Published online Mar 6, 2021. doi: 10.12998/wjcc.v9.i7.1532
Peer-review started: July 28, 2020
First decision: November 23, 2020
Revised: December 7, 2020
Accepted: December 23, 2020
Article in press: December 23, 2020
Published online: March 6, 2021
Processing time: 216 Days and 0.1 Hours
As transjugular intrahepatic portosystemic shunt (TIPS) creation alters the hemodynamic status of the portal system, whether reduced portal blood supply affects the synthetic reserve function of the liver has been the focus of clinical attention. Since the Viatorr stent entered the Chinese market in 2015, it has not yet been widely used in clinical practice. Further, unlike other countries, the main cause of liver cirrhosis in China is viral hepatitis. Therefore, use of the Viatorr stent to establish a TIPS channel in patients with liver cirrhosis with differing etiologies is of great clinical interest.
To investigate factors affecting changes in liver reserve function after TIPS Viatorr stent implantation.
Clinical data from 200 patients with cirrhotic portal hypertension who received TIPS treatment from March 2016 to March 2020 were analyzed retrospectively. The patients were divided into three groups (A-C), according to their disease etiology, with post-hepatitis, autoimmune, and alcoholic cirrhosis, respectively. Preoperative and postoperative liver and renal function and coagulation data, Child-Pugh grade, and model for end-stage liver disease (MELD) scores were collected. Statistical analyses were performed using the t-test or chi-square test. The incidence and of hepatic encephalopathy and patient survival were calculated using Kaplan-Meier method.
The surgical success rate was 100%, with mean portal pressure gradient (mmHg) decreasing from 25.5 ± 5.22 to 10.04 ± 2.76 (t = 45.80; P < 0.001). After 24 mo, the cumulative incidence of hepatic encephalopathy in group A was significantly lower than that in group B/C, while the cumulative survival rate was significantly higher in group A than in group B/C (P < 0.05 for both). The Child-Pugh score for group A was 6.96 ± 1.21, which was significantly better than those of groups B (7.42 ± 0.99; t = -2.44; P = 0.016) and C (7.52 ± 1.12; t = -2.67; P = 0.009). Further, the MELD score for group A (9.62 ± 2.19) was significantly better than those for groups B (10.64 ± 1.90; t = -2.92; P = 0.004) and C (10.82 ± 2.01; t = -3.29; P = 0.001).
Insertion of 8 mm internal diameter Viatorr stent has no significant effects on liver reserve function. Changes of liver reserve function in the medium and long term may be related to the etiology and treatment of portal hypertension.
Core Tip: As transjugular intrahepatic portosystemic shunt (TIPS) creation alters the hemodynamic status of the portal system, whether reduced portal blood supply affects the synthetic reserve function of the liver has been the focus of clinical attention. Since the Viatorr stent entered the Chinese market in 2015, it has not yet been widely used in clinical practice. Further, unlike other countries, the main cause of liver cirrhosis in China is viral hepatitis. Therefore, use of the Viatorr stent to establish a TIPS channel in patients with liver cirrhosis with differing etiologies is of great clinical interest. The purpose of this study was to investigate factors affecting changes in liver reserve function after TIPS Viatorr stent implantation, and to find ways to reduce the occurrence of liver failure and improve long-term survival rates.
- Citation: Yao X, Zhou H, Huang S, Tang SH, Qin JP. Effects of transjugular intrahepatic portosystemic shunt using the Viatorr stent on hepatic reserve function in patients with cirrhosis. World J Clin Cases 2021; 9(7): 1532-1542
- URL: https://www.wjgnet.com/2307-8960/full/v9/i7/1532.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i7.1532
Transjugular intrahepatic portosystemic shunt (TIPS) can effectively reduce portal hypertension. It is a commonly used minimally invasive interventional therapy for many patients with complications of cirrhosis, which is safe, effective, and repeatable. As TIPS creation alters the hemodynamic status of the portal system, whether reduced portal blood supply affects the synthetic reserve function of the liver has been the focus of clinical attention. Early clinical studies were based on the Fluency stents in establishing a shunt in the portal vein; stents should be placed in the portal vein so that sufficient length remains for the stent to shunt blood from the portal trunk, and affect portal vein branch blood supply. The Viatorr stent can be used to establish a TIPS shunt channel, where the bare stent region is established in a branch of the portal vein, to ensure smooth blood flow in the portal vein and avoid excess blood that has not passed directly through the liver entering the systemic circulation. Since the Viatorr stent entered the Chinese market in 2015, it has not yet been widely used in clinical practice. Further, unlike other countries, the main cause of liver cirrhosis in China is viral hepatitis. Therefore, use of the Viatorr stent to establish a TIPS channel in patients with liver cirrhosis with differing etiologies is of great clinical interest. The purpose of this study was to investigate factors affecting changes in liver reserve function after TIPS Viatorr stent implantation, and to find ways to reduce the occurrence of liver failure and improve long-term survival rates.
Data was collected from 280 patients with portal hypertension and cirrhosis treated by Viatorr stent TIPS from March 2016 to December 2019. The inclusion criteria were: (1) Patients with decompensated portal hypertension treated by TIPS for the first time; (2) Only one stent (the Viatorr stent) was used; and (3) TIPS operation using a stent with a diameter of 8 mm. The exclusion criteria were as follows: (1) Patients were excluded based on the results of imaging before surgery; (2) History of splenectomy; (3) Stent dysfunction; (4) Preoperative liver function Child-Pugh score > 13; (5) Portal vein cavernous change or portal vein thrombosis; (6) History of hepatic encephalopathy, hepatorenal syndrome, or hepatopulmonary syndrome before operation; (7) Previous liver lobectomy, surgical shunt, liver transplantation, or other major liver surgery; (8) Severe coagulation disorder; (9) Severe right heart failure and pulmonary hypertension; (10) Uncontrolled intrahepatic or systemic infection; (11) Pregnancy or lactation; (12) Follow-up < 24 mo; or (13) Cirrhosis treated with warfarin or other anticoagulants. Finally, 200 patients with cirrhosis, of whom 119 were men and 81 women, with an average age 56.2 ± 8.8 years, were included in the study. The patients were divided into groups according to disease etiology, as follows: n = 71, post-hepatitis cirrhosis (group A); n = 67, alcoholic cirrhosis (group B); n = 62, autoimmune cirrhosis (group C). All patients were treated in accordance with the United States TIPS guidelines[1]. This study was approved by the Hospital Ethics Committee. The patients and their families were fully informed and provided written consent for surgery.
The digital subtraction angiography equipment used was the AXIOM-Artist DSA system (Siemens, Germany) and Mark V type high pressure syringe. The surgical materials used included RUPS-100 puncture kit, straight-headed multi-sided foramen catheter, Opta Pro balloon catheter, embolic spring coil, Cobra catheter (Cook Inc., United States), stiff exchange guide wire, and Viatorr stent (Goreand Associates Inc., United States).
Preoperative preparation: The patients underwent routine blood evaluation, liver and kidney function tests, and prothrombin time examination. All patients underwent contrast-enhanced computed tomography (CT) of the liver and three-dimensional reconstruction of hepatic vein-portal vein vessels to analyze the anatomical relationship between the hepatic and portal veins and guide intraoperative puncture of the portal vein.
TIPS approach: All patients underwent surgery to the right jugular vein, by puncture through the superior vena cava, right atrium to right hepatic vein, or inferior vena cava, according to the patient's preoperative contrast-enhanced liver CT and three-dimensional reconstruction image of hepatic vein-portal vein, to guide portal vein branch puncture. Puncture safety was evaluated and portal vein pressure measured. Portal vein puncture and pressure parameter measurements were conducted as previously described[2,3]. An 8 mm diameter balloon and Viatorr stent were used for all patients. All operations were successfully completed by the same group of professionals, with no serious complications of surgery.
Postoperative follow-up: Patient data, including liver and renal function and coagulation data, were collected from preoperative examination, then at 1 wk and 1, 3, 6, 12, and 24 mo postoperative follow-up. Data from ultrasound examination and medical records were used to assess ascites and hepatic encephalopathy. Child-Pugh grade and model for end-stage liver disease (MELD) score were obtained. The end point of follow-up was defined as death or two consecutive follow-ups without response, liver transplantation, or deadline (December 2019). The methods used for follow-up assessment were published previously[4-6].
All data are expressed as the mean ± SD or percentages. Portal pressure was measured before and after treatment and the results were compared using the paired t test or chi-square test. The incidence of hepatic encephalopathy and patient survival were calculated using the Kaplan-Meier method. All statistical analyses were conducted using SPSS 22.0 software. P < 0.05 was considered statistically significant.
There were no significant differences in sex ratio, age, etiology, preoperative Child-Pugh grade, preoperative MELD score, or preoperative portal pressure gradient among the three patient groups (P > 0.05 for all) (Table 1).
| Clinical factor | Hepatitis cirrhosis | Alcoholic cirrhosis | Autoimmune cirrhosis | Statistic | P value |
| Gender (n) | χ2 = 3.43 | 0.18 | |||
| Male | 46 | 42 | 31 | ||
| Female | 25 | 25 | 31 | ||
| Age (yr, mean ± SD) | 55.5 ± 8.7 | 54.7 ± 8.5 | 55.3 ± 9.6 | F = 0.15 | 0.87 |
| Child-Pugh Classification | χ2 = 3.19 | 0.53 | |||
| A | 32 | 25 | 19 | ||
| B | 34 | 35 | 37 | ||
| C | 5 | 7 | 6 | ||
| Child-Pugh score | 7.25 ± 1.54 | 7.55 ± 1.45 | 7.48 ± 1.29 | F = 0.82 | 0.44 |
| MELD score | 10.24 ± 3.52 | 9.67 ± 2.90 | 10.47 ± 2.97 | F = 1.11 | 0.33 |
| Prothrombin time (s) | 14.59 ± 2.79 | 14.98 ± 2.46 | 14.74 ± 2.92 | F = 0.36 | 0.69 |
| ALT (IU/L) | 35.28 ± 16.40 | 37.75 ± 12.17 | 36.95 ± 10.82 | F = 0.42 | 0.67 |
| AST (IU/L) | 48.87 ± 24.43 | 50.13 ± 23.10 | 50. 75 ± 24.34 | F = 0.06 | 0.94 |
| Albumin (g/L) | 35.09 ± 6.33 | 34.14 ± 4.52 | 34.23 ± 4.44 | F = 0.69 | 0.50 |
| Total bilirubin (μmol/L) | 33.53 ± 12.99 | 32.75 ± 14.88 | 32.47 ± 16.84 | F = 0.04 | 0.96 |
| PPG before TIPS (mmHg) | 24.58 ± 4.79 | 25.45 ± 5.16 | 25.27 ± 5.65 | F = 1.78 | 0.17 |
| PPG after TIPS (mmHg) | 9.94 ± 2.55 | 10.22 ± 3.07 | 10.01 ± 2.65 | F = 0.22 | 0.80 |
| Deaths in 24 mo, n (%) | 8 (11.3) | 12 (17.9) | 9 (14.5) | χ2 = 1.23 | 0.54 |
| HE in 24 mo, n (%) | 15 (21.1) | 17 (25.4) | 18 (29.0) | χ2 = 1.11 | 0.57 |
All patients had TIPS approached from the right jugular vein, and all 200 operations were successful. There was no abdominal cavity, bile duct hemorrhage, or other serious intraoperative complications. Collateral circulation and stent placement induction of esophageal and gastric varices were reduced by successful embolization. The success rate of operation was 100%, as was the recent hemostasis rate. Portal vein pressure (mmHg) decreased from 25.5 ± 5.22 preoperatively to 10.04 ± 2.76 postoperatively (t = 45.80, P = 0.001).
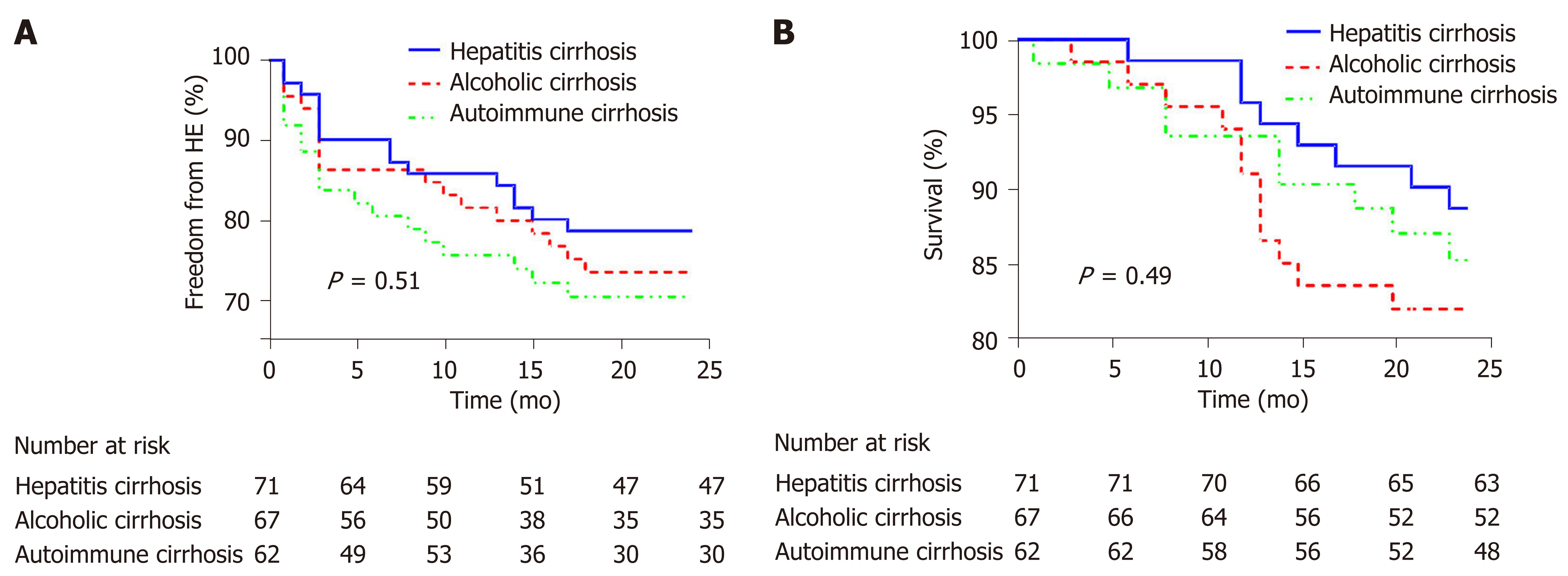
The cumulative incidences of hepatic encephalopathy in the three groups combined were 18.8% and 25.4% at 12 and 24 mo, respectively. There were 36 cases of hepatic encephalopathy after 12 mo, with a group A/B/C ratio of 5%/6%/7%, and cumulative incidence of 14.1%/18.3%/24.2%. There were 50 cases of hepatic encephalopathy at 24 mo, with a group A/B/C ratio of 6%/8%/11%, and cumulative incidence 21.2%/26.3%/29.4%. Among these cases, 22 cases of grade I hepatic encephalopathy and 28 cases of grade II were relieved by drug treatment (Figure 1A).
The median follow-up time was 24 mo, and the overall cumulative survival rate was 93.5% at 12 mo and 85.4% at 24 mo. The cumulative survival rate was significantly higher in group A than in group B/C after 24 mo (P < 0.05) (Figure 1B). There were 29 deaths during follow-up, comprising 8 (11.3%), 12 (17.9%), and 9 (12.9%) in groups A, B, and C, respectively. Of these, 11 patients died of liver failure, comprising 2 (2%), 5 (7%), and 4 (6%) in groups A, B, and C, respectively.
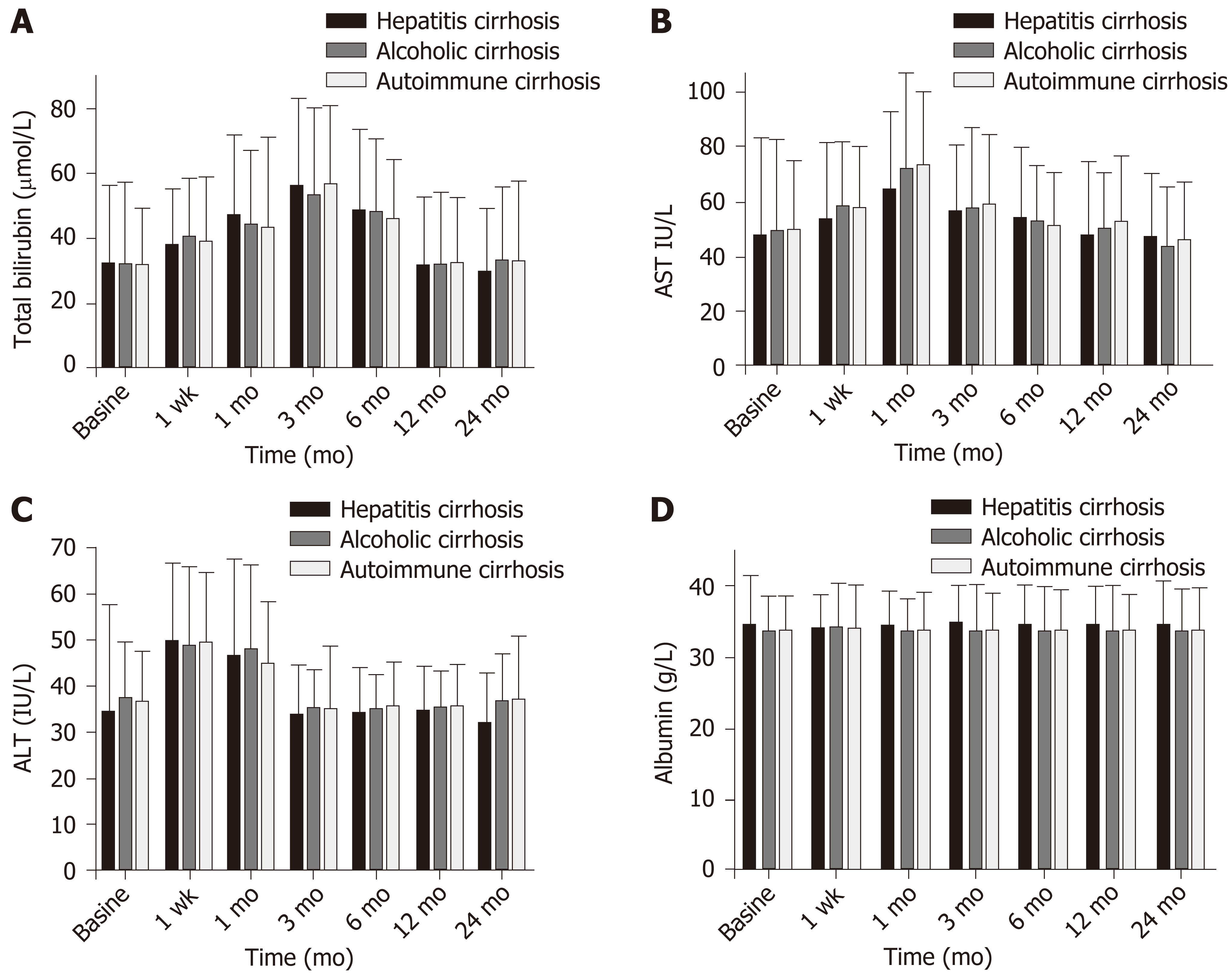
Total bilirubin (mainly indirect bilirubin) increased gradually in all three groups after surgery. The mean preoperative value was 32.93 ± 16.83 μmol/L, and the levels reached a peak value at 3 mo (56.07 ± 20.73 μmol/L; t = -10.86, P = 0.001). At 12 mo, the levels had recovered to baseline (33.49 ± 19.89; t = -0.27, P = 0.78). There was no significant difference between the two groups (Figure 2A).
During the first week following surgery, aspartate aminotransferase (AST) levels increased gradually in all the three groups. Mean preoperative AST was 50.82 ± 32.75 IU/L, compared with a peak of 69.95 ± 27.8 IU/L (t = -5.91, P < 0.001). Baseline value was 53.67 ± 1.56 IU/L (t =1.15, P = 0.25). At 1-mo follow-up, the level in patients with Child-Pugh class A was 55.67 ± 20.2 IU/L, which was significantly higher than the level in those with Child-Pugh class B (48.28 ± 16.1 IU/L; t = 2.83; P = 0.005) or C (44.8 ± 15.18 IU/L; t = 2.22; P = 0.029) (Figure 2B).
Alanine aminotransferase (ALT) levels increased gradually during the first week following surgery in all the three groups. Mean preoperative ALT value was 36.73 ± 16.17 IU/L, compared with a peak of 49.88 ± 17.34 IU/L (t = -9.41; P < 0.001). The mean baseline value was 35.30 ± 10.43 IU/L (t = 1.15; P = 0.25). At 1 wk after surgery compared with preoperative value, ALT level in patients with Child-Pugh class A (77.10 ± 30.38 IU/L) was significantly higher than that of patients with Child-Pugh class B (67.74 ± 26.14; t =2.15; P = 0.03) or C (60.61 ± 18.92 IU/L; t = 2.07; P = 0.024) (Figure 2C).
Mean albumin level in the three groups combined was 36.11 ± 6.9 g/L, which was significantly higher than that before surgery (t = -2.91; P = 0.004). At 24 mo postoperatively, mean albumin level in group A (35.90 ± 5.36 g/L) was significantly higher than those in B group (33.34 ± 6.46 g/L; t = 2.54; P = 0.01) and group C (33.53 ± 5.46 g/L; t = 2.52; P = 0.01) (Figure 2D).
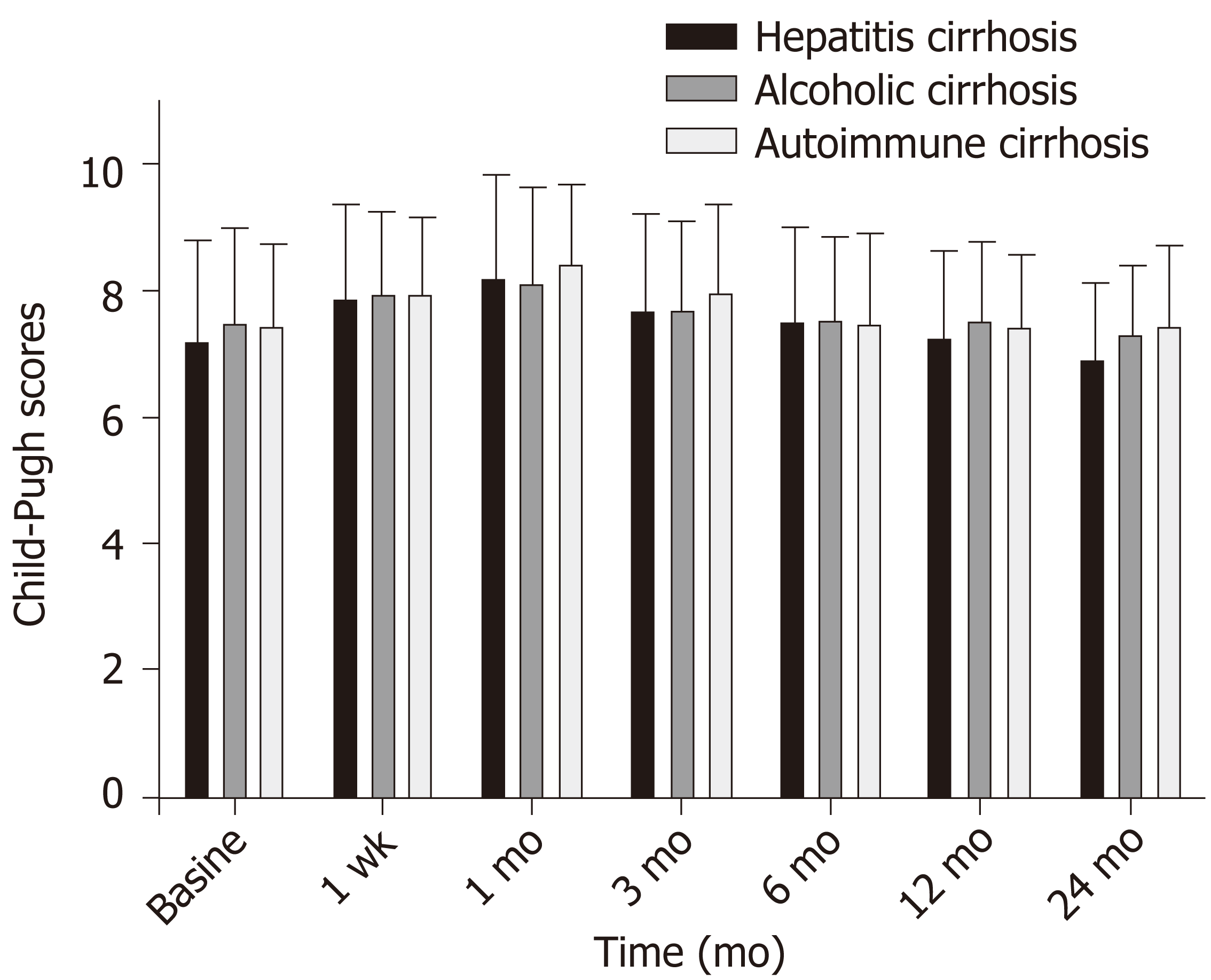
Mean Child-Pugh score for the three groups combined was 7.43 ± 1.43, with a maximum of 8.28 ± 1.45 (t = -6.10, P < 0.001). The scores returned to baseline value (7.48 ± 1.91; t = -0.46) at 12 mo (P = 0.65). The mean Child-Pugh score for group A was 6.96 ± 1.21, which was significantly better than those for groups B (7.42 ± 0.99; t = -2.44; P = 0.016) and C (7.52 ± 1.12; t = -2.67; P = 0.009) (Figure 3).
There was no significant difference in the Child-Pugh grade ratio among the three groups (A/B/C; P > 0.05). The composition ratio of the Child-Pugh score was 54% in group A, which was significantly higher than that preoperatively (25.4%; χ2 = 11.51; P = 0.001). The composition ratio for group B was 44.4%, which was lower than that preoperatively (63.4%; χ2 = 4.83; P = 0.03). Similarly, the composition ratio for group C was 1.6%, which was significantly lower than that preoperatively (11.3%; χ2 = 4.99; P = 0.02). There was no significant difference in the Child-Pugh A/B/C grade composition ratio or preoperative composition ratio in group B (χ2 = 0.17; P = 0.92), nor was there a significant difference between the Child-Pugh score A/B/C grade composition ratio in group C (χ2 = 0.93; P = 0.63) (Table 2).
| Baseline | 1 wk | 1 mo | 3 mo | 6 mo | 12 mo | 24 mo | |
| Hepatitis cirrhosis (n) | 18/45/8 (71) | 14/46/11 (71) | 10/52/9 (71) | 9/53/9 (71) | 16/49/5 (70) | 23/42/3 (68) | 34/28/1 (63) |
| Child-Pugh A/B/C (%) | 25.4/63.4/11.3 | 19.7/64.8/15.5 | 14.1/73.2/12.7 | 12.7/74.6/12.7 | 22.9/70.0/7.1 | 33.8/61.8/4.4 | 54.0/44.4/1.6 |
| Alcoholic cirrhosis (n) | 14/46/7 (67) | 7/52/8 (67) | 8/51/8 (67) | 6/49/12 (67) | 12/45/8 (65) | 15/41/5 (61) | 13/37/5 (55) |
| Child-Pugh A/B/C (%) | 20.8/68.7/10.4 | 10.4/77.6/11.9 | 11.9/76.1/12.0 | 8.9/73.2/17.9 | 18.5/69.2/12.3 | 24.6/67.2/8.2 | 23.6/67.3/9.1 |
| Autoimmune cirrhosis (n) | 12/45/5 (62) | 7/49/6 (62) | 10/43/8(61) | 8/41/12 (61) | 11/43/6 (60) | 13/43/2 (58) | 14/35/4 (53) |
| Child-Pugh A/B/C (%) | 19.4/72.6/8.1 | 11.3/79.0/9.7 | 16.4/70.5/13.1 | 13.1/67.2/19.7 | 18.3/71.7/10.0 | 22.4/74.1/3.5 | 26.4/66.1/7.5 |
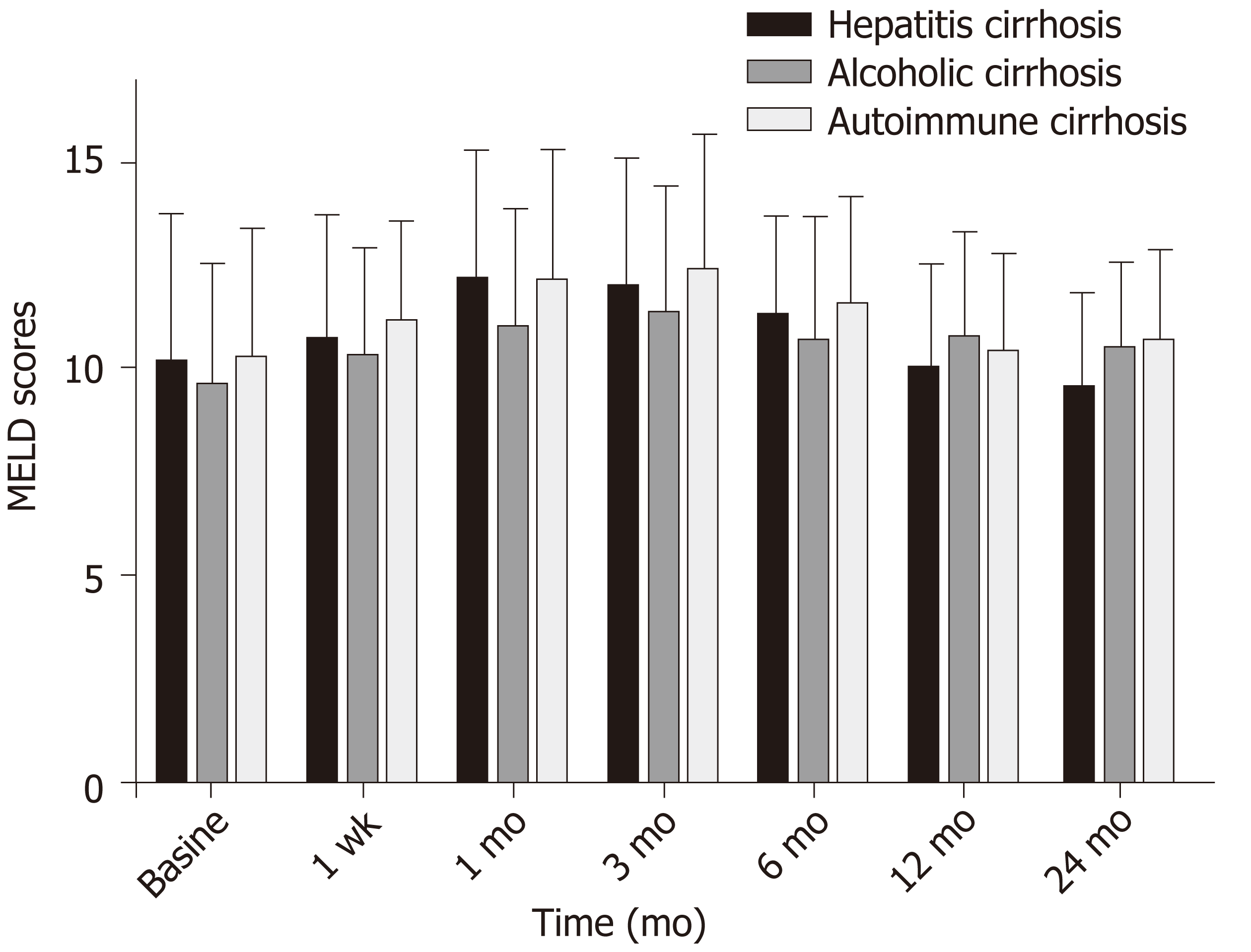
Before surgery, the mean MELD score for the three patient groups combined was 10.12 ± 3.1, rising to a peak of 12.01 ± 3.02 (t = -6.45; P = 0.001) at 3 mo following surgery, and recovering to the baseline value (10.50 ± 2.36; t = -1.44; P = 0.15) at 12 mo. The mean MELD score for patients in group A was 9.62 ± 2.19 after 24 mo, which was significantly better than those for group B (10.64 ± 1.90; t = -2.92; P = 0.004) and group C (10.82 ± 2.01; t = -3.29; P = 0.001) (Figure 4).
In recent years, the treatment of portal hypertension complications of cirrhosis has entered the era of minimally invasive intervention, as represented by TIPS surgery. The application of Viatorr stents in the clinic has basically resolved the major complication of postoperative stent dysfunction[7], and the effects on the heart and changes in liver reserve function have become the focus of clinical attention. Reports from outside China[8] indicate that a small number of patients develop postoperative liver infarction and acute liver failure following TIPS, resulting in high mortality rates. Experts in China[9] report that hepatic function can be affected by the use of contrast enhanced liver CT and three-dimensional reconstruction of the hepatic vein and portal vein, instead of indirect portal angiography, and controlled stent diameter reduction. Our center began applying 8 mm diameter balloon and Viatorr stents in the clinic in March 2016. Child-Pugh grade and MELD score were used to assess liver reserve function.
TIPS treatment of cirrhosis with different causes can lead to portal hypertension complications, where portal vein pressure gradient decreases significantly. No postoperative hepatic infarction or acute liver failure occurred in any of the patients included in this study. Previous clinical studies in China have been based on the establishment of a shunt channel in the portal vein using Fluency covered stents. These stents should be placed in the length of the portal vein, where they influence the blood supply of the portal branch. Viatorr supports have excellent flexibility and strong radial support, provided the stent is covered by liver parenchyma. To avoid stent obstruction of the portal branch or trunk blood supply, sufficient length in the hepatic vein is required to prevent liver infarction, which can prevent the occurrence of postoperative acute liver failure.
Liver reserve function is an indicator of the potential size of the liver and is the sum of all liver cells with normal physiological function. In patients with cirrhosis, liver function damage following TIPS surgery has been linked to a reduction of portal blood supply by scholars outside China[6,10] as reported in the literature[11].
According to the 2019 Guidelines for Clinical Practice of Transjugular Intrahepatic Portal Splitting with Portal High Pressure in China[12], the hemodynamic changes associated with TIPS can lead to liver ischemia, which is more common in patients with high Child-Pugh scores. In this study we found that the liver function 1 wk after TIPS surgery in patients with liver cirrhosis with different etiologies were more serious in patients with class A Child-Pugh scores than in those with class B/C. ALT and AST were initially elevated. We propose that the reduction of pressure caused by TIPS in the posterior vein and hepatic sinus can lead to congestion of the spleen and gastrointestinal tract. Patients with Child-Pugh grade A have better liver reserve function, which increases the amount of circulating blood in the periphery. This means that they are less likely to experience sudden ischemia, acute injury, or impaired liver function, than patients with Child-Pugh class B/C.
Researchers from outside China[13] have proposed that increased bilirubin and the establishment of a TIPS shunt, can result in puncture of the liver, and placement of the stent can lead to the extrusion of liver tissue, impeding normal bile discharge. Simultaneously, portal vein blood shunt decreases liver perfusion and metabolic function. In this study, we found that postoperative bilirubin (mainly indirect bilirubin) gradually increased within 1 wk of surgery, then recovered to the baseline level at 12 mo, which was later than the recovery of enzymatic liver injury indices. We consider that the observed short-term increase in bilirubin may be related to partial shunt of blood non-liver metabolism, since 70%-80% of bilirubin is derived from the decomposition of hemoglobin from aging red blood cells. Liver cells are required for the uptake, transport, esterification, and excretion of bilirubin. Following shunt surgery, partial non-liver metabolism of bilirubin in the systemic circulation causes an increase in bilirubin. Surgically-induced liver injury is infrequent and self-limited, or requires only conservative treatment, in most patients. Due to limitation of the stent shunt to 8 mm diameter, bilirubin will gradually return to baseline levels, leading to a rebalancing of the systemic blood metabolism. Albumin levels were better in all patients after 12 mo of follow-up than those before the operation, and there were no significant differences in Child-Pugh or MELD scores, indicating that the use of an 8 mm Viatorr stent to establish a shunt channel had no clear effect on liver reserve function in the short term, while some liver function indices could be improved.
Current Chinese guidelines for TIPS do not explain the effects of TIPS on long-term liver reserve function following surgery, while studies from other countries have shown that TIPS surgery has no effect on long-term liver reserve function[14]. In this study, follow-up examination demonstrated that the incidence of hepatic encephalopathy was lower in group A 24 mo after surgery than in group B/C, while the cumulative survival rate was higher in group A than in group B/C.
Effective perfusion of the liver and levels of portal vein endotoxin are important factors affecting liver function and influencing the development of hepatic encephalopathy under normal conditions. In view of the consistency of preoperative Child-Pugh class, preoperative MELD score, and preoperative portal pressure gradient, and the consistency of the internal diameter of the shunt stent, we propose that this difference may not be caused by the TIPS-mediated decrease in hepatic perfusion in the portal vein, consistent with numerous previous publications[15,16]. We suggest that the differences described above may be related to the compensatory capacity of residual liver cells in patients with liver cirrhosis with different etiologies. Hepatocyte damage can be reduced, or even terminated, after anti-hepatitis virus treatment in patients with post-hepatitis cirrhosis, and liver reserve function can be improved by hepatocyte regeneration. Patients with hepatitis B and hepatitis C were treated with antiviral and basic therapy following surgery. Alcoholic cirrhosis is a form of small nodular cirrhosis, and the regeneration of liver cells in nodules is often not clinically significant, because patients are unable to abstain from alcohol in long term, leading to a decline in liver reserve function, and death in severe cases, due to liver failure. Once in the cirrhosis phase, hepatocytes are the target of immune attack in autoimmune liver disease, and immune damage will continue to occur following stent placement, since immune intervention maintenance treatment is rare because of a fear of the negative effects of hormone therapy. Clinical practice has proven that etiological and basic treatments for alcoholic and autoimmune forms of cirrhosis are particularly important, and key to reducing the incidence of hepatic encephalopathy and improving post-surgery survival rates. Management of patients after surgery using individualized regimens is worthy of further clinical exploration.
We conclude that the use of Viatorr stent for TIPS surgery in patients with cirrhosis complications of portal hypertension has no significant effect on postoperative liver reserve function. For patients with cirrhosis, portal pressure is continuously and effectively controlled and, while etiological and basic treatment are equally important, comprehensive treatment can significantly improve survival rates and reduce the occurrence of hepatic encephalopathy. The results presented here are from a single-center retrospective study and, as the long-term efficacy of TIPS surgery is related to clinical factors, postoperative patient management, and many other variables, clinical research from multi-center studies with large samples is required to further support our findings.
As transjugular intrahepatic portosystemic shunt (TIPS) creation alters the hemodynamic status of the portal system, whether reduced portal blood supply affects the synthetic reserve function of the liver has been the focus of clinical attention.
Early clinical studies were based on the Fluency stents in establishing a shunt in the portal vein; stents should be placed in the portal vein so that sufficient length remains for the stent to shunt blood from the portal trunk, and affect portal vein branch blood supply. The Viatorr stent can be used to establish a TIPS shunt channel, where the bare stent region is established in a branch of the portal vein, to ensure smooth blood flow in the portal vein and avoid excess blood that has not passed directly through the liver entering the systemic circulation. Since the Viatorr stent entered the Chinese market in 2015, it has not yet been widely used in clinical practice. Further, unlike other countries, the main cause of liver cirrhosis in China is viral hepatitis. Therefore, use of the Viatorr stent to establish a TIPS channel in patients with liver cirrhosis with differing etiologies is of great clinical interest.
The purpose of this study was to investigate factors affecting changes in liver reserve function after TIPS Viatorr stent implantation, and to find ways to reduce the occurrence of liver failure and improve long-term survival rates.
Clinical data from 200 patients with cirrhotic portal hypertension who received TIPS treatment from March 2016 to March 2020 were analyzed retrospectively. The patients were divided into three groups (A-C), according to their disease etiology, with post-hepatitis, autoimmune, and alcoholic cirrhosis, respectively. Preoperative and postoperative liver and renal function and coagulation data, Child-Pugh class, and model for end-stage liver disease (MELD) scores were collected. Statistical analyses were performed using the t-test or chi-square test. The incidence of hepatic encephalopathy and patient survival were calculated using the Kaplan-Meier method.
The surgical success rate was 100%, with mean portal pressure gradient (mmHg) decreasing from 25.5 ± 5.22 to 10.04 ± 2.76 (t = 45.80; P < 0.001). After 24 mo, the cumulative incidence of hepatic encephalopathy in group A was significantly lower than that in group B/C, while the cumulative survival rate was significantly higher in group A than in group B/C (P < 0.0.5 for both). The Child-Pugh score for group A was 6.96 ± 1.21, which was significantly better than those of groups B (7.42 ± 0.99; t = -2.44; P = 0.016) and C (7.52 ± 1.12; t = -2.67; P = 0.009). Further, the MELD score for group A (9.62 ± 2.19) was significantly better than those for groups B (10.64 ± 1.90; t = -2.92; P = 0.004) and C (10.82 ± 2.01; t = -3.29; P = 0.001).
Insertion of a Viatorr stent with an internal diameter of 8 mm has no significant effects on liver reserve function.
Changes of liver reserve function in the medium and long term may be related to the etiology and treatment of portal hypertension.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tsoulfas G S-Editor: Huang P L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Boyer TD, Haskal ZJ; American Association for the Study of Liver Diseases. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology. 2010;51:306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 405] [Article Influence: 27.0] [Reference Citation Analysis (1)] |
| 2. | Qin JP, Tang SH, Jiang MD, He QW, Chen HB, Yao X, Zeng WZ, Gu M. Contrast enhanced computed tomography and reconstruction of hepatic vascular system for transjugular intrahepatic portal systemic shunt puncture path planning. World J Gastroenterol. 2015;21:9623-9629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Tang S, Qin J, Jiang M, He Q, Yao X, Zeng W, Gu M. [The agreement and clinical value of hepatic vein pressure gradient and portal vein pressure in patients with portal hypertension]. Zhonghua Ganzang Bing Zazhi. 2015;23:354-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Tripathi D, Stanley AJ, Hayes PC, Patch D, Millson C, Mehrzad H, Austin A, Ferguson JW, Olliff SP, Hudson M, Christie JM; Clinical Services and Standards Committee of the British Society of Gastroenterology. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. 2015;64:1680-1704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 454] [Cited by in RCA: 416] [Article Influence: 41.6] [Reference Citation Analysis (2)] |
| 5. | Chinese Society of Hepatology, Chinese Medical Association. [Guidelines on the management of ascites and complications in cirrhosis]. Zhonghua Ganzang Bing Zazhi. 2017;25:664-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Bercu ZL, Fischman AM, Kim E, Nowakowski FS, Patel RS, Schiano TD, Chang CY, Lookstein RA. TIPS for refractory ascites: a 6-year single-center experience with expanded polytetrafluoroethylene-covered stent-grafts. AJR Am J Roentgenol. 2015;204:654-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Hausegger KA, Karnel F, Georgieva B, Tauss J, Portugaller H, Deutschmann H, Berghold A. Transjugular intrahepatic portosystemic shunt creation with the Viatorr expanded polytetrafluoroethylene-covered stent-graft. J Vasc Interv Radiol. 2004;15:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Lopera JE, Katabathina V, Bosworth B, Garg D, Kroma G, Garza-Berlanga A, Suri R, Wholey M. Segmental liver ischemia/infarction after elective transjugular intrahepatic portosystemic shunt creation: clinical outcomes in 10 patients. J Vasc Interv Radiol. 2015;26:835-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Qin JP, Jiang MD, Tang W, Wu XL, Yao X, Zeng WZ, Xu H, He QW, Gu M. Clinical effects and complications of TIPS for portal hypertension due to cirrhosis: a single center. World J Gastroenterol. 2013;19:8085-8092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Tan HK, James PD, Sniderman KW, Wong F. Long-term clinical outcome of patients with cirrhosis and refractory ascites treated with transjugular intrahepatic portosystemic shunt insertion. J Gastroenterol Hepatol. 2015;30:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Gaba RC, Khiatani VL, Knuttinen MG, Omene BO, Carrillo TC, Bui JT, Owens CA. Comprehensive review of TIPS technical complications and how to avoid them. AJR Am J Roentgenol. 2011;196:675-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Chinese College of Interventionalists. [CCI clinical practice guidelines: management of TIPS for portal hypertension (2019 edition)]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:582-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 13. | Busk TM, Bendtsen F, Poulsen JH, Clemmesen JO, Larsen FS, Goetze JP, Iversen JS, Jensen MT, Møgelvang R, Pedersen EB, Bech JN, Møller S. Transjugular intrahepatic portosystemic shunt: impact on systemic hemodynamics and renal and cardiac function in patients with cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2018;314:G275-G286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Teng D, Zuo H, Liu L, Dong J, Ding L. Long-term clinical outcomes in patients with viral hepatitis related liver cirrhosis after transjugular intrahepatic portosystemic shunt treatment. Virol J. 2018;15:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Preibsch H, Spira D, Thaiss WM, Syha R, Nikolaou K, Ketelsen D, Lauer UM, Horger M. Impact of transjugular intrahepatic portosystemic shunt implantation on liver perfusion measured by volume perfusion CT. Acta Radiol. 2017;58:1167-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Busk TM, Bendtsen F, Henriksen JH, Fuglsang S, Clemmesen JO, Larsen FS, Møller S. Effects of transjugular intrahepatic portosystemic shunt (TIPS) on blood volume distribution in patients with cirrhosis. Dig Liver Dis. 2017;49:1353-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |