Published online Feb 26, 2021. doi: 10.12998/wjcc.v9.i6.1433
Peer-review started: October 7, 2020
First decision: December 21, 2020
Revised: December 30, 2020
Accepted: January 8, 2021
Article in press: January 8, 2021
Published online: February 26, 2021
Processing time: 122 Days and 2.8 Hours
Granulomatosis with polyangiitis (GPA) is a rare autoimmune disease that involves small-to-medium-sized vessels and forms necrotizing vasculitis with granulomatous inflammation. The formation of a large vessel lesion in GPA patients has been scarcely reported, and it can cause confusion in the diagnosis.
A 27-year-old man presented with mild left-sided pleuritic chest pain that started one year prior. An imaging study revealed up to 2.5 cm-sized two irregular nodular consolidation nodule in the left lower lobe. Both nodules showed central necrosis. Also, there was a periaortic mass occluding the branching porting of the subclavian artery. He had positive anti-neutrophil cytoplasmic antibodies (ANCAs), but myeloperoxidase-ANCAs and proteinase 3-ANCAs were negative. The patient also developed symptoms of subclavian vein syndrome during the follow-up. Wedge resection of the lung revealed necrotizing vasculitis, destructive parenchymal abscess and surrounding granuloma, and therefore diagnosed of GPA. The patient started on methotrexate and steroid therapy with a relief of symptomatic.
Here, we present an unusual manifestation of GPA with periaortitis and consequent subclavian steal syndrome, which has never been previously described. This case alerts us that we should include GPA in the differential diagnosis of large vessel vasculitis as well as subclavian steal syndrome.
Core Tip: Granulomatosis with polyangiitis (GPA) is a systemic process characterized by necrotizing vasculitis and granulomatous inflammation. The large vessel involvement by GPA is not only rare but also causes major diagnostic difficulty. We present a rare case of GPA, showing both lung and periaortic lesion in a male patient. Periaortic lesion of the patient caused vascular occlusion and consequent subclavian vein syndrome. On pathologic examination, the lung nodules had typical histologic features of GPA. This case reminds us that the GPA should not be excluded from the diagnosis because of a large vascular lesion present in an otherwise suspicious setting.
- Citation: Cho U, Kim SK, Ko JM, Yoo J. Unusual presentation of granulomatosis with polyangiitis causing periaortitis and consequent subclavian steal syndrome: A case report. World J Clin Cases 2021; 9(6): 1433-1438
- URL: https://www.wjgnet.com/2307-8960/full/v9/i6/1433.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i6.1433
Granulomatosis with polyangiitis (GPA), formerly called Wegener granulomatosis, is a systemic process characterized by necrotizing vasculitis and granulomatous reaction involving small- to medium-sized vessels. It is rare for the GPA to present with lesions in the large blood vessels. Moreover, GPA as a cause of periaortitis and consequent subclavian steal syndrome has never been described. Here, we present a GPA case showing such rare findings that evoke physicians of a new aspect of the disease.
A 27-year-old man presented with mild left-sided pleuritic chest pain.
The patient’s symptom started one year prior, and it was aggravated by breathing and laughing. He also had occasional substernal area discomfort.
He had no past medical history except for allergic rhinitis.
He had no family history. He had a smoking history of 12 pack-years, and he was a social drinker.
The patient’s lungs were clear on auscultation, and the other physical examination findings, including skin, neurologic and otorhinolaryngologic examinations, were within normal limits.
Laboratory studies revealed an elevated level of C-reactive protein but normal levels of creatinine and blood urea nitrogen. White blood cell count was within the normal limit. Antinuclear antibodies were positive, with a low titer of 1:40. Enzyme-linked immunosorbent assay for anti-neutrophil cytoplasmic antibodies (ANCAs) was positive, but myeloperoxidase-ANCAs and proteinase 3-ANCAs were negative. Other autoimmune antibodies were not found. The urine analysis results were within normal limits.
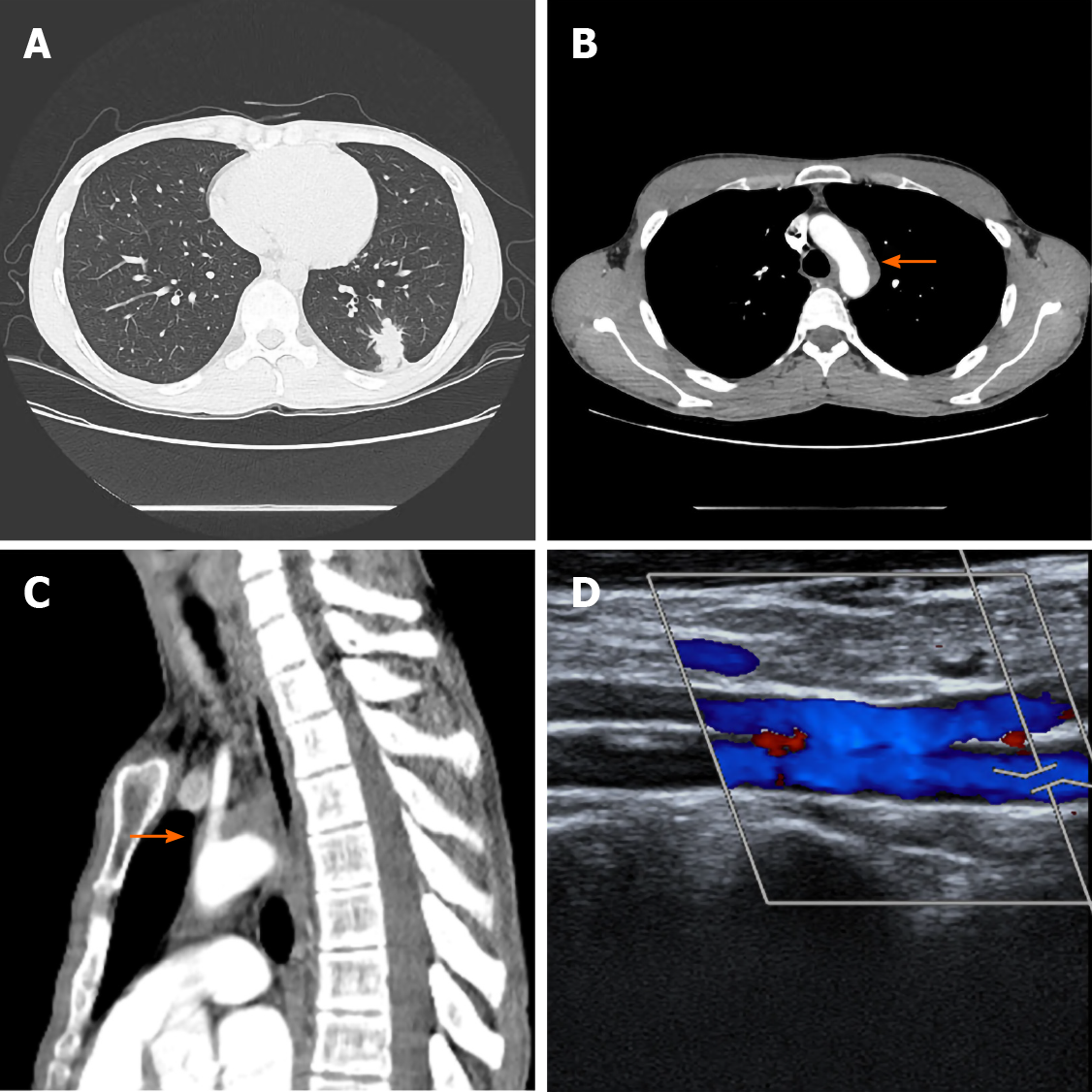
Chest computed tomography revealed a peribronchial, 2.5 cm-sized irregular nodular consolidation nodule in the left lower lobe (Figure 1A). Another solid nodule was located adjacent to the main nodule. Both nodules showed central necrosis. Vascular wall thickening from the distal portion of the aortic arch to the proximal descending aorta was also noted (Figure 1B and C). This periaortic lesion occluded the branching portion of the left subclavian artery. The bronchial arteries had no occlusions.
Transbronchial lung biopsy showed nonspecific interstitial inflammation and fibrosis. Tests for tuberculosis, including the polymerase chain reaction and culture, were all negative. An open lung biopsy was recommended, but the patient refused. During follow-up, the patient complained of exercise-induced arm fatigue and paresthesia. The blood pressure of the left brachial artery was 105/65 mmHg, which was more than 15 mmHg lower than that of the right brachial artery blood pressure (129/74 mmHg). Continuous Doppler ultrasonography showed reversed flow in the left vertebral artery (Figure 1D). Brain magnetic resonance imaging and angiography also revealed occlusion of the left proximal subclavian artery along with retrograde filling of the distant subclavian artery. Subclavian steal syndrome was diagnosed based on the patient’s symptoms and imaging studies. The patient underwent lung wedge resection.
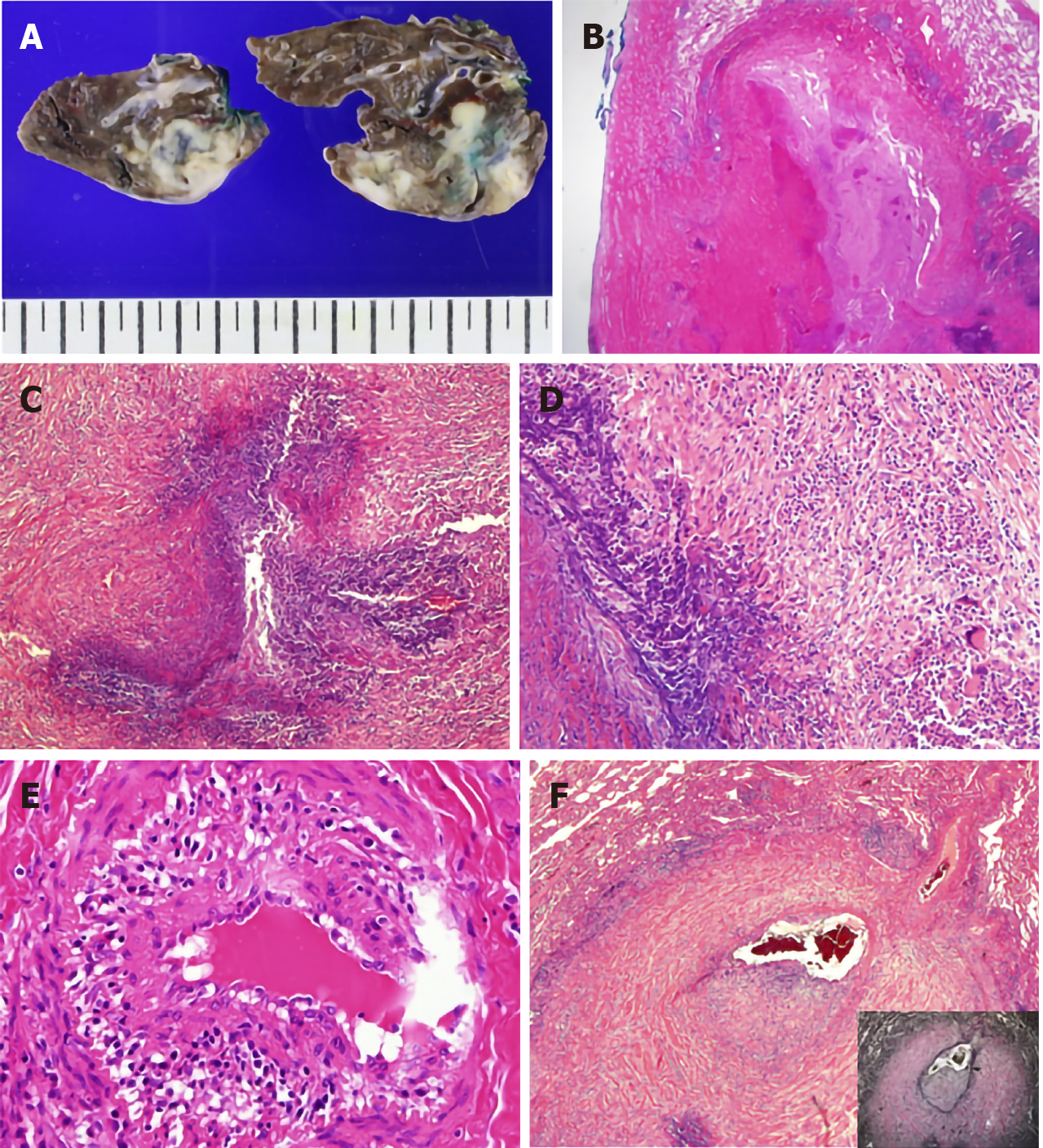
On gross examination, the nodules in the lung parenchymal consolidation showed irregular edges and pale-brown cut surfaces (Figure 2A). Histologically, there was bronchocentric chronic inflammation, extensive geographic parenchymal necrosis, granulomatous inflammation with vasculitis. The areas of necrosis featured microabscesses and large basophilic zones of geographical necrosis with serpiginous borders. Epithelioid macrophages were palisading around the borders of the necrosis, and a few multinucleated giant cells were also present. Vasculitis involved the bronchial arteries and small vessels. Lymphocytes infiltrated the vascular wall and destroyed the elastic laminae (Figure 2B-F). Special staining and culture techniques showed no fungal or bacterial infection, and all the tests for Mycobacteria, including Ziehl-Neelsen stain, culture and polymerase chain reaction, were also negative.
The pathology findings were consistent with granulomatosis with polyangiitis.
The patient was started on methotrexate and steroid therapy.
During 11 mo follow up, the patient did well with a relief of chest pain. Fatigue and paresthesia on the left arm did not worsen but remained. Twelve months after the lung wedge resection, the patient developed headache and aphagia and visited the hospital emergency room. Computed tomography angiography and magnetic resonance imaging revealed middle cerebral artery territory infarct of the left brain. The left proximal carotid coronary artery, proximal subclavian artery, and left inferior division of the middle cerebral artery M2 segment were occluded. Treatments of the acute brain infarct, e.g., aspirin, clopidogrel, rosuvastatin, low-molecular-weight heparin, were added to the methotrexate and steroid therapy. The patient was discharged after the symptomatic relief and had been following up eventless.
Many pulmonary conditions can cause vasculitis, but most are secondary conditions, such as necrotizing granulomatous infection. Granulomatosis with polyangiitis[1], microscopic polyangiitis, and eosinophilic granulomatous polyangiitis are the few primary idiopathic vasculitis diseases that affect the lung.
Because of the patient’s subclavian steal syndrome, the major differential diagnosis, in this case, was Takayasu arteritis (TA). Subclavian steal syndrome is a syndrome with symptoms like exercise-induced arm pain, fatigue, and paresthesia caused by flow reversal in the vertebral artery ipsilateral to stenosis of the subclavian artery[2]. Atherosclerosis is the most common cause of subclavian steal syndrome. Other conditions, such as TA, compression of the subclavian artery in the thoracic outlet, and aortic or heart surgery complications, may also lead to subclavian steal syndrome[3].
Lung aberrations in TA are stenosis of the main pulmonary artery branch associated with the parenchymal ischemic change[4]. Vasculitis with geographic necrotizing granuloma is not a feature of TA[5]. Besides, concentric wall thickening of the large vessel on imaging is the typical finding in TA[6], but in our case, the aortic lesion was not concentric. Thus, the patient’s overall pathologic and radiologic findings suggested against this form of vasculitis.
Involvement of large vessels by GPA is a rare phenomenon, and less than 40 cases have been reported in the literature to date[7-9]. Although the most frequent site of involvement is an abdominal aorta, there have been some case reports of GPA presenting in the thoracic aorta as periaortitis or aortic aneurism[8]. Histologic examination of the large vessel lesions demonstrated necrotizing granulomatous inflammation of the vessel wall itself or periaortic tissue extending to the aortic wall[8,10]. The pathogenic mechanism of the large vessel involvement by GPA is yet to be elucidated. ANCA may play a role in this process by inducing vasculitis in the vasa vasorum[9,11]. Large vessel involvement may also be explained by polyangiitis overlap syndrome or a novel clinical overlap syndrome[12]. However, histologic and clinical observations in the previous studies suggest that large vessel involvement belongs to the spectrum of ANCA-associated vasculitis rather than overlap with other large vessel vasculitides[9,11,13].
Interestingly, our patient had a GPA with associated periaortitis, which led to the consequent subclavian steal syndrome and brain infarct, which has never been previously described. Such unusual presentations can result in delayed diagnosis since GPA is known to involve not large vessels but small- to medium-sized vessels. This is an instructive case showing that GPA should be included in the differential diagnosis of large vessel vasculitis as well as subclavian steal syndrome.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alshewered ASH S-Editor: Zhang L L-Editor: A P-Editor: Ma YJ
| 1. | Lazim QJ, Atrah SSG, Mutlag KJ, Alhilfi HSQ, Fahad AM, Alshewered AS. Granulomatosis (Wegener's granulomatosis) with polyangiitis presented as pulmonary manifestation: a case report. Respirol Case Rep. 2020;8:e00674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Fisher CM. A new vascular syndrome-"the subclavian steal. N Engl J Med. 1961;265:912-913. [DOI] [Full Text] |
| 3. | Osiro S, Zurada A, Gielecki J, Shoja MM, Tubbs RS, Loukas M. A review of subclavian steal syndrome with clinical correlation. Med Sci Monit. 2012;18:RA57-RA63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | He Y, Lv N, Dang A, Cheng N. Pulmonary Artery Involvement in Patients with Takayasu Arteritis. J Rheumatol. 2020;47:264-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Nakajima N. Takayasu arteritis: consideration of pulmonary involvement. Ann Vasc Dis. 2008;1:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Zhu FP, Luo S, Wang ZJ, Jin ZY, Zhang LJ, Lu GM. Takayasu arteritis: imaging spectrum at multidetector CT angiography. Br J Radiol. 2012;85:e1282-e1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Miłkowska-Dymanowska J, Laskowska P, Rzuczkowski M, Białas AJ, Piotrowski WJ, Górski P. Untypical Manifestations of Granulomatosis with Polyangiitis—A Review of the Literature. SN Compr Clin Med. 2019;1:616-626. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Ozaki T, Maeshima K, Kiyonaga Y, Torigoe M, Imada C, Hamasaki H, Haranaka M, Ishii K, Shibata H. Large-vessel involvement in granulomatosis with polyangiitis successfully treated with rituximab: A case report and literature review. Mod Rheumatol. 2017;27:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Chirinos JA, Tamariz LJ, Lopes G, Del Carpio F, Zhang X, Milikowski C, Lichtstein DM. Large vessel involvement in ANCA-associated vasculitides: report of a case and review of the literature. Clin Rheumatol. 2004;23:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Blockmans D, Baeyens H, Van Loon R, Lauwers G, Bobbaers H. Periaortitis and aortic dissection due to Wegener's granulomatosis. Clin Rheumatol. 2000;19:161-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Carels T, Verbeken E, Blockmans D. p-ANCA-associated periaortitis with histological proof of Wegener's granulomatosis: case report. Clin Rheumatol. 2005;24:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Ohta H, Shirai S, Nasu K, Tei M, Kambara H, Ono T, Shintaku M. Ga-67 uptake in the aorta in Wegener's granulomatosis: overlap with Takayasu's arteritis? Clin Nucl Med. 1998;23:859-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Zoma AA. Clinical overlap in proximal artery stenosis: two disorders or one? Lancet. 2016;387:2350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |