Published online Mar 6, 2021. doi: 10.12998/wjcc.v9.i7.1554
Peer-review started: August 11, 2020
First decision: December 14, 2020
Revised: December 18, 2020
Accepted: December 30, 2020
Article in press: December 30, 2020
Published online: March 6, 2021
Processing time: 201 Days and 19.9 Hours
Some patients with the novel 2019 coronavirus disease (COVID-19) display elevated liver enzymes. Some antiviral drugs that can be used against COVID-19 are associated with a risk of hepatotoxicity.
To analyze the clinical significance of the dynamic monitoring of the liver function of patients with COVID-19.
This was a retrospective study of patients diagnosed with COVID-19 in January and February 2020 at the Department of Infection, Shantou Central Hospital. The exclusion criteria for all patients were: (1) History of chronic liver disease; (2) History of kidney disease; (3) History of coronary heart disease; (4) History of malignancy; or (5) History of diabetes. The serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyltransferase, and total bilirubin of patients with COVID-19 were measured on days 1, 3, 7 and 14 after admission, and compared to non-COVID-19 patents.
Twelve patients with COVID-19 (seven men and five women) and twelve controls (eight men and four women) were included. There were one, two, and nine patients with severe, mild, and moderate COVID-19, respectively. There were no differences in age and sex between the two groups (both P > 0.05). No significant differences were found in albumin, ALT, AST, γ-glutamyltransferase, or total bilirubin between the controls and the patients with COVID-19 on day 1 of hospitalization (all P > 0.05). Serum albumin showed a decreasing trend from days 0 to 7 of hospitalization, reaching the lowest level on day 7. Total bilirubin was higher on day 3 than on day 7. ALT, AST, and γ-glutamyltransferase did not change significantly over time. The severe patient was observed to have ALT levels of 67 U/L and AST levels of 75 U/L on day 7, ALT of 71 U/L and AST of 35 U/L on day 14, and ALT of 210 U/L and AST of 123 U/L on day 21.
Changes in serum liver function indicators are not obvious in the early stage of COVID-19, but clinically significant changes might be observed in severe COVID-19.
Core Tip: Twelve patients with 2019 coronavirus disease and twelve controls were included. There were one, two, and nine patients with severe, mild, and moderate 2019 coronavirus disease, respectively. Serum albumin showed a decreasing trend from days 0 to 7 of hospitalization, reaching the lowest level on day 7. Total bilirubin was higher on day 3 than on day 7. Alanine aminotransferase, aspartate aminotransferase, and γ-glutamyltransferase did not change significantly over time.
- Citation: Lin H, Wu LJ, Guo SQ, Chen RL, Fan JR, Ke B, Pan ZQ. Dynamic monitoring of serum liver function indexes in patients with COVID-19. World J Clin Cases 2021; 9(7): 1554-1562
- URL: https://www.wjgnet.com/2307-8960/full/v9/i7/1554.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i7.1554
The novel 2019 coronavirus disease (COVID-19) has become one of the major epidemic diseases seriously endangering human health and public safety[1]. On February 11, 2020, the World Health Organization officially named the disease caused by the SARS-CoV-2 virus COVID-19[2]. The main features of COVID-19 are pulmonary, and the common signs are fever, cough, and shortness of breath, which can aggravate to respiratory failure requiring oxygen therapy or mechanical ventilation. Death may occur due to acute respiratory distress syndrome, sepsis, coagulopathy, or multiorgan failure, and the overall mortality was 2.3% in China[3], but could reach 34% in nursing homes[4,5], 50% in intensive care units[6], and 88% in patients receiving mechanical ventilation[7].
Besides the pulmonary manifestation of COVID-19, previous studies reported that COVID-19 might be associated with liver dysfunction[1,8-13]. This could be clinically significant because the “Novel Coronavirus Infected Pneumonia Treatment Scheme (Trial 5th Edition)” issued by the National Health Commission of China suggest that antiviral drugs such as lopinavir-ritonavir can be used in patients with COVID-19, and those drugs have adverse effects such as diarrhea, nausea, vomiting, and hepatotoxicity[14,15], which could aggravate pre-existing liver function damage. Despite reports of elevated liver enzymes in patients with COVID-19, data are lacking regarding the changes in liver function indicators during the course of COVID-19.
Therefore, the aim of the present retrospective study was to analyze the clinical significance of the dynamic monitoring of the liver function of patients with COVID-19. The results could provide an objective basis for the use of potentially hepatotoxic drugs and the management of liver injury in these patients.
This was a retrospective study of patients diagnosed with COVID-19 in January and February 2020 at the Department of Infection, Shantou Central Hospital. The study was approved by the ethics committee of Shantou Central Hospital [(2020)-Research No.003]. The need for individual consent was waived because of the retrospective nature of the study.
COVID-19 was diagnosed according to the “Novel Coronavirus Infected Pneumonia Treatment Scheme (Trial 5th Edition)” issued by the National Health Commission of China. The exclusion criteria for all patients were: (1) History of chronic liver disease; (2) History of kidney disease; (3) History of coronary heart disease; (4) History of malignancy; or (5) History of diabetes. The outpatient medical examination participants with no history of chronic liver disease, kidney disease, coronary heart disease, tumor, and other viral infectious diseases in our hospital were collected as controls during the same period.
Fasting venous blood was collected from patients with COVID-19 on days 1, 3, 7, and 14 after admission. The venous blood from the healthy controls was collected when they visited the hospital. Serum was separated to determine albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyltransferase (GGT), total bilirubin (TBIL), and other biochemical indices. A biochemical analyzer (AU5800, Beckman, Brea, CA, United States) was used to determine the liver markers, using the manufacturer’s reagents and according to the manufacturer’s instructions.
All data were collected from the medical charts, including age, sex, comorbidities, and laboratory values. The severity of COVID-19 was classified as: (1) Mild: With only mild clinical manifestations and imaging examinations showing no signs of pneumonia; (2) Moderate: With fever and respiratory symptoms and imaging examinations showing signs of pneumonia; (3) Severe: The patient met one or more of the following items: (a) With respiratory distress and respiratory rate ≥ 30 times/min; (b) Finger oxygen saturation ≤ 93% in the resting state; and (c) Arterial partial pressure of oxygen/concentration of oxygen inhalation ≤ 300 mmHg (1 mmHg = 0.133 kPa) {for high altitude areas (altitude > 1000 m), the arterial partial pressure of oxygen/ concentration of oxygen inhalation was adjusted according to the following equation: arterial partial pressure of oxygen/concentration of oxygen inhalation × [atmospheric pressure (mmHg)/760]}; in addition, the patients in whom pulmonary imaging showed that the lesion progressed > 50% within 24-48 h were also managed as severe cases; and (4) Critical: The patient met one or more of the following items: (a) Respiratory failure requiring mechanical ventilation; (b) Shock; and (c) Failure of other organs and required monitoring and treatment in the intensive care unit.
SPSS 22.0 (IBM, Armonk, NY, United States) was used for data analysis. Continuous variables were presented as means ± standard deviation or as median and interquartile range according to their distribution, as determined by the Kolmogorov-Smirnov test. The biochemical indexes were compared between two groups using the Student t-test. The biochemical indexes were compared among different time groups using analysis of variance and the LSD post hoc test. P values < 0.05 were considered as statistically significant.
Twelve patients with COVID-19 (seven men and five women) were included. They were 12-69 years of age (median of 37). There were one, two, and nine patients with severe, mild, and moderate conditions, respectively. All the patients were clustering cases, except patient 10, who had a travel history. All the patients were cured and discharged from hospital. The detailed information of patients was listed in the Table 1. Twelve controls (eight men and four women) were included. They were 16-65 years of age (median of 36.5). There were no significant differences in age and sex between the two groups (both P > 0.05).
| No. | Sex | Age | Severity of diseases | Symptoms | Comorbidities | CT characteristic | AST at baseline, U/L | ALT at baseline, U/L |
| 1 | M | 69 | 2 | Fever, cough | DM, COPD | Interstitial changes | 31 | 31 |
| 2 | F | 66 | 2 | Fever, cough | DM | Interstitial changes | 22 | 38 |
| 3 | F | 12 | 1 | Sore throat | - | 27 | 19 | |
| 4 | M | 28 | 1 | Sore throat | - | 26 | 30 | |
| 5 | M | 53 | 2 | Fever, cough | HBP | Interstitial changes | 10 | 19 |
| 6 | M | 58 | 3 | Fever, cough | - | Interstitial changes | 23 | 37 |
| 7 | F | 47 | 2 | Sore throat | - | Interstitial changes | 32 | 31 |
| 8 | M | 31 | 1 | Fever, cough | - | 10 | 14 | |
| 9 | F | 37 | 2 | Fever, cough | - | GGO | 17 | 22 |
| 10 | M | 37 | 1 | Sore throat | - | GGO | 23 | 22 |
| 11 | F | 33 | 2 | Fever, cough | DM | Interstitial changes | 28 | 25 |
| 12 | M | 14 | 2 | Sore throat | - | Interstitial changes | 52 | 28 |
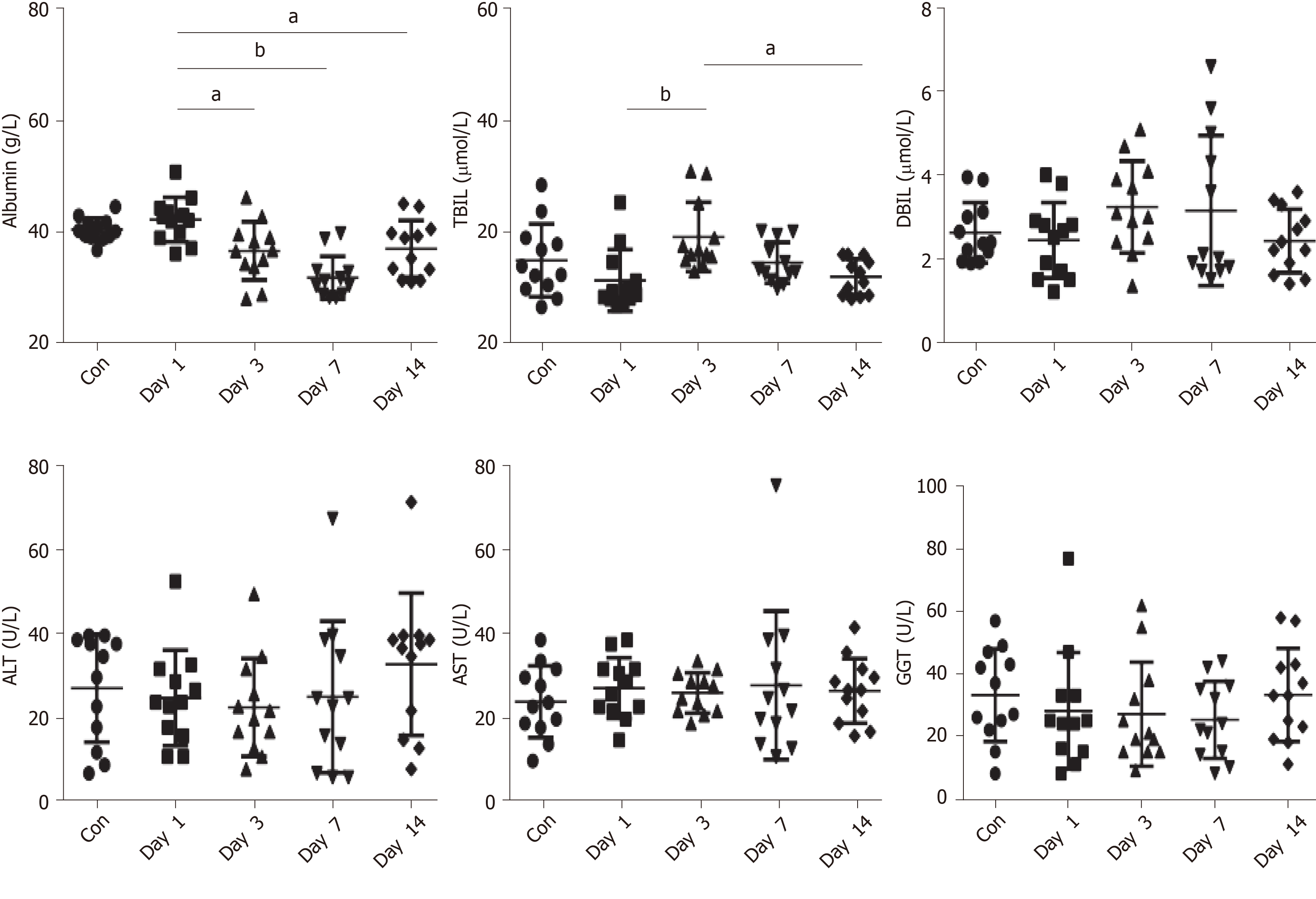
There were no significant differences in albumin, ALT, AST, GGT, and TBIL between the controls and the patients with COVID-19 on day 1 of hospitalization (all P > 0.05) (Figure 1 and Table 2).
| Indexes | Control group | Disease group | ||||
| Day 1 | Day 3 | Day 7 | Day 14 | P value | ||
| Albumin in g/L | 40.42 ± 2.06 | 42.22 ± 3.99 | 36.59 ± 5.2a | 31.80 ± 3.83b | 36.98 ± 5.08a | < 0.001 |
| TBIL in µmol/L | 14.97 ± 6.54 | 11.38 ± 5.53 | 19.19 ± 6.25b | 14.55 ± 3.68 | 12.00 ± 3.22c | 0.025 |
| DBIL in µmol/L | 2.62 ± 0.72 | 2.44 ± 0.90 | 3.24 ± 1.10 | 3.15 ± 1.80 | 2.42 ± 0.76 | 0.199 |
| ALT in U/L | 27.22 ± 13.16 | 24.08 ± 11.46 | 21.82 ± 12.00 | 24.29 ± 20.06 | 32.00 ± 23.46 | 0.753 |
| AST in U/L | 23.21 ± 8.59 | 26.50 ± 7.28 | 25.36 ± 4.38 | 27.14 ± 19.36 | 25.80 ± 7.66 | 0.954 |
| GGT in U/L | 33.23 ± 14.95 | 28.17 ± 18.70 | 27.00 ± 16.95 | 25.29 ± 12.68 | 33.23 ± 14.95 | 0.682 |
Serum albumin showed a decreasing trend from days 0 to 7 of hospitalization, reaching the lowest level on the seventh day (P < 0.001). TBIL was higher on day 3 of hospitalization than on day 7 (P = 0.025). There were no significant changes for the other markers (all P > 0.05). ALT, AST, and GGT did not change significantly over time (Figure 1 and Table 2). The severe patient was observed to have ALT levels of 67 U/L and AST levels of 75 U/L on day 7, ALT of 71 U/L and AST of 35 U/L on day 14, and ALT of 210 U/L and AST of 123 U/L on day 21. No abnormalities of ALT, AST, and GGT were observed in the other 11 patients. After treatment with bicyclol and compound glycyrrhizic acid preparation, liver function injury in the severe patient was improved.
Some patients with COVID-19 display elevated liver enzymes[1,8-13]. Some antiviral drugs that can be used against COVID-19 are associated with a risk of hepatoto-xicity[14,15]. Therefore, the aim of the present study was to analyze the clinical significance of the dynamic monitoring of the liver function of patients with COVID-19. The results suggest that the changes in serum liver function indicators are not obvious in the early stage of COVID-19, but clinically significant changes might be observed in severe COVID-19.
Patients with COVID-19 usually present with respiratory symptoms, such as fever, chest tightness, and cough. Some of them might also display liver biochemical abnormalities at different degrees[1,8-13]. In this study, 11 patients diagnosed with COVID-19 but with mild or moderate symptoms at admission showed no abnormalities in serum TBIL, ALB, ALT, AST, and GGT levels. Abnormalities were observed in one patient with severe COVID-19. This is supported by a previous study that reported little changes in liver function in mild and moderate COVID-19, but that severe cases should be monitored more closely[16]. In addition, Zhang et al[17] suggested that patients with a pre-existing liver condition and severe COVID should be managed more closely. However, this will require more observations to determine the most appropriate course of action. In acute respiratory distress syndrome, liver cirrhosis was independently associated with mortality[18]. Because the two viruses are close parents, the impact of cirrhosis should also be examined in COVID-19. A recent review indicated that liver marker abnormalities are common during the course of COVID-19, but that the clinically relevant liver abnormalities are rare[19]. It also showed that although patients with chronic liver diseases were not at a higher risk of being infected with SARS-CoV-2, patients with cirrhosis, liver cancer, fatty liver disease, liver transplant, or autoimmune hepatic diseases were at higher risk of severe COVID-19[19].
A study speculated that the mechanism of SARS-CoV-2-induced liver function injury might be related to a direct effect of the virus on angiotensin-converting enzyme 2, a receptor for SARS-CoV-2 that is highly expressed in the bile duct epithelium[20]. Previous clinical data showed that alkaline phosphatase and GGT levels reflecting bile duct injury in patients with COVID-19 were not significantly increased[8,9]. Nevertheless, 30 of 56 (54%) patients with COVID-19 had elevated GGT[17], but an exact cause of elevated GGT and alkaline phosphatase could not be found and was attributed to COVID-19. In the present study, all serum-related liver biochemical indices at admission for COVID-19 were not elevated compared with the control group, suggesting that SARS-CoV-2 has only a small direct effect on liver cells.
All patients in this study were treated with antiviral therapy using lopinavir and ritonavir, and the biochemical liver indices were monitored. One patient deteriorated on day 7 of hospitalization and was transferred to the intensive care unit for treatment. Liver biochemical indices were monitored, showing slightly increased ALT and AST. With the progression of the disease, ALT and AST were progressively increased, but GGT and alkaline phosphatase were normal. It is presumed that the causes of liver function injury might be related to the deterioration of his condition, to immune inflammation injury of heart, lung, and liver caused by systemic inflammatory response syndrome, and to deterioration of the condition presenting with respiratory failure and leading to hypoxic liver injury. When the patient’s condition was aggravated, considering a possible combination of bacterial infection, antibiotic treatment was given with lopinavir. In addition, the patient was already treated with lopinavir/ritonavir, and drug-induced liver injury could not be excluded. The patient was treated with high-flow moist oxygen, anti-infection therapy, compound glycyrrhizin, bicyclol, and other treatments. The patient eventually improved, recovered, and was discharged. Several studies reported that elevated ALT, AST, and TBIL in patients with COVID-19 are mainly observed in severe patients[1,8-13]. From the reports and clinical practice, COVID-19 liver injury is likely a secondary liver injury related to a severe inflammatory reaction and hypoxic liver injury. This study dynamically observed a case of severe COVID-19 with liver injury. Because of the retrospective nature of the study, we failed to carry out a liver biopsy to understand the pathological liver changes. Clinically, it was speculated that the main cause of liver injury might be severe systemic inflammatory response syndrome combined with ischemia and hypoxia.
Dynamic observation of other liver biochemical indices revealed that the serum albumin level of all patients was decreased significantly from day 3 to 7 after admission compared with day 1. Serum albumin is synthesized by the hepatic parenchymal cells and has a half-life of about 15-19 d in plasma. Decreased serum albumin is associated with chronic moderate to severe hepatic inflammation, cirrhosis, malnutrition, excessive weight loss, and increased alcohol consumption. All patients in this study had no chronic liver or kidney diseases but a progressive decrease in serum albumin, which might be due to albumin leakage into the interstitial tissues with edema. As our computed tomography characteristics show in Table 1 and other research has shown[21,22], COVID-19 was mainly manifested as interstitial pulmonary edema, and edema is generally accompanied with the leakage of serum albumin[23]. After active treatment of the primary disease and albumin supplementation, all COVID-19 patients showed improvement in serum albumin levels after 14 d. In this study, TBIL was increased in patients with COVID-19 on day 3 of admission compared with day 1. Serum DBIL did not change. The significance of TBIL changes is not clear and needs to be further investigated. Some studies reported that the liver markers recovered without specific treatments[1,24,25]. Future studies should examine whether treatments could help liver recovery in severe patients.
This study has limitations. First, the sample size was small, and only one patient had severe COVID-19. The retrospective nature of the study prevented the analysis of variables that were not routinely collected.
In conclusion, liver cell injury might occur in the early stage of COVID-19. Changes in serum liver function indicators are not obvious in the early stage of COVID-19, but clinically significant changes might be observed in severe COVID-19. Close monitoring on the liver function in severe patients can allow timely intervention for liver damage, help organ function recovery, and avoid deterioration of liver function.
Some patients with the novel 2019 coronavirus disease (COVID-19) display elevated liver enzymes. Some antiviral drugs that can be used against COVID-19 are associated with a risk of hepatotoxicity.
To analyze the clinical significance of the dynamic monitoring of the liver function of patients with COVID-19.
The main objectives of this retrospective trial study was to analyze the clinical significance of the dynamic monitoring of the liver function of patients with COVID-19.
We retrospectively analyzed the liver indexes of patients diagnosed with COVID-19 in our hospital. The serum levels of alanine amino-transferase (ALT), aspartate aminotransferase (AST), γ-glutamyltransferase, and total bilirubin of patients with COVID-19 were measured on days 1, 3, 7, and 14 after admission, and compared to non-COVID-19 patents. We analyzed the dynamic changes in liver function index before and after treatment.
There were no significant differences found in albumin, ALT, AST, γ-glutamyltransferase, and total bilirubin between the controls and the patients with COVID-19 on day 1 of hospitalization (all P > 0.05). Serum albumin showed a decreasing trend from days 0 to 7 of hospitalization, reaching the lowest level on day 7. Total bilirubin was higher on day 3 than on day 7. ALT, AST, and γ-glutamyltransferase did not change significantly over time. The severe patient was observed to have ALT levels of 67 U/L and AST levels of 75 U/L on day 7, ALT of 71 U/L and AST of 35 U/L on day 14, and ALT of 210 U/L and AST of 123 U/L on day 21.
Changes in serum liver function indicators are not obvious in the early stage of COVID-19, but clinically significant changes might be observed in severe COVID-19.
Close monitoring on the liver function in severe patients can allow timely intervention for liver damage, help organ function recovery, and avoid deterioration of liver function.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El Kassas M, Lambrecht NW S-Editor: Zhang L L-Editor: Filipodia P-Editor: Xing YX
| 1. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12977] [Article Influence: 2595.4] [Reference Citation Analysis (1)] |
| 2. | World Health Organization. Novel Coronavirus (2019-nCoV). Situation Reoirt - 22. February 11th, 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-sitrep-22-ncov.pdf?sfvrsn=fb6d49b1_2. Accessed 05-05-2020. |
| 3. | Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1341] [Reference Citation Analysis (0)] |
| 4. | McMichael TM, Clark S, Pogosjans S, Kay M, Lewis J, Baer A, Kawakami V, Lukoff MD, Ferro J, Brostrom-Smith C, Riedo FX, Russell D, Hiatt B, Montgomery P, Rao AK, Currie DW, Chow EJ, Tobolowsky F, Bardossy AC, Oakley LP, Jacobs JR, Schwartz NG, Stone N, Reddy SC, Jernigan JA, Honein MA, Clark TA, Duchin JS; Public Health – Seattle and King County; EvergreenHealth; and CDC COVID-19 Investigation Team. COVID-19 in a Long-Term Care Facility - King County, Washington, February 27-March 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:339-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 282] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 5. | McMichael TM, Currie DW, Clark S, Pogosjans S, Kay M, Schwartz NG, Lewis J, Baer A, Kawakami V, Lukoff MD, Ferro J, Brostrom-Smith C, Rea TD, Sayre MR, Riedo FX, Russell D, Hiatt B, Montgomery P, Rao AK, Chow EJ, Tobolowsky F, Hughes MJ, Bardossy AC, Oakley LP, Jacobs JR, Stone ND, Reddy SC, Jernigan JA, Honein MA, Clark TA, Duchin JS; Public Health–Seattle and King County; EvergreenHealth; and CDC COVID-19 Investigation Team. Epidemiology of Covid-19 in a Long-Term Care Facility in King County, Washington. N Engl J Med. 2020;382:2005-2011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 938] [Cited by in RCA: 926] [Article Influence: 185.2] [Reference Citation Analysis (0)] |
| 6. | Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, Greninger AL, Pipavath S, Wurfel MM, Evans L, Kritek PA, West TE, Luks A, Gerbino A, Dale CR, Goldman JD, O'Mahony S, Mikacenic C. Covid-19 in Critically Ill Patients in the Seattle Region - Case Series. N Engl J Med. 2020;382:2012-2022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1819] [Cited by in RCA: 1862] [Article Influence: 372.4] [Reference Citation Analysis (0)] |
| 7. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium; Barnaby DP; Becker LB; Chelico JD; Cohen SL; Cookingham J; Coppa K; Diefenbach MA; Dominello AJ; Duer-Hefele J; Falzon L; Gitlin J; Hajizadeh N; Harvin TG; Hirschwerk DA; Kim EJ; Kozel ZM; Marrast LM; Mogavero JN; Osorio GA; Qiu M; Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6518] [Article Influence: 1303.6] [Reference Citation Analysis (0)] |
| 8. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18878] [Article Influence: 3775.6] [Reference Citation Analysis (7)] |
| 9. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30123] [Article Influence: 6024.6] [Reference Citation Analysis (3)] |
| 10. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14767] [Article Influence: 2953.4] [Reference Citation Analysis (0)] |
| 11. | Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2493] [Cited by in RCA: 2310] [Article Influence: 462.0] [Reference Citation Analysis (0)] |
| 12. | Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, Li SB, Wang HY, Zhang S, Gao HN, Sheng JF, Cai HL, Qiu YQ, Li LJ. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1298] [Cited by in RCA: 1263] [Article Influence: 252.6] [Reference Citation Analysis (0)] |
| 13. | Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6231] [Cited by in RCA: 6660] [Article Influence: 1332.0] [Reference Citation Analysis (0)] |
| 14. | Canta F, Marrone R, Bonora S, D'Avolio A, Sciandra M, Sinicco A, De Rosa FG, Di Perri G. Pharmacokinetics and hepatotoxicity of lopinavir/ritonavir in non-cirrhotic HIV and hepatitis C virus (HCV) co-infected patients. J Antimicrob Chemother. 2005;55:280-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Núñez M. Hepatotoxicity of antiretrovirals: incidence, mechanisms and management. J Hepatol. 2006;44:S132-S139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 16. | Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 357] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 17. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 18. | Gacouin A, Locufier M, Uhel F, Letheulle J, Bouju P, Fillatre P, Le Tulzo Y, Tadié JM. Liver Cirrhosis is Independently Associated With 90-Day Mortality in ARDS Patients. Shock. 2016;45:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Garrido I, Liberal R, Macedo G. Review article: COVID-19 and liver disease-what we know on 1st May 2020. Aliment Pharmacol Ther. 2020;52:267-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 20. | Chai X, Hu L, and Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Fan J, Lan F. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. BioRxiv. 2020;. [DOI] [Full Text] |
| 21. | Solaimanzadeh I. Acetazolamide, Nifedipine and Phosphodiesterase Inhibitors: Rationale for Their Utilization as Adjunctive Countermeasures in the Treatment of Coronavirus Disease 2019 (COVID-19). Cureus. 2020;12:e7343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 22. | Lu W, Zhang S, Chen B, Chen J, Xian J, Lin Y, Shan H, Su ZZ. A Clinical Study of Noninvasive Assessment of Lung Lesions in Patients with Coronavirus Disease-19 (COVID-19) by Bedside Ultrasound. Ultraschall Med. 2020;41:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 23. | Sharma HS, Miclescu A, Wiklund L. Cardiac arrest-induced regional blood-brain barrier breakdown, edema formation and brain pathology: a light and electron microscopic study on a new model for neurodegeneration and neuroprotection in porcine brain. J Neural Transm (Vienna). 2011;118:87-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 25. | Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 575] [Article Influence: 115.0] [Reference Citation Analysis (0)] |