Published online Feb 26, 2021. doi: 10.12998/wjcc.v9.i6.1386
Peer-review started: September 24, 2020
First decision: December 4, 2020
Revised: December 8, 2020
Accepted: December 23, 2020
Article in press: December 23, 2020
Published online: February 26, 2021
Processing time: 134 Days and 19.2 Hours
In Wilson disease lack of biliary copper excretion causes hepatocellular injury by accumulation of free toxic copper. Its overspill to serum accounts for neuronal damage as second common manifestation. Therapy with copper chelators or zinc targets the removal of this free copper. However, in some patients liver disease persists for unknown reason despite normalized free copper. The discovery of a hyperimmunity as a contributing pathogenetic factor was discovered in this case report with implication also for other liver diseases.
A 9-year-old girl was diagnosed in August 2009 by family screening of having asymptomatic Wilson disease with elevated transaminases. Already at time of diagnosis antinuclear antibodies (ANA) were elevated without hyperimmunoglobulinemia (immunoglobulin G, IgG). After one year of therapy with D-penicillamine transaminases normalized together with free serum copper. Under continuous therapy with copper chelators free copper remained normal until today, whereas transaminases raised to alanine aminotransferase values of 571 U/L in December 2019. For hyperimmunity a tentative steroid course on top of D-penicillamine improved transaminases. Thus, hyperimmunity may have impact on liver inflammation after control of the metabolic disturbance. A retrospective cohort study confirmed the common association of elevated transaminases with ANA, but no IgG elevation.
This hyperimmune-triggered condition may represent a new entity which per se or on top of other liver diseases induces liver inflammation responsive to steroids.
Core Tip: Variable courses of metabolic liver diseases remain obscure. A 9-year-old girl with Wilson disease had concomitant antinuclear antibodies elevation without immunoglobulin G elevation. Already after one year of therapy with copper chelators the free copper as treatment target was normalized as well as transaminases. Six years later transaminases (alanine aminotransferase) rose despite normalized free copper up to 571 U/L in December 2019. A short-term steroid therapy, improved transaminases significantly. As underlying course, such a neglected hyperimmune state without immunoglobulin elevation was verified in a cohort of 5.789 liver disease patients and may represent a new entity explaining liver disease activation.
- Citation: Stremmel W, Longerich T, Liere R, Vacata V, van Helden J, Weiskirchen R. Wilson disease — the impact of hyperimmunity on disease activity: A case report. World J Clin Cases 2021; 9(6): 1386-1393
- URL: https://www.wjgnet.com/2307-8960/full/v9/i6/1386.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i6.1386
In liver disease physicians are puzzled by the versatile clinical presentation within the same entity. Why only a fraction of heavy drinkers develop overt alcoholic liver diseases[1] and why only 25% of non-alcoholic fatty liver disease (NAFLD) patients come down with non-alcoholic steatohepatitis (NASH)[2]? This is also obvious in genetic liver diseases, e.g., HFE-related hemochromatosis, such that in former times some argued it is the concomitant alcohol consumption leading to disease[3]. Wilson disease presents with a plethora of symptoms, which reveal a predominant hepatic or neurologic phenotype, but sometimes patients are also asymptomatic despite the identical ATP7B mutation[4]. Epigenetic factors were suggested to be responsible although this could never be proven[5]. In this report, we present a case of Wilson disease where an added co-factor effect determined the course of clinical presentation.
A today 20-year-old female patient was diagnosed in August 2009 of having asymptomatic Wilson disease by a genetic family screening. Her transaminases were elevated.
The patient had no clinical presentation.
The patient had a free previous history.
Her 1-year older sister was just diagnosed, and family screening revealed in both girls compound heterozygous mutations for the ATP7B gene at positions H1069Q and R778P.
Height: 137.7 cm; body weight: 30.5 kg. Good physical and mental condition, skin and mucus membranes unremarkable, soft abdomen, no liver or spleen enlargement. Heart, lung, lymph-node status normal, neurological evaluation normal, no Kayser-Fleischer corneal rings.
Initially, the serum copper of the patient was 8.9 µmol/L (normal 12.6-25.1), ceruloplasmin 0.1 g/L (normal 0.2-0.6), “free” copper (non-ceruloplasmin bound copper) 26.8 µg/dL (normal < 15 µg/dL) and the daily urinary copper excretion 116 µg/d (normal 60 µg/d). The liver enzymes were elevated: aspartate aminotransferase (AST) 65 U/L (normal -39 U/L) and alanine aminotransferase (ALT) 114 U/L (normal -35 U/L).
At time of diagnosis the antinuclear antibodies (ANA)-titer was 1:2560 (sparkled pattern) and varied during the course of the disease down to 1:640, extractable nuclear antigens (ENA) were positive at that time, but intermittently also negative later in the course. In August 2011 for the first time double standard DNA was determined with 178.0 IU/mL (normal < 40 IU/mL). Immunoglobulins were in the normal range. A slight proteinuria with 167 mg protein (57.5 mg albumin) per day was detected. A later laboratory workup did not reveal an underlying cause. A kidney biopsy was not performed. The urinary protein excretion varied over the course of the disease and was periodically not detectable anymore. The alkaline phosphatase varied due to physiologic periods of growth in adolescence. Accordingly, these values are not provided. All other laboratory values were in the normal range.
Wilson disease with concomitant ANA elevation without hyperimmunoglobulinemia.
The therapy was started with D-penicillamine together with 40 mg vitamin B6 on August 21st, 2009 in a dose of 150 mg daily and was weekly increased by 150 mg until 2 × 300 mg at September 11th, 2009.
Overall, the therapy was well tolerated. Liver enzymes started to drop at end of November 2009 (AST 49 U/L, ALT 90 U/L), and became completely normal in June 2010 (Table 1). Ceruloplasmin remained in the range at the time of diagnosis. Serum copper fell simultaneously to transaminases as well as the “free” (non-ceruloplasmin bound) copper which became normal in June 2010 and remained there throughout the entire further course of treatment. Urinary copper under D-penicillamine was in August 2009 3.88 mmol/d (= 248 mg/d) and weekly dropped over time finally to normal values (< 0.94 mmol/d or < 60 mg/d) recorded after a 2 d D-penicillamine holiday in November 2010 and remained in normal range thereafter.
| Date of testing | ALT (U/L) | AST (U/L) | GGT (U/L) | Non-CP-bound copper (µg/dL) |
| 08/09 | 114 | 65 | 28 | 27 |
| 10/09 | 113 | 55 | 30 | 21 |
| 03/10 | 61 | 43 | 27 | 16 |
| 06/10 | 28 | 31 | 20 | < 15 |
| 10/10 | 33 | 27 | 21 | < 15 |
| 12/10 | 24 | 25 | 19 | < 15 |
| 03/11 | 19 | 24 | 18 | < 15 |
| 10/11 | 17 | 25 | 13 | < 15 |
In August 2011 a cutaneous lupus with hypopigmentation in the right axilla and at the presternal area was clinically diagnosed (without biopsy) by a dermatologist. At that time the ANA titer was 1:1280 and double stranded DNA was 178 IU/mL. It was assumed to be due to D-penicillamine medication which was discontinued and trientine-2HCl was put on with increasing doses reaching finally 1200 mg. Vitamin B6 was stopped.
From February 2016, ALT fluctuated around 40-50 U/L despite persistent normal “free” non-ceruloplasmin bound copper (Table 2). An elevated urinary copper excretion up to 239 µg/d was observed. However, urine was collected under chelator therapy. The ANA titer was 1:1280 and immunoglobulin (Ig) G was normal. To optimize the copper metabolism, the therapy with 600 mg trientine-2HCl in the morning was supplemented with zinc (Wilzin 50 mg) provided 3x daily (given separately from trientine) in January 2018.
| Therapy | Date of testing | ALT (U/L) | AST (U/L) | GGT (U/L) | Non-CP-bound copper (µg/dL) |
| Trientine-2HCl | 02/16 | 38 | 24 | < 40 | < 15 |
| 03/16 | 47 | 28 | < 40 | < 15 | |
| 07/16 | 44 | 30 | < 40 | < 15 | |
| 09/16 | 42 | 30 | < 40 | < 15 | |
| 10/16 | 39 | 21 | < 40 | < 15 | |
| 11/17 | 34 | 24 | < 40 | < 15 | |
| 05/17 | 39 | 24 | < 40 | < 15 | |
| 10/17 | 56 | 30 | < 40 | < 15 | |
| Trientine-2HCl + zinc | 01/18 | 69 | 36 | < 40 | < 15 |
| 04/18 | 24 | 23 | < 40 | < 15 | |
| 08/18 | 41 | 40 | < 40 | < 15 | |
| 10/18 | 66 | 30 | < 40 | < 15 | |
| Trientine-2HCl | 05/19 | 174 | 61 | ND | < 15 |
| Trientine-4HCl | 07/19 | 245 | 74 | 51 | < 10 |
| 08/19 | 285 | 80 | 76 | < 15 | |
| 09/19 | 297 | 90 | 67 | < 15 | |
| 10/19 | 367 | 81 | 87 | < 15 | |
| 11/19 | 417 | 107 | 76 | < 15 | |
| D-penicillamine | 04/12/19 | 505 | 128 | 79 | < 15 |
| 10/12/19 | 571 | 147 | 77 | < 15 | |
| 17/12/19 | 409 | 109 | 78 | < 15 | |
| D-penicillamine+Prednisolone | |||||
| 40 mg | 15/01/20 | 416 | 135 | 59 | < 15 |
| 40 mg | 30/01/20 | 268 | 79 | 56 | < 15 |
| 20 mg | 18/02/20 | 170 | 47 | 54 | < 15 |
| 5 mg | 15/03/20 | 84 | 37 | 33 | < 15 |
| D-penicillamine alone1 | 25/03/20 | 168 | 60 | ND | < 15 |
| 20/4/20 | 117 | 48 | |||
| 29/5/20 | 127 | 46 |
The regimen did not dramatically change the course of the disorder. Indeed, the patient did not tolerate zinc very well and complained about abdominal pain. Therefore, zinc administration was stopped in October 2018.
The slightly elevated ALT started to increase dramatically from May 2019. At that time ALT was 174 U/L, AST 61 U/L and ANA titer was 1:640.
In July 2019 trientine-2HCl was switched to trientine-4HCl (Cuprior 150 1-0-1). Nevertheless, liver function tests further deteriorated and constantly increased until December 4th, 2019 to ALT 505 U/L, AST 128 U/L, GGT 79 U/L. Furthermore, the patient lost during the last 2 years in total 7 kg body weight with fluctuating liver enzymes. She also had frequent headache episodes and often a bad (almost depressive) mood. One hypothesis argued that this transaminase elevation might be due to trientine and the treatment was stopped and D-penicillamine (the presumable lupus inducer) was reinstalled on December 5th with 600 mg daily together with 100 mg vitamin B6 wkly. However, thereafter the transaminases remained highly elevated despite normal parameters of copper metabolism. The ANA titer was at 1:640 with still normal γ-globulins. Liver stiffness was increased to 6.8 kPa.
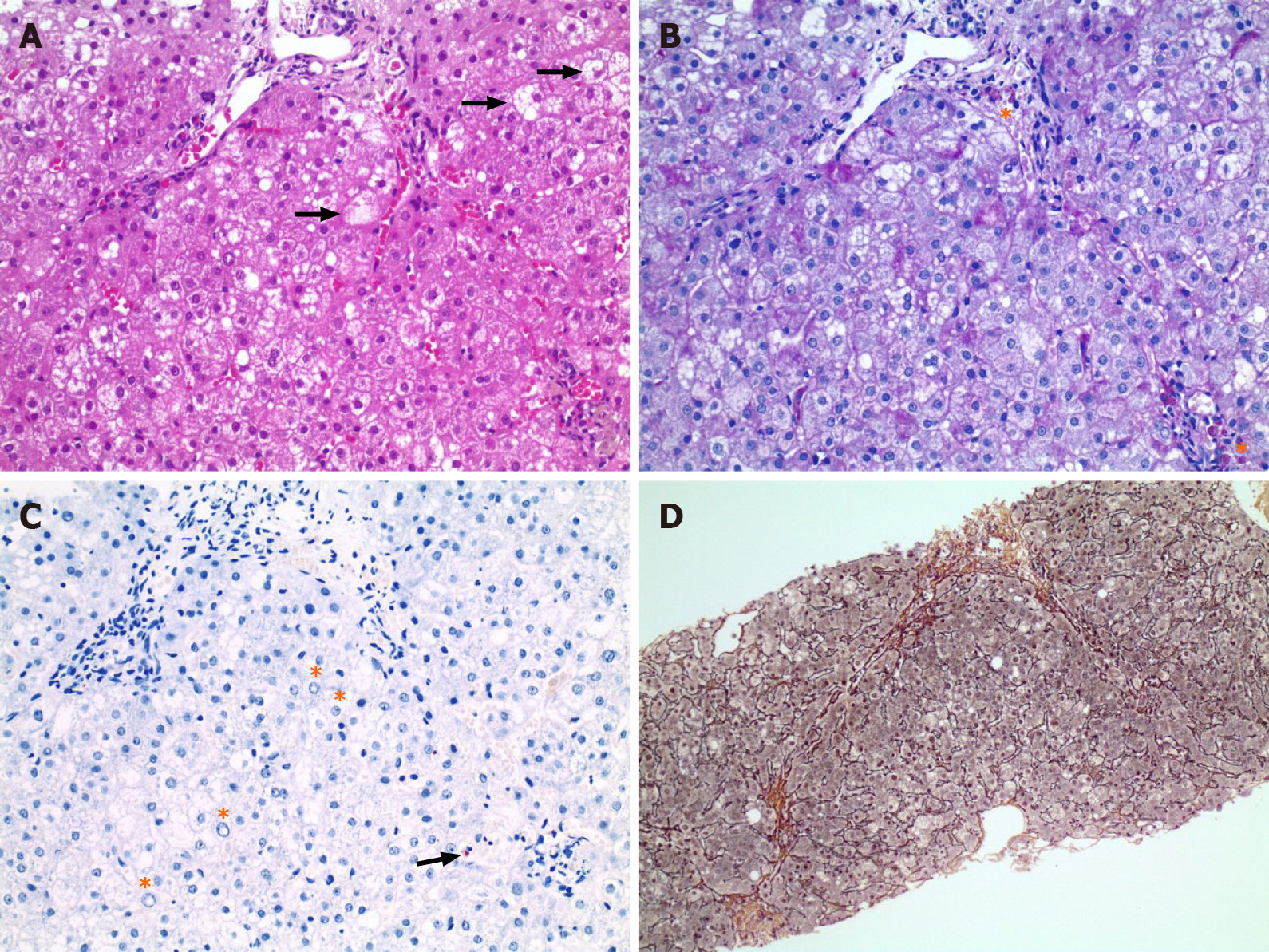
The liver biopsy that was conducted in December 2019 was dominated by ballooned hepatocytes and revealed discrete steatosis as well as portal, perisinusoidal and initial septal fibrosis, and only very discrete copper deposits (Figure 1).
In January 2020 the question arose whether this all may be due to Wilson disease and its therapy. The diagnosis was doubted because the therapy varied so much without change for the patient and persistent normal parameters of copper metabolism, but deterioration of the liver disease. It was considered whether this might be an atypical autoimmune liver disease. Fitting to this option was the observation of an elevated ANA titer, but the autoimmune hepatitis (AIH) score was only 15. Normal concentrations of immunoglobulins and histology were also not consistent with AIH. Nevertheless, a therapeutic trial with a short-term course of a moderate dose prednisolone with weekly deescalating doses (40, 20, 15, 10, 5 mg daily) was started.
The rapid decrease of transaminases after installment of prednisolone is remarkable. After a period of only 2 wk, the values were significantly improved and continued to drop down thereafter. Withdrawal of prednisolone immediately worsened the case and transaminases raised and maintained elevated (Table 2).
She was born 1999. After a viral infection with nasopharyngeal manifestation, bronchitis and fever up to 38.8 °C in April 2009, transaminases were elevated (ALT 126 and AST 78 U/L). A liver biopsy showed a prominent small droplet steatosis, portal fibrosis without cirrhosis and an elevated liver copper content of 1335 µg/g liver (normal below 50 µg/g).
At time of diagnosis ceruloplasmin was 0.1 g/L, serum copper 63.4 mg/dL, calculated free non-ceruloplasmin bound copper 33.4 mg/dL and the urinary copper excretion 96 µg/d. The ANA titer (1:80) was borderline. Antibodies to smooth muscles (ASMA) were elevated to a titer of 1:20 up to 1:80 throughout the course. Intermittently elevated ds-DNA (79 IU/mL), a discrete proteinuria and microhematuria were registered. In May 2009 a therapy with D-penicillamine (750 mg) together with vitamin B6 (40 mg) was started. The ALT dropped continuously to 75 U/L in November 2009, 44 U/L in March 2010 and 30 U/L in June 2010. Later on, ALT was normal or slightly elevated (27-51 U/L). AST was initially elevated until August 2009. Thereafter, values dropped to 43-47 U/L until end of 2009 and became normal in 2010. Non-cerulosplasmin bound copper and urinary copper were constantly normal. Due to a vitiligo in March 2011, D-penicillamine was stopped and trientine-2HCl (900 mg) was started. The copper parameters and transaminases remained stable until February 2016. Without any explainable reason, ALT raised to 145 and AST to 63 U/L and in March to 155 and 61 U/L, respectively. The patient was then treated with trientine-2HCl in reduced dosage of 600 mg together with an oral zinc preparation (Wilzin, 3-times 50 mg zinc). Until January 2017 ALT and AST remained in that range. Thereafter values dropped with only marginal elevated ALT (24-48 U/L). Trientine-2HCl was stopped in November 2017 and she maintained a zinc monotherapy until today.
One could argue that it is not only Wilson disease alone but also another disorder in this patient causing deterioration of liver injury. The diagnosis of Wilson disease is established by genetic analysis, typical laboratory features and good response to the initial therapy with chelators[6,7]. The biopsy taken after decoppering therapy revealed only few copper deposits compatible with Wilson disease[6]. It may reflect over the course of treatment a shift from lysosomal copper (Rhodamine staining positive) to harmless non-visible metallothionein bound cytoplasmatic copper which is harmless[4]. A quantitative copper determination was not performed. However, in histology there were signs of cell damage. The patient did not receive any other liver affecting medications, including phytotherapeutics, nutritional additives or paramedications. Metabolic diseases were excluded, e.g., hemochromatosis, NASH due to hypercholesterinemia or diabetes, alpha1-antitrypsin deficiency and celiac disease. Inflammation of the biliary system (primary sclerosing cholangitis or primary biliary cirrhosis) was also not detectable.
The constantly deteriorating clinical and laboratory course could not be reversed by any of the applied copper depleting therapies which were taken most trustworthy (Table 2). Apparently, none of the applied drugs was reported to induce transaminases, including the new trientine-4HCl[6,7]. During the entire course, copper metabolism was well compensated which excludes exacerbation of Wilson disease due to ineffective therapy.
However, before the diagnosis of Wilson disease was established in this patient ANA levels were found to be increased. Could it be an atypical autoimmune hepatitis (AIH) variant? It seemed unlikely because γ-globulins (IgG) were always normal and the later performed liver biopsy showed no interface hepatitis[8]. During the course of the disease autoimmune parameters were recorded, namely ANA-titer up to 1:2560 and intermittently double stranded DNA (178 IU/mL), detection of ENA, proteinuria and a cutaneous lupus. The AIH score yielded just 15 points which does not suggest overt AIH[8]. Despite this uncertainty, the patient was put on 40 mg prednisolone and significantly improved in regard to her physical and mental condition. Most importantly, the transaminases dropped within two weeks to significantly lower levels and continued to drop over the course of steroid therapy.
Two disorders attacked the liver: Wilson disease and a concomitant idiopathic (non therapeutically-induced) ANA elevation. The later was shown to be responsible for fluctuating liver inflammation (ALT raise), because copper metabolism was normalized. It is unclear whether there are two separate disorders or a condition of mutual aggravation. Until today, it was unknown that elevation of ANA without hyperimmunoglobulinemia (possibly not visible due to its minor extend) causes active liver disease. It has been fallen through the grid of attention because the definition criteria for autoimmune hepatitis, even atypical courses, may be too strict to cover all aspects of a hyperimmune triggered pathophysiology. AIH is defined by elevation of ANA or ASMA (type 1) and LKM-antibodies (type 2), elevated gamma-globulins and interface hepatitis in histology[8].
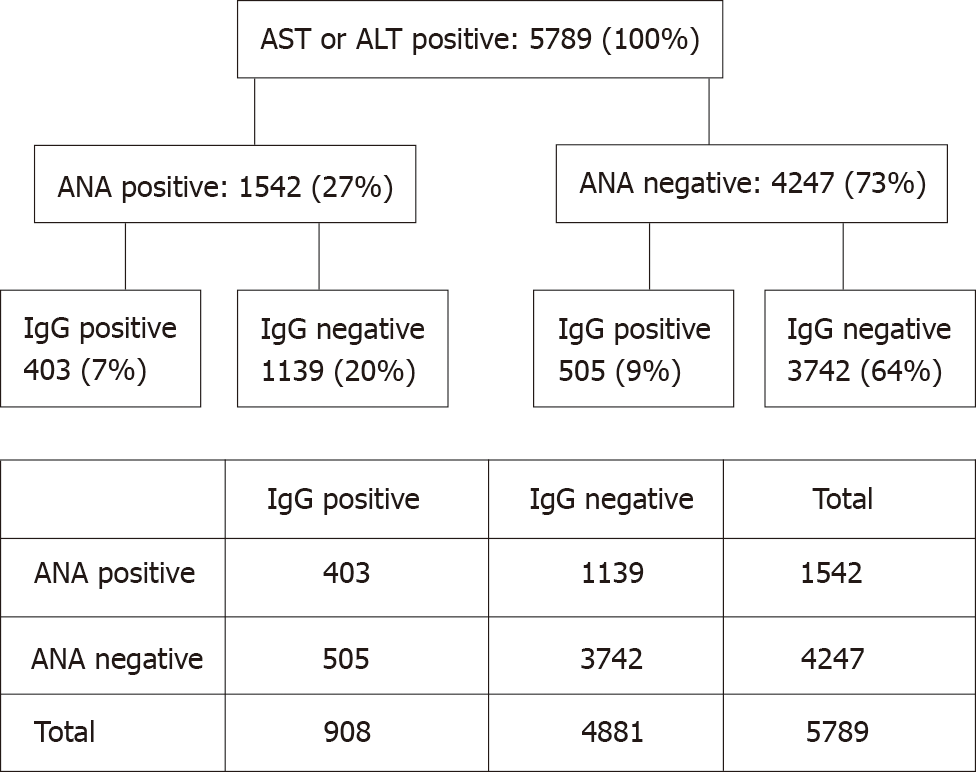
To follow the hypothesis of a hyperimmune triggered liver disease, we evaluated 26096 blood samples of different patients where ANA, IgG, AST, and ALT were simultaneously determined, irrespective of underlying diagnoses. Of these patients 5789 (22%) showed elevated transaminases, representing the group of interest. Among them, ANA and IgG negative patients were predominant, representing metabolic, toxic or infectious diseases affecting the liver. The ANA and IgG positive pattern is a characteristic feature of autoimmune hepatitis (AIH)[8]. In presence of cirrhosis a relative increase of IgG compared to albumin is common in absence of ANA elevation. The ANA positive but IgG negative patients resemble patients with hyperimmune-triggered liver disease as described in this report. This group accounted for 20% of the ANA positive cohort which is significantly higher as the AIH group (P < 0.00001) (Figure 2).
The observation that patients with elevation of ANA or ASMA (as in type 1 AIH) but normal immunoglobulins often present with elevation of ALT as the most prominent transaminase, opens the perspective for a new disease entity. In case it can be confirmed by other studies, its immunologic and genetic background, pathogenesis and reliable diagnostic criteria have to be explored. Furthermore, as shown in this case, the efficiency of immunosuppressive therapy (e.g., steroids, budesonide, azathioprine) has to be evaluated in regard to doses and length of treatment. Furthermore, the impact of therapy on the natural course and prognosis needs clinical trials and the evaluation of beneficial effects vs adverse events.
It would be a challenge if it represents a key to treat those until now not therapeutically targetable inflammatory liver diseases, e.g., NASH. At present no medication against this progressive NASH is available. Only metabolic risk factors for NAFLD are defined and preventable.
We describe a new entity of hepatocellular injury on top of other metabolic disorders, like Wilson disease, which leads to an inflammatory phenotype with transaminase elevation, predominantly ALT: A hyperimmune state with autoantibody elevation, i.e., ANA, without immunoglobulin elevation. It responses well to steroid therapy.
The cohort study of patients with simultaneous determination of transaminases, ANA and immunoglobulins revealed that those with transaminases and ANA, but no hyperimmunoglobulinemia are more frequent as expected compared to those with concomitant IgG elevation. Thus, it may be a novel entity not yet described as such. A more detailed analysis of this entity is required. However, a course of steroid therapy was shown to be effective and may be considered in these cases. This is still far from an evidence-based recommendation.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: German Society for Gastroenterology, Digestive and Metabolic Diseases.
Specialty type: Medicine, research and experimental
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Quarleri J S-Editor: Gao CC L-Editor: A P-Editor: Liu JH
| 1. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J Hepatol. 2018;69:154-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 588] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 2. | European Association for the Study of the Liver (EASL). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3182] [Article Influence: 353.6] [Reference Citation Analysis (4)] |
| 3. | Fletcher LM, Powell LW. Hemochromatosis and alcoholic liver disease. Alcohol. 2003;30:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Stremmel W, Weiskirchen R. Therapeutic strategies in Wilson disease: pathophysiology and mode of action. Annals Trans Med. 2021;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Medici V, LaSalle JM. Genetics and epigenetic factors of Wilson disease. Ann Transl Med. 2019;7:S58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 6. | European Association for Study of Liver. EASL Clinical Practice Guidelines: Wilson's disease. J Hepatol. 2012;56:671-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 971] [Cited by in RCA: 784] [Article Influence: 60.3] [Reference Citation Analysis (1)] |
| 7. | Roberts EA, Schilsky ML; American Association for Study of Liver Diseases (AASLD). Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008;47:2089-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 884] [Cited by in RCA: 814] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 8. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63:971-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 848] [Article Influence: 84.8] [Reference Citation Analysis (0)] |