3C and D).
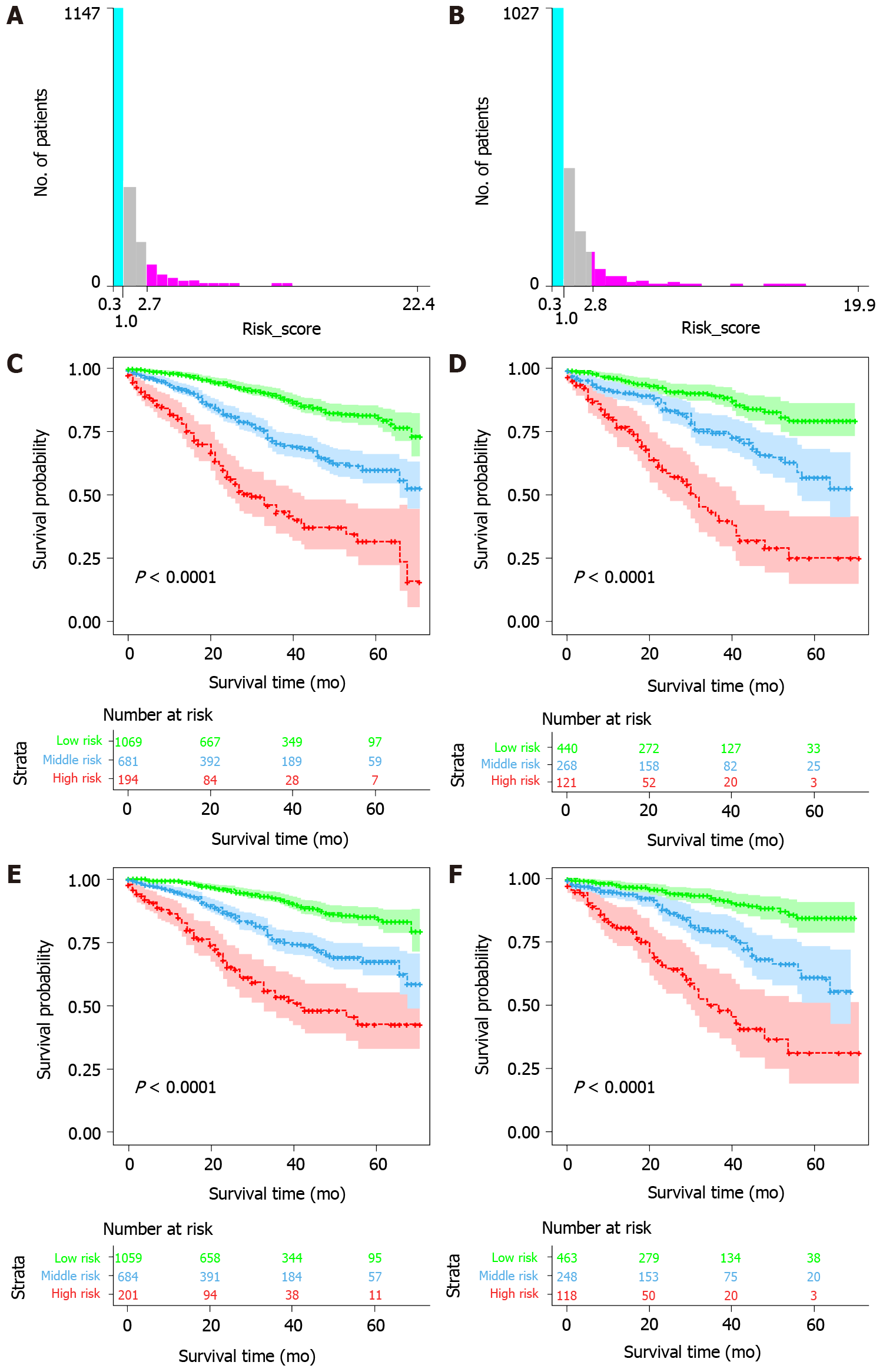
The optimal OS risk score cutoffs were 1.0 and 2.7. The optimal CSS risk score cutoffs were 1.0 and 2.8. The study population was divided into three risk groups according to the size of the cut-off value: high, medium, and low, and the corresponding survival curves were plotted. The survival curve showed that there was a significant survival difference between the training cohort and the validation cohort among the three groups (Figure 4). Therefore, the OS prediction model was found to successfully distinguish low, medium, and high all-cause mortality, and the CSS prediction model was found to successfully distinguish low, medium, and high cancer-specific mortality in patients.
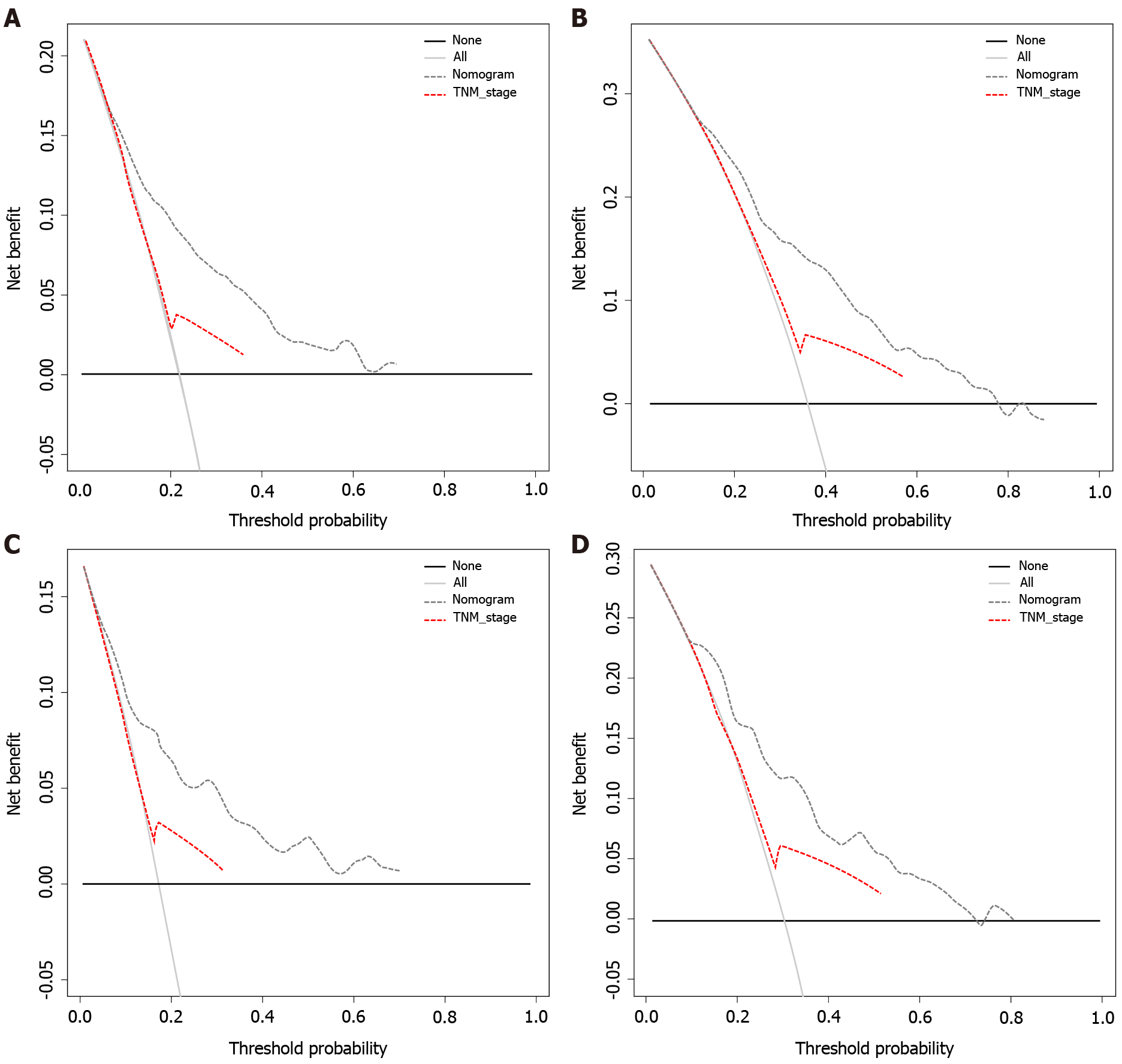
Decision curves were constructed for the OS and CSS nomogram validation cohorts (Figure 5) with threshold probabilities of < 71% and < 72% at 3 years, respectively. With a threshold probability of < 89% and < 82% at 5 years, the OS and CSS nomograms showed a net benefit over the "all treatment" and "no treatment" strategies, respectively. Therefore, the proposed nomogram aids in good clinical decision-making ability. Comparing the nomogram with TNM staging, we can see that its use in clinical decision efficacy is significantly better than that using TNM staging.
In this study, the prognostic factors of OS and CSS in the study population were found by analyzing the relevant clinical data, and two nomogram prediction models were constructed. The original intention of the clinical prediction models was to predict the status and prognosis of a disease with readily available practical predictors[14]. We constructed new nomograms that aid in effectively judging the prognosis of patients using more comprehensive and practical clinical indicators in this study.
In this study, the OS and CSS nomogram prediction models were successfully constructed. The model shows the extent to which different predictors affect the study population and provides an accurate score. In previous studies by Brenner et al[15] and Fu et al[16], age was a significant prognostic factor, similar to the results of this study. However, we found that race and sex were not significant prognostic factors in this group.
Among the clinical features, previous studies have shown that the stage, grade, and size of the tumor are prognostic factors in patients with rectal cancer[17,18]. In this study population, clinical stage and tumor size were known prognostic factors for OS and CSS. However, grade was not a significant prognostic factor in the current study. Massarweh et al[19] showed that CRM is an important prognostic factor for rectal adenocarcinoma and that high CEA levels indicate a poor prognosis, similar to our study results. TD refers specifically to a solitary tumor nodule present within the lymphatic drainage area of the primary tumor. Most TD originate from angiolymphatic invasion, in which there are no identifiable lymph nodes, blood vessels, or neural structures[20]. Wang et al[21] concluded that TD did not influence the clinical prognostic impact, unlike in our study and that by Liu et al[22] wherein TD was a significant prognostic factor. PNI is an important factor in the prognosis of rectal cancer, and the prognosis of node-negative patients with PNI-positive tumors is significantly lower than that of node-positive patients[23]. We also obtained similar results. In the abovementioned studies on prognostic factors of CRC, the common indicators CEA, TD, CRM, and PNI have clinical utility and are the key to determining prognostic factors of the disease. The nomogram showed that poor prognostic factors were as follows: high preoperative CEA levels, positive TD, positive PNI, and positive CRM. These key predictors have gradually become standard reporting practice in clinicopathological reports, and our nomograms show the important predictive ability and scoring proportion of these indicators.
Lymph node dissection is a crucial step in surgery, and the completeness and number of dissections often affect the prognosis of cancer patients[24,25]. Lymph node status is a key factor used to predict the prognosis of cancer patients and guide postoperative treatment[26]. The higher the pLN, the greater the chance of recurrence, metastasis, and poor prognosis[27]. More DLNs are helpful to clarify the pathological stage of CRC[28,29]. Although DLNs and pLNs influence treatment outcome with survival outcome in patients with rectal adenocarcinoma, DLNs and pLNs were not prognostic factors in our study population. However, we found that LNR played an important role in the prognosis of this group of patients. Although the N-staging system of the 8th edition of the AJCC is the most widely used lymph node staging method, the accuracy of this system remains controversial[30]. Some scholars have proposed that LNR can be used as a prognostic factor in patients with rectal cancer and, thus, constructed a new staging system[31]. LNR synthesizes the effects of DLNs and pLN and avoids the drawbacks of insufficient DLNs. This has a more practical guiding significance in clinical application. We found that LNR was an important factor affecting the independent prognosis of surgical patients in this study population. The LNR-based staging method is promising as a new staging or adjuvant staging method for the AJCC staging system, which can more accurately predict the prognosis of patients with stage III rectal cancer presenting with postoperative recurrence, thus providing an important basis for further treatment and planning of postoperative management. We derived an LNR cutoff of 0.05 and 0.43, but the optimal cutoff still needs further confirmation and consensus.
By comprehensively analyzing the effect of predictors on the prognosis of the study population, nomograms based on clinically practical prognostic factors were constructed to assess the three- and five-year CSS and OS of patients. This predictive model has shown excellent predictive ability with clinical decision-making ability, as validated in a number of ways. Moreover, this nomogram was superior to TNM staging in survival prediction. These findings still need further validation in clinical practice.
In this study, we evaluated the prognostic impact of multiple factors on the study population due to the large sample size and long overall follow-up time of patients in multiple centers. Through validation, the constructed model may have good application value and evaluation advantages in clinical practice. First, the prediction model constructed in this study is based on the results of a large sample study of the target population. Second, the data used to construct the prediction model for this study are clinically easy to obtain and have good clinical utility. Third, we found that the optimal cutoff values for some factors differed from previous studies. Instead of using a common cutoff, we need to find relevant cutoff values for different target populations for clinical application.
The study has some limitations. First, the study was retrospective in nature and there was an inherent selection bias due to the strict inclusion and exclusion criteria used. Second, the included patients were treated by different surgeons, which may have an impact due to the different experience and level of surgeons as well as pathologists. Third, data on some novel predictors were not recorded, such as tumor sprouting and molecular changes. Fourth, the current database information does not contain the latest clinical data of patients diagnosed with rectal adenocarcinoma, and other databases are needed to further validate the findings of this paper. Finally, some indicators, including nutritional status, detailed chemoradiotherapy information, and complications, were not recorded in the database.
We constructed three-year and five-year CSS and OS nomogram models for middle-aged and elderly patients with stage III rectal adenocarcinoma. Both prediction models demonstrated excellent clinical decision-making ability and survival prediction ability. The results indicate that LNR is more important for the prognosis of the target population than pLN and DLNs. This nomogram can be used for individualized survival prediction in the target population. It is a convenient tool for general practitioners and surgeons as it can help in more accurate evaluation of the patient’s status.
Patients with colorectal cancer have fewer nomograms for prognosis prediction, and prognostic indicators change with age. Middle-aged and elderly people frequently develop rectal adenocarcinoma, and clinical patients are mainly stage III patients.
Providing a prognostically essential tool for the target population can help physicians make correct clinical decisions regarding related treatments and benefit physicians and patients.
To construct a prognostic nomogram for in the target population, a prognostic tool that can predict OS and CSS in this population. This prognostic tool should have good predictive power and clinical utility.
First, patients in the Surveillance, Epidemiology, and End Results Program database were screened according to the inclusion and exclusion criteria; second, the prognostic factors of OS and CSS in the target population were determined by univariate analysis and multivariate analysis; third, a predictor-based clinical survival prediction model was constructed; and fourth, the predictive power and clinical efficacy of the nomogram were verified.
The 95%CI was 0.719 (0.690-0.749) and 0.733 (0.702-0.74) in the OS and CSS nomogram prediction model training groups, respectively, compared with 0.739 (0.696-0.782) and 0.750 (0.701-0.800) in the validation group. In the validation, the area under the receiver operating characteristic curve (AUC) for the OS and CSS nomograms for the three-year survival rate was 0.762 and 0.770, respectively, while the AUC for the five-year survival rate was 0.722 and 0.744, respectively. Predictive models can distinguish all-cause mortality from cancer-specific mortality in patients with different risk grades. Time-dependent AUC and decision curve analysis showed that the nomogram had excellent clinical prediction and decision-making capabilities, significantly better than the tumor-node-metastases staging system.
Lymph node-positive rate is more important for the prognosis of the target population than the number of positive lymph nodes and number of examined lymph nodes. The nomogram survival prediction model we constructed is helpful in assessing the clinical prognosis of this population and providing guidance for the optimization of clinical treatment plans.
The nomograms constructed can be used for individualized survival prediction in the target population. It is a convenient tool for general practitioners and surgeons as it can help to evaluate the patient's status more accurately and provide help for the relevant treatment of the target population in clinical practice.
The authors thank the members of the Department of General Surgery of the Affiliated Hospital of Qingdao University for sharing their data analysis knowledge.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Elshaarawy O, Saglam S, Srinivasamurthy B, Tsukanov V S-Editor: Zhang H L-Editor: Webster JR P-Editor: Yuan YY
| 1. | Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 905] [Cited by in RCA: 1034] [Article Influence: 206.8] [Reference Citation Analysis (0)] |
| 2. | Zhu X, Wang D, Lin Q, Wu G, Yuan S, Ye F, Fan Q. Screening key lncRNAs for human rectal adenocarcinoma based on lncRNA-mRNA functional synergistic network. Cancer Med. 2019;8:3875-3891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Jia J, Zhang P, Gou M, Yang F, Qian N, Dai G. The Role of Serum CEA and CA19-9 in Efficacy Evaluations and Progression-Free Survival Predictions for Patients Treated with Cetuximab Combined with FOLFOX4 or FOLFIRI as a First-Line Treatment for Advanced Colorectal Cancer. Dis Markers. 2019;2019:6812045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Reinau D, Surber C, Jick SS, Meier CR. Epidemiology of basal cell carcinoma in the United Kingdom: incidence, lifestyle factors, and comorbidities. Br J Cancer. 2014;111:203-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Song W, Miao DL, Chen L. Nomogram for predicting survival in patients with pancreatic cancer. Onco Targets Ther. 2018;11:539-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Du Q, Wang Y, Guan S, Hu C, Li M, Zhou L, Zhang M, Chen Y, Mei X, Sun J, Zhou Y. Retraction Note: The diagnostic nomogram of platelet-based score models for hepatic alveolar echinococcosis and atypical liver cancer. Sci Rep. 2020;10:16405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173-e180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 2397] [Article Influence: 239.7] [Reference Citation Analysis (0)] |
| 8. | Duggan MA, Anderson WF, Altekruse S, Penberthy L, Sherman ME. The Surveillance, Epidemiology, and End Results (SEER) Program and Pathology: Toward Strengthening the Critical Relationship. Am J Surg Pathol. 2016;40:e94-e102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 342] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 9. | Wang Y, Li J, Wen S, Yang X, Zhang Y, Wang Z, Zhang Z. CHRM3 is a novel prognostic factor of poor prognosis in patients with endometrial carcinoma. Am J Transl Res. 2015;7:902-911. [PubMed] |
| 10. | Guan X, Chen W, Jiang Z, Liu Z, Miao D, Hu H, Zhao Z, Yang R, Wang X. Exploration of the Optimal Minimum Lymph Node Count after Colon Cancer Resection for Patients Aged 80 Years and Older. Sci Rep. 2016;6:38901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Greaves P, Clear A, Coutinho R, Wilson A, Matthews J, Owen A, Shanyinde M, Lister TA, Calaminici M, Gribben JG. Expression of FOXP3, CD68, and CD20 at diagnosis in the microenvironment of classical Hodgkin lymphoma is predictive of outcome. J Clin Oncol. 2013;31:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Shah RV, Truong QA, Gaggin HK, Pfannkuche J, Hartmann O, Januzzi JL Jr. Mid-regional pro-atrial natriuretic peptide and pro-adrenomedullin testing for the diagnostic and prognostic evaluation of patients with acute dyspnoea. Eur Heart J. 2012;33:2197-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Hijazi Z, Oldgren J, Lindbäck J, Alexander JH, Connolly SJ, Eikelboom JW, Ezekowitz MD, Held C, Hylek EM, Lopes RD, Yusuf S, Granger CB, Siegbahn A, Wallentin L; ARISTOTLE and RE-LY Investigators. A biomarker-based risk score to predict death in patients with atrial fibrillation: the ABC (age, biomarkers, clinical history) death risk score. Eur Heart J. 2018;39:477-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 14. | Zhou ZR, Wang WW, Li Y, Jin KR, Wang XY, Wang ZW, Chen YS, Wang SJ, Hu J, Zhang HN, Huang P, Zhao GZ, Chen XX, Li B, Zhang TS. In-depth mining of clinical data: the construction of clinical prediction model with R. Ann Transl Med. 2019;7:796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 203] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 15. | Purim O, Gordon N, Brenner B. Cancer of the colon and rectum: potential effects of sex-age interactions on incidence and outcome. Med Sci Monit. 2013;19:203-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Fu WJ. Racial-Sex Disparities--A Challenging Battle Against Cancer Mortality in the USA. J Racial Ethn Health Disparities. 2015;2:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Li X, Yu W, Liang C, Xu Y, Zhang M, Ding X, Cai X. INHBA is a prognostic predictor for patients with colon adenocarcinoma. BMC Cancer. 2020;20:305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Cai D, Huang ZH, Yu HC, Wang XL, Bai LL, Tang GN, Peng SY, Li YJ, Huang MJ, Cao GW, Wang JP, Luo YX. Prognostic value of preoperative carcinoembryonic antigen/tumor size in rectal cancer. World J Gastroenterol. 2019;25:4945-4958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 19. | Massarweh NN, Hu CY, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, Cantor SB, Cormier JN, Feig BW, Chang GJ. Risk-adjusted pathologic margin positivity rate as a quality indicator in rectal cancer surgery. J Clin Oncol. 2014;32:2967-2974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Mirkin KA, Kulaylat AS, Hollenbeak CS, Messaris E. Prognostic Significance of Tumor Deposits in Stage III Colon Cancer. Ann Surg Oncol. 2018;25:3179-3184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 21. | Wang S, Guan X, Ma M, Zhuang M, Ma T, Liu Z, Chen H, Jiang Z, Chen Y, Wang G, Wang X. Reconsidering the prognostic significance of tumour deposit count in the TNM staging system for colorectal cancer. Sci Rep. 2020;10:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Liu F, Zhao J, Li C, Wu Y, Song W, Guo T, Chen S, Cai S, Huang D, Xu Y. The unique prognostic characteristics of tumor deposits in colorectal cancer patients. Ann Transl Med. 2019;7:769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Liebig C, Ayala G, Wilks J, Verstovsek G, Liu H, Agarwal N, Berger DH, Albo D. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol. 2009;27:5131-5137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 371] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 24. | Salehi B, Fokou PVT, Yamthe LRT, Tali BT, Adetunji CO, Rahavian A, Mudau FN, Martorell M, Setzer WN, Rodrigues CF, Martins N, Cho WC, Sharifi-Rad J. Phytochemicals in Prostate Cancer: From Bioactive Molecules to Upcoming Therapeutic Agents. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 25. | Zhou YY, Zhang QW, Huang J, Yan XL, Chen C, Xu FF, Du XJ, Jin R. Additional lymphadenectomy might not improve survival of patients with resectable metastatic colorectal adenocarcinoma of T4 stage, proximal location, poor/undifferentiation, or N3/N4 stages: a large population-based study. J Cancer. 2018;9:2428-2435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1965] [Cited by in RCA: 2294] [Article Influence: 208.5] [Reference Citation Analysis (1)] |
| 27. | Govindarajan A, Gönen M, Weiser MR, Shia J, Temple LK, Guillem JG, Paty PB, Nash GM. Challenging the feasibility and clinical significance of current guidelines on lymph node examination in rectal cancer in the era of neoadjuvant therapy. J Clin Oncol. 2011;29:4568-4573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Compton CC, Greene FL. The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin. 2004;54:295-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 311] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 29. | Kidner TB, Ozao-Choy JJ, Yoon J, Bilchik AJ. Should quality measures for lymph node dissection in colon cancer be extrapolated to rectal cancer? Am J Surg. 2012;204:843-7; discussion 847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Lykke J, Jess P, Roikjaer O; Danish Colorectal Cancer Group. The prognostic value of lymph node ratio in a national cohort of rectal cancer patients. Eur J Surg Oncol. 2016;42:504-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Persiani R, Cananzi FC, Biondi A, Paliani G, Tufo A, Ferrara F, Vigorita V, D'Ugo D. Log odds of positive lymph nodes in colon cancer: a meaningful ratio-based lymph node classification system. World J Surg. 2012;36:667-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |