Published online Feb 26, 2021. doi: 10.12998/wjcc.v9.i6.1343
Peer-review started: August 13, 2020
First decision: August 21, 2020
Revised: August 24, 2020
Accepted: October 26, 2020
Article in press: October 26, 2020
Published online: February 26, 2021
Processing time: 176 Days and 22.6 Hours
In clinical work, 85%-90% of malignant thyroid diseases are papillary thyroid cancer (PTC); thus, clinicians neglect other types of thyroid cancer, such as medullary thyroid carcinoma (MTC).
We report a 53-year-old female patient with a preoperative calcitonin level of 345 pg/mL. There was no definitive diagnosis of MTC by preoperative fine-needle aspiration cytology or intraoperative frozen pathology, but the presence of PTC and MTC was confirmed by postoperative paraffin pathology. The patient underwent total thyroidectomy and bilateral central lymph node dissection. Close follow-up at 1.5 years after surgery revealed no signs of recurrence or metastasis.
The issue in clinical work-up regarding types of thyroid cancer provides a novel and challenging idea for the surgical treatment of MTC. In the absence of central lymph node metastasis, it is worth addressing whether patients with high calcitonin can undergo total thyroidectomy and bilateral central lymph node dissection without bilateral lateral neck lymph node dissection.
Core Tip: Medullary thyroid carcinoma accounts for a small proportion of all types of thyroid cancer and is rarely encountered clinically. Therefore, medullary thyroid carcinoma requires further study. For patients with medullary thyroid cancer and high calcitonin levels, it may be possible to choose a surgical approach with a small range of operations. By closely monitoring the trend of calcitonin changes and survival follow-up results for 1.5 years, we herein propose a new question about the surgical scope of medullary thyroid carcinoma: Do thyroid medullary carcinoma patients with high calcitonin require lateral neck lymphadenectomy?
- Citation: Gan FJ, Zhou T, Wu S, Xu MX, Sun SH. Do medullary thyroid carcinoma patients with high calcitonin require bilateral neck lymph node clearance? A case report. World J Clin Cases 2021; 9(6): 1343-1352
- URL: https://www.wjgnet.com/2307-8960/full/v9/i6/1343.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i6.1343
Thyroid carcinoma is a secretory tumour. According to the latest global cancer statistics, the incidence of thyroid carcinoma is increasing significantly[1,2]. Papillary thyroid carcinoma (PTC) accounts for a large proportion of thyroid carcinomas (85-90%) and originates from the follicular epithelium[3]. At present, PTC treatment is mainly based on surgery combined with endocrine therapy. Medullary thyroid carcinoma (MTC) is a rare tumour arising from para-follicular C-cells[4], accounting for only 2-3% of all thyroid cancers. Treatment is currently based on surgery. According to relevant case data for thyroid disease patients admitted to our hospital since 2010, the present patient was the only one to have both MTC and PTC, even though 400 thyroid disease patients are admitted to our hospital every year. In addition, according to the relevant literature, there are few reports on simultaneous MTC and PTC. Kim et al[5] collected patients with MTC in their hospital from 1996 to 2006 and found that 10 of 53 MTC patients had PTC (19%). In their study, the coexistence of MTC and PTC was only identified as a coincidence and was considered an accidental phenomenon with no special significance. There are few cases of simultaneous PTC and MTC as well as different types of thyroid carcinoma appearing simultaneously; thus, treatment principles still need to be explored. Currently, specific and sensitive tumour markers for thyroid carcinoma are not available. Most thyroid nodules are identified in physical examination, and the diagnosis is confirmed by fine needle aspiration biopsy (FNAC). MTC has some special markers compared with PTC. MTC originates from para-follicular C-cells, which can secrete calcitonin. Therefore, most of the MTCs exhibit an abnormal increase in calcitonin and an abnormal anti-carcinoembryonic antigen (CEA) level. Surgical operation of MTC is the only treatment. Postoperative monitoring of calcitonin[6-10] and CEA[11,12] is particularly important.
A 53-year-old female patient was admitted to our hospital because a thyroid nodule was found 2 mo prior.
A thyroid nodule without any symptoms was found 2 mo prior in a physical examination.
The patient had no past medical history.
The patient did not have any family history of other diseases.
At admission, the patient was conscious. Her body temperature was 36.5 °C, with a regular heart rate of 70 bpm, respiratory rate of 20 breaths per minute, and blood pressure of 118/70 mmHg. The physical examination was normal.
When the patient was admitted to the hospital, blood biochemistry and urine analysis were normal. Preoperative thyroid function was normal, and no hyperthyroidism or hypothyroidism was observed. Parathyroid hormone and serum calcium were normal. Electrocardiogram, chest X-ray, and arterial blood gas were also normal. Preoperative calcitonin was 345 pg/mL (typically less than 6.4 pg/mL), and CEA was 6.44 µg/L (typically less than 3.0 µg/L).
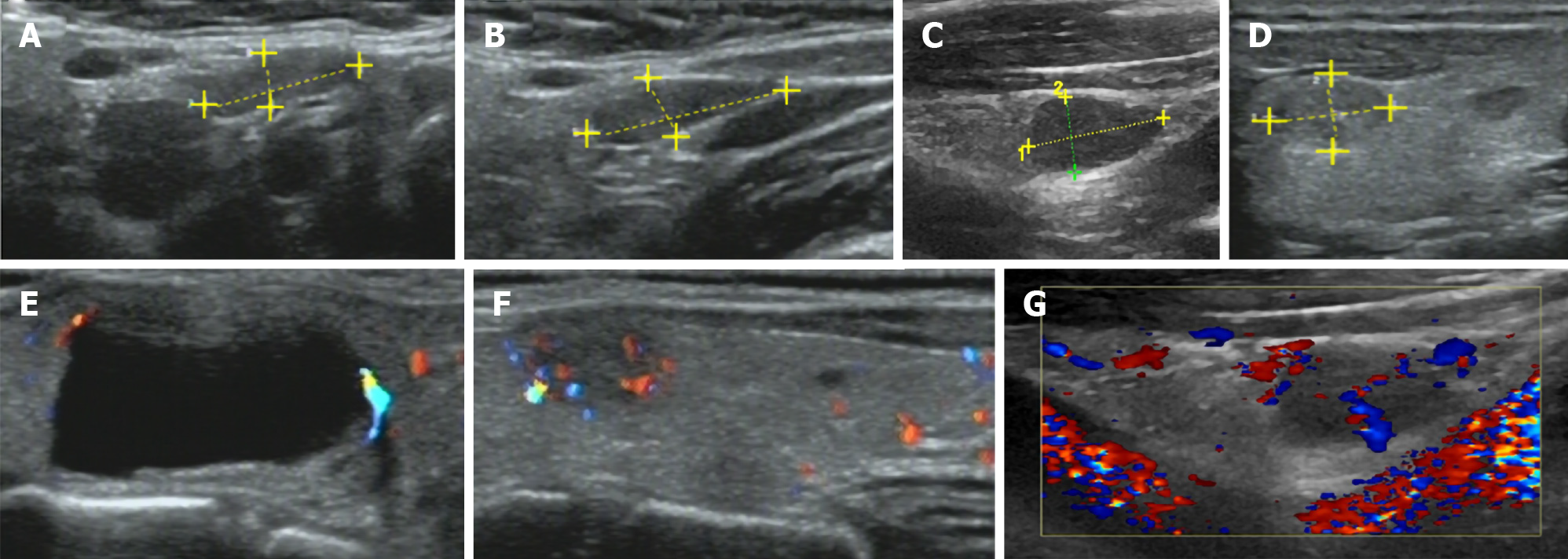
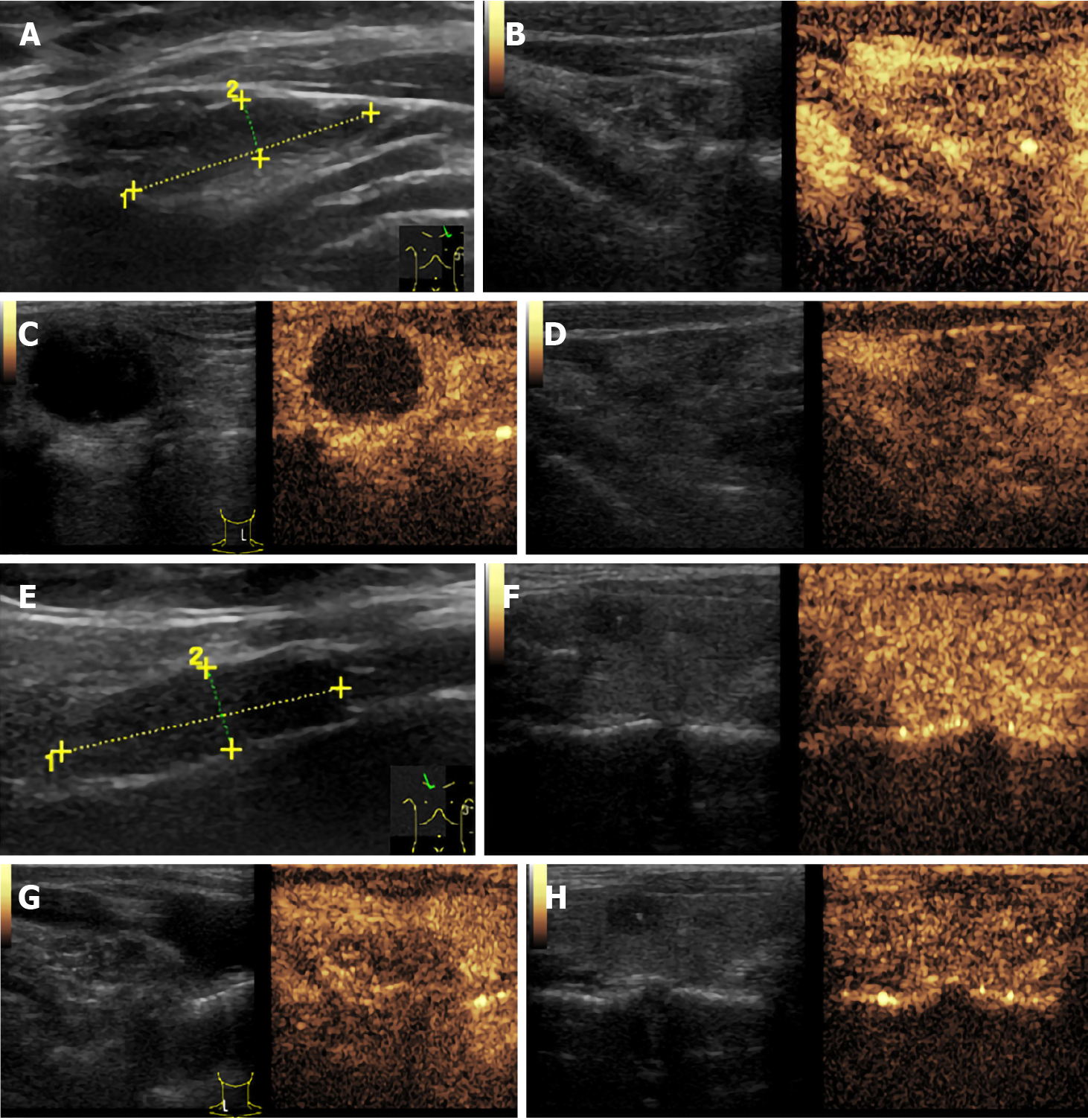
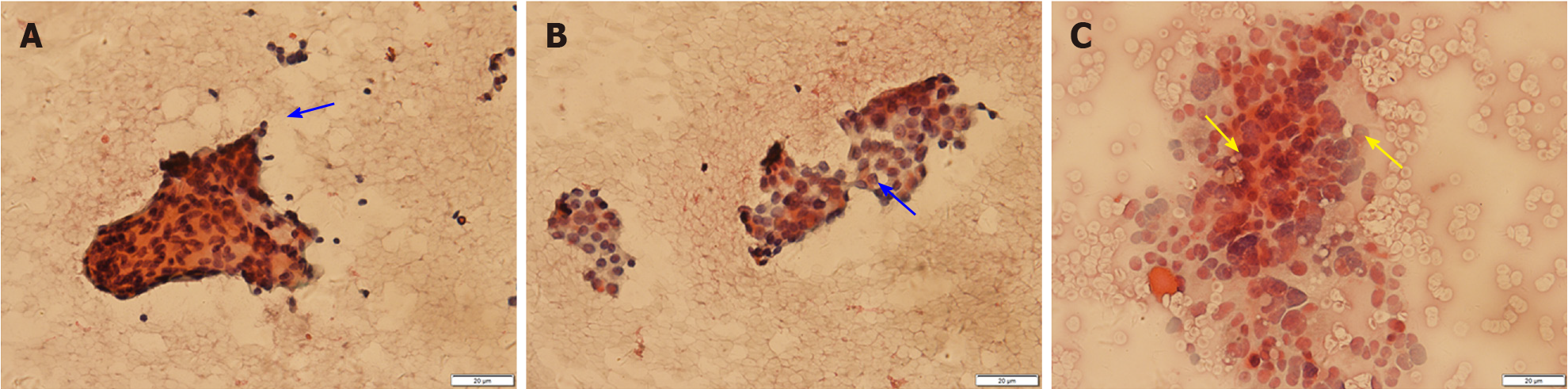
Thyroid ultrasound (US) suggested hypoechoic nodules located in the right lobe of the thyroid, with a size of 5 mm × 5 mm and clear boundaries. The patient was then treated conservatively, and she exhibited no symptoms during long-term follow-up monitoring. On December 18, 2018, the patient visited our hospital again for re-examination, and thyroid US indicated multiple hypoechoic nodules located in the right lobe, the largest of which was approximately 10 mm × 6 mm, with clear boundaries, uneven internal echoes, and punctured blood flow signals. The left lobe showed cystic-solid mixed hypoechoic nodules with a size of approximately 22 mm × 11 mm, clear boundaries, mainly cystic features, and cord-like blood flow signals around the nodules. On both sides of the neck, multiple solid hypoechoic nodules, with clear borders and flat shape, and a clear boundary between the cortex and medulla were detected. She was diagnosed with bilateral thyroid nodules, thyroid imaging reporting and data system (TI-RADS) grade 4 (Figure 1). Subsequent further thyroid contrast-enhanced ultrasonography still suggested that the bilateral lymph nodes in the neck were more likely to be reactive hyperplasia (Figure 2). To confirm the diagnosis, FNAC of bilateral thyroid nodules was performed, and cytopathology showed the following: Suspicious PTC [the Bethesda system for reporting thyroid cytopathology (TBSRTC) V] in the right thyroid nodule Left thyroid nodule cytology suggested a malignancy (TBSRTC V); however, its type could not be determined (Figure 3).
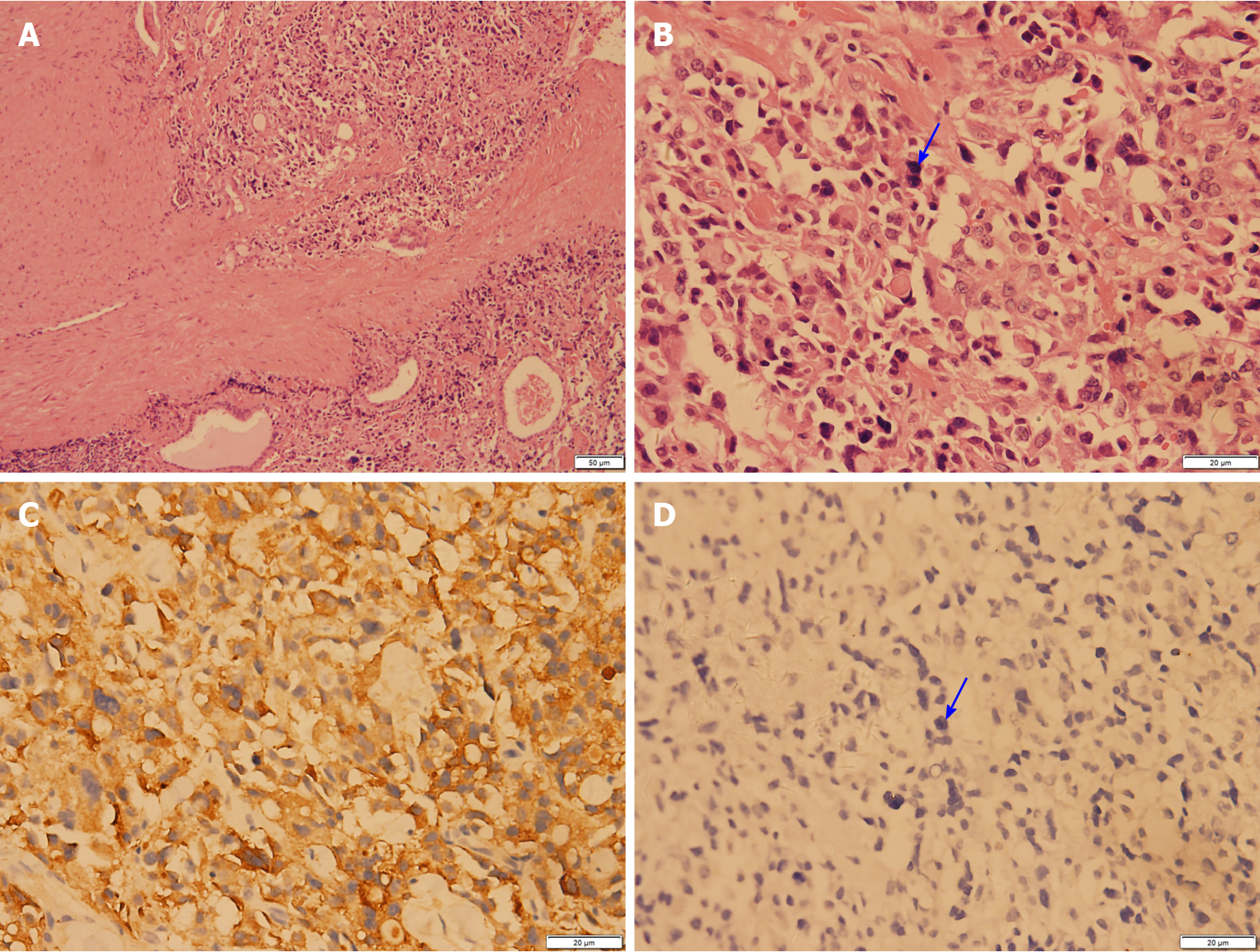
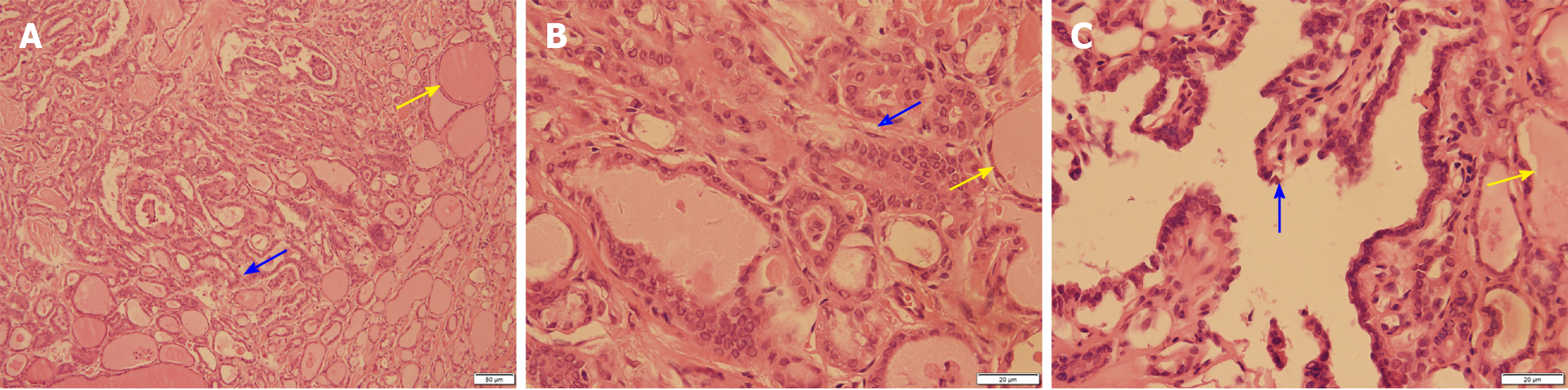
After the relevant examinations were completed, the surgical contraindications were excluded. On December 24, 2018, thyroidectomy (thyroidectomy, bilateral central lymph node dissection, and bilateral recurrent laryngeal nerve exploration) was performed in the Thyroid Breast Surgery Department of the Affiliated Hospital of Zunyi Medical University. Intraoperative pathological freezing analysis suggested bilateral papillary thyroid micropapillary carcinoma, and the bilateral central lymph nodes had no cancer metastasis. However, the postoperative paraffin pathology report was inconsistent with the intraoperative frozen pathology report. Postoperative paraffin pathology suggested MTC in the left lobe, with the following immunohistochemistry results: Calcitonin (+), CK (+), thyroid transcription factor (TTF1) (+), CK19 (-), thyroglobulin (-), galectin-3 (-), Ki-67 < 1%, and MC (-) (Figure 4). PTC with a BRAF V600E mutation was detected in the right lobe (Figure 5). No metastasis was noted in the bilateral central (VI) lymph nodes (left 0/2, right 0/3). Therefore, according to the TNM staging system of American Joint Committee on Cancer (AJCC) version 8, the patient's final clinical diagnosis was PTC of the right lobe (T1N0M0, I stage) and MTC of the left lobe (T1N0M0, I stage). The calcitonin level was 345 pg/mL preoperatively, which decreased to 15.74 pg/mL on the first day after the operation. CEA was not detected before operation and was 6.440 µg/L on the first day after the operation (normally less than 3.0 µg/L). The clinical and pathological data of the patient are presented in Table 1.
| Project | Content |
| Biographical data | 53 years old and female |
| Family history | No |
| Chief complaint | Thyroid nodule discovered 2 mo prior |
| Specialized physical examination | The trachea was in the middle. The thyroid gland moved up and down with swallowing action, and no nodules were felt in the bilateral lobes. There was no hoarseness in the voice and no choking cough when drinking water. |
| US | Nodules were found in bilateral lobes of the thyroid gland, and the right lobe had a well-defined hypoechoic nodule with a size of approximately 10 mm × 6 mm. The left lobe had a cystic and solid mixed hypoechoic nodule with a size of approximately 22 mm × 11 mm. The US grade was TI-RADS 4. Multiple solid hypoechoic nodules were detected in bilateral I-IV areas of the neck, and the boundaries of the medulla and cortex were not clear |
| CEUS | Lymph nodes were observed on both sides of the neck. T-CEUS suggested that the cortical stage of the lymph nodes was enhanced by uneven medulla, while the medulla was slightly enhanced. Enlarged lymph nodes were considered as reactive hyperplasia |
| FNAC | Right thyroid nodule was suspected as a thyroid papillary carcinoma (TBSRTC V). Left thyroid nodule cytology suggested a malignancy (TBSRTC V), but the type was not determined |
| Surgery | Thyroidectomy, bilateral central lymph node dissection, and bilateral recurrent laryngeal nerve exploration |
| Frozen pathology | Bilateral papillary thyroid micropapillary carcinoma and bilateral central lymph nodes showed no cancer metastasis |
| Paraffin pathology | Left medullary thyroid carcinoma (Figure 2), with immunohistochemistry results of calcitonin (+), CK (+), TTF1 (+), CK19 (-), thyroglobulin (-), galectin-3 (-), Ki -67 < 1%, and MC (-); right thyroid papillary carcinoma, BRAF V600E mutation |
| Final diagnosis | Papillary thyroid carcinoma of the right lobe (T1N0M0, stage I) and medullary thyroid carcinoma of the left lobe (T1N0M0, stage I) |
| Postoperative treatment | Oral levothyroxine sodium tablets (Euthyrox): 100 µg/time/day |
Postoperatively, the patient was treated with levothyroxine sodium tablets supplemented with thyroid hormone.
The patient was followed after the operation, and her calcium calcitonin, thyroid function, and CEA changes were assessed regularly. The dose of levothyroxine tablets was adjusted based on changes in thyroid-stimulating hormone (TSH) to maintain TSH at < 0.1 mu/L. The follow-up results are provided in Table 2.
| Time | CT (pg/mL) | TSH (μIU/mL) | TgAb (IU/mL) | Tg (ng/mL) | CEA (µg/L) |
| Normal | < 6.4 | 0.5-4.8 | 0-115 | 3.5-77 | < 3.0 |
| Preoperative | 345 | 4.2 | < 10.0 | 16.820 | / |
| 1 d after surgery | 15.74 | 2.545 | < 10.0 | 3.370 | 6.440 |
| 1 wk after surgery | 2.69 | 0.634 | < 10.0 | 0.497 | / |
| 1 mo after surgery | 0.84 | 0.096 | 14.4 | 0.062 | / |
| 2 mo after surgery | 0.55 | 0.048 | 14.0 | 0.040 | / |
| 5 mo after surgery | < 0.5 | 0.473 | < 10.0 | 0.040 | / |
| 8 mo after surgery | < 0.5 | 0.343 | < 10.0 | 0.110 | < 3.0 |
| 1 yr after surgery | < 0.5 | 0.343 | < 10.0 | 0.100 | < 3.0 |
| 1.5 yr after surgery | < 0.5 | 0.008 | < 10.0 | 0.100 | 1.18 |
We performed long-term follow-up of this case of simultaneous PTC and MTC to monitor postoperative serum calcitonin, thyroid function, and CEA.
At present, non-invasive thyroid examination mainly involves US examination, and risk stratification is performed based on different US manifestations. The American Institute of Radiology designed the TI-RADS in 2017 to reduce biopsy of benign nodules and improve the overall diagnostic accuracy. The US features in the ACR TI-RADS[13] are categorized as benign, minimally suspicious, moderately suspicious, or highly suspicious for malignancy. Points are given for all the ultrasound features in a nodule, with more suspicious features being awarded additional points. The point total determines the nodule’s ACR TI-RADS level, which ranges from TR1 (benign) to TR5 (high suspicion of malignancy). The use of the ACR TI-RADS classification significantly reduces unnecessary thyroid punctures and improves the sensitivity and specificity of benign and malignant diagnoses of thyroid nodules[14]. Occasionally, US cannot effectively determine the nature of nodules. To avoid excessive invasive examination, relevant studies[15,16] have found that contrast-enhanced ultrasound (CEUS) can further determine whether nodules are benign or malignant on the basis of US; thus invasive examinations can be reduced. It is recommended that patients with grade 4 and above undergo a thyroid biopsy to diagnose the nodules with pathology. In this article, the patient's thyroid US grade was 4, and further FNAC was performed. The diagnosis of thyroid cancer mainly depends on thyroid US combined with FNAC. Pathology is the gold standard for diagnosing any type of cancer, but FNAC occasionally does not clearly diagnose the pathological type of thyroid nodules. Thus, further diagnosis by paraffin pathology is needed. The diagnosis of FNAC is mainly through cytological diagnosis. TBSRTC established a standardized, category-based reporting system for thyroid FNAC specimens. Related studies have[17-19] shown that the implementation of the TBST report reduces the number of ambiguous reports, positively increases the deterministic value of thyroid malignancy for surgery, and reduces the rate of surgery for benign thyroid nodules. In this case, the diagnosis of bilateral thyroid FNAC was TBSRTC V, which is highly suspected of malignant tumours, but there was no clear diagnosis of the thyroid cancer subtype. Therefore, when managing patients with thyroid nodules, the existence of MTC cannot be ignored due to its extremely low incidence. Preoperative routine thyroid ultrasonography combined with FNAC should be performed. If necessary, preoperative calcitonin, CEA, and other relatively specific biomarkers should be assessed to help doctors select a more suitable surgical treatment and postoperative treatment for patients and predict the prognosis[20,21]. Overall variation in MTC cells makes FNAC diagnosis difficult, and histopathological evaluation and relevant immunohistochemical markers are necessary for a definitive diagnosis. Given that cancer cell changes in MTC are associated with a poor prognosis, the correct diagnosis and appropriate treatment are necessary.
MTC is a type of malignant neuroendocrine tumour that arises from para-follicular C-cells[4]. Most MTC cases are caused by mutations in the RET proto-oncogene. MTC[22] accounts for 2-3% of thyroid carcinomas. Females are significantly more often affected than males. The patient in this case was a middle-aged female patient who was admitted to the hospital due to thyroid nodules found in physical examination without any signs or endocrine-related symptoms. There was no family history of MTC and RET gene mutations were not detected in this patient. The patient is at present undergoing long-term follow-up monitoring, and there are no signs of recurrence or distant metastasis. In clinical practice, avoiding the wrong preoperative diagnosis and selecting the best treatment plan are important. In clinical work, 85-90% of malignant thyroid nodules are PTC, which is likely to lead to imperfect preoperative preparation, resulting in incorrect surgical methods and leading to secondary surgery. In this case, the initial values of CEA were not sufficiently monitored due to the inability to clarify the pathological type based on FNAC before the operation. The negligence in this clinical work once again provided a warning. To better treat thyroid nodules, it is recommended that all patients admitted to the hospital for suspected malignant thyroid nodules be routinely tested for serum calcitonin and CEA before surgery. This point is worthy of every clinician’s attention. However, it should be noted that the possibility of MTC cannot be completely excluded when preoperative calcitonin and CEA are negative. Gambardell et al[23] have reported cases of calcitonin and CEA-negative MTC.
MTC is not sensitive to iodine-131 radiation therapy and chemotherapy, and endocrine therapy is ineffective. Thus, surgical resection is the first choice for early MTC. The surgical treatments for MTC include preventive surgery and therapeutic surgery. For patients with hereditary MTC (mostly children)[24,25], prophylactic surgery is the mainstay based on genetic mutations, and the resection range varies according to age and the preoperative calcitonin levels (Table 3). Patients[24,25] undergoing therapeutic surgery usually have primary thyroid lesions, and different ranges of cervical lymph node dissection are performed based on preoperative calcitonin levels (Table 4). ATA guidelines (2015 version) suggest that MTC treatment should be primarily surgical depending on the preoperative evaluation for distant metastasis and preoperative level of procalcitonin. The guidelines recommend complete thyroidectomy, bilateral central cervical lymph node dissection, and ipsilateral cervical lymph node dissection when preoperative US examination indicates no cervical lymph node or distant metastasis, and preoperative procalcitonin is 200 ng/L. However, the guidelines also indicate that when procalcitonin is reduced to normal levels after MTC surgery (< 10 ng/L), it is considered a biological cure, with a 10-year survival rate of 97.7%. In this case, although the preoperative calcitonin level was 345 ng/L, indicating an abnormal increase, the diagnosis of MTC was not suggested by intraoperative cryotherapy but by postoperative paraffin pathology. Therefore, the operation did not follow the guidelines, which recommended total thyroidectomy with bilateral central area lymph node and bilateral cervical lateral lymph node removal. In this patient, only thyroidectomy and bilateral middle cervical lymph node dissection were performed. Procalcitonin levels were reduced to normal 1 wk after surgery, and have been maintained to date. Recurrence or distant metastasis has not been observed. Based on the results of the close follow-up of the patient in the previous period, we pose a bold question about MTC: When preoperative calcitonin level is greater than 200 pg/mL and metastasis is not noted in central lymph nodes, is extensive bilateral neck lymph node dissection necessary?
| Risk classification | Patient population | Treatment |
| ATA-HST | MEN2B; RET codon M918T mutation | Thyroidectomy ± central lymph node dissection in the neck within 1 year after birth |
| ATA-H | MEN2A; RET codon C634 mutation | Five years old or earlier thyroidectomy ± central lymph node dissection |
| ATA-MOD | Others | Every year from the age of 5, physical examination, neck US, and serum calcitonin |
| Condition | Treatment |
| Patients without evidence of cervical lymph nodes and distant metastases before surgery | Preventive central lymph node dissection |
| Preoperative calcitonin 40-150 pg/mL, even if no suspected lymph node metastasis were found | Central lymph node and ipsilateral II-IV lymph node dissection |
| Patients with preoperative calcitonin > 200 pg/mL | Ipsilateral neck II-VI lymph node dissection, and contralateral cervical lymph nodes should also be considered for removal |
Prognostic factors of thyroid cancer, PTC after main endocrine therapy, and the presence of TSH inhibition can effectively predict recurrence or metastasis. MTC is currently followed by monitoring serum calcitonin and CEA levels.
For patients with nodular thyroid disease, serum calcitonin should be routinely tested before surgery to exclude the possibility of MTC, to develop a better preoperative treatment plan and reduce unnecessary second surgery. According to guidelines, cervical lymph node dissection is recommended for preoperative MTC with calcitonin > 200 pg/mL. For MTC, we propose the following challenging question: When preoperative calcitonin is > 200 pg/mL, is it necessary to remove lymph nodes in ipsilateral neck II-VI regions and ipsilateral neck II-VI regions for all MTCs?
Nevertheless, the long-term follow-up of this case has provided us with a novel and challenging treatment strategy. For patients with MTC with high calcitonin levels and no cancer metastasis in cervical region VI, can the scope of the operation be reduced? Specifically, dissection of lateral cervical lymph node (regions II-V) is not required. Therefore, surgical trauma and the possibility of postoperative complications are reduced.
It is worth thinking about!
The authors thank the Chief Physician Hua-Qing Liu from the Department of Pathology, Affiliated Hospital of Zunyi Medical University for his support with all the pathological images in this article.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ou YW S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55827] [Article Influence: 7975.3] [Reference Citation Analysis (132)] |
| 2. | Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3585] [Cited by in RCA: 4900] [Article Influence: 700.0] [Reference Citation Analysis (1)] |
| 3. | Hay ID, Thompson GB, Grant CS, Bergstralh EJ, Dvorak CE, Gorman CA, Maurer MS, McIver B, Mullan BP, Oberg AL, Powell CC, van Heerden JA, Goellner JR. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940-1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg. 2002;26:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 491] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 4. | Vinciguerra GL, Noccioli N, Cippitelli C, Minucci A, Capoluongo E, Bartolazzi A. Oncocytic Variant of Medullary Thyroid Carcinoma: A Rare Case of Sporadic Multifocal and Bilateral RET Wild-Type Neoplasm with Revision of the Literature. Rare Tumors. 2016;8:6537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Kim WG, Gong G, Kim EY, Kim TY, Hong SJ, Kim WB, Shong YK. Concurrent occurrence of medullary thyroid carcinoma and papillary thyroid carcinoma in the same thyroid should be considered as coincidental. Clin Endocrinol (Oxf). 2010;72:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W; European Thyroid Cancer Taskforce. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154:787-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1495] [Cited by in RCA: 1311] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 7. | Trimboli P, Nigri G, Romanelli F, Cicciarella Modica DD, Crescenzi A, Valabrega S, Giovanella L. Medullary thyroid nodules by measurement of calcitonin (Ct) in aspiration needle washout in patients with multinodular goiter and moderately elevated serum Ct. Exp Clin Endocrinol Diabetes. 2012;120:234-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | de Crea C, Raffaelli M, Maccora D, Carrozza C, Canu G, Fadda G, Bellantone R, Lombardi CP. Calcitonin measurement in fine-needle aspirate washouts vs. cytologic examination for diagnosis of primary or metastatic medullary thyroid carcinoma. Acta Otorhinolaryngol Ital. 2014;34:399-405. [PubMed] |
| 9. | Trimboli P, Cremonini N, Ceriani L, Saggiorato E, Guidobaldi L, Romanelli F, Ventura C, Laurenti O, Messuti I, Solaroli E, Madaio R, Bongiovanni M, Orlandi F, Crescenzi A, Valabrega S, Giovanella L. Calcitonin measurement in aspiration needle washout fluids has higher sensitivity than cytology in detecting medullary thyroid cancer: a retrospective multicentre study. Clin Endocrinol (Oxf). 2014;80:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Trimboli P, Treglia G, Guidobaldi L, Romanelli F, Nigri G, Valabrega S, Sadeghi R, Crescenzi A, Faquin WC, Bongiovanni M, Giovanella L. Detection rate of FNA cytology in medullary thyroid carcinoma: a meta-analysis. Clin Endocrinol (Oxf). 2015;82:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Machens A, Ukkat J, Hauptmann S, Dralle H. Abnormal carcinoembryonic antigen levels and medullary thyroid cancer progression: a multivariate analysis. Arch Surg. 2007;142:289-93; discussion 294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Raue F, Frank-Raue K. Epidemiology and Clinical Presentation of Medullary Thyroid Carcinoma. Recent Results Cancer Res. 2015;204:61-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, Cronan JJ, Beland MD, Desser TS, Frates MC, Hammers LW, Hamper UM, Langer JE, Reading CC, Scoutt LM, Stavros AT. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J Am Coll Radiol. 2017;14:587-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 1406] [Article Influence: 175.8] [Reference Citation Analysis (0)] |
| 14. | Hoang JK, Middleton WD, Farjat AE, Langer JE, Reading CC, Teefey SA, Abinanti N, Boschini FJ, Bronner AJ, Dahiya N, Hertzberg BS, Newman JR, Scanga D, Vogler RC, Tessler FN. Reduction in Thyroid Nodule Biopsies and Improved Accuracy with American College of Radiology Thyroid Imaging Reporting and Data System. Radiology. 2018;287:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 15. | Ma JJ, Ding H, Xu BH, Xu C, Song LJ, Huang BJ, Wang WP. Diagnostic performances of various gray-scale, color Doppler, and contrast-enhanced ultrasonography findings in predicting malignant thyroid nodules. Thyroid. 2014;24:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Jin Y, He YS, Zhang MM, Parajuly SS, Chen S, Zhao HN, Peng YL. Value of contrast-enhanced ultrasonography in the differential diagnosis of enlarged lymph nodes: a meta-analysis of diagnostic accuracy studies. Asian Pac J Cancer Prev. 2015;16:2361-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Theoharis CG, Schofield KM, Hammers L, Udelsman R, Chhieng DC. The Bethesda thyroid fine-needle aspiration classification system: year 1 at an academic institution. Thyroid. 2009;19:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 18. | Crowe A, Linder A, Hameed O, Salih C, Roberson J, Gidley J, Eltoum IA. The impact of implementation of the Bethesda System for Reporting Thyroid Cytopathology on the quality of reporting, "risk" of malignancy, surgical rate, and rate of frozen sections requested for thyroid lesions. Cancer Cytopathol. 2011;119:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Ozluk Y, Pehlivan E, Gulluoglu MG, Poyanli A, Salmaslioglu A, Colak N, Kapran Y, Yilmazbayhan D. The use of the Bethesda terminology in thyroid fine-needle aspiration results in a lower rate of surgery for nonmalignant nodules: a report from a reference center in Turkey. Int J Surg Pathol. 2011;19:761-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Elisei R, Bottici V, Luchetti F, Di Coscio G, Romei C, Grasso L, Miccoli P, Iacconi P, Basolo F, Pinchera A, Pacini F. Impact of routine measurement of serum calcitonin on the diagnosis and outcome of medullary thyroid cancer: experience in 10,864 patients with nodular thyroid disorders. J Clin Endocrinol Metab. 2004;89:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 276] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 21. | Bugalho MJ, Santos JR, Sobrinho L. Preoperative diagnosis of medullary thyroid carcinoma: fine needle aspiration cytology as compared with serum calcitonin measurement. J Surg Oncol. 2005;91:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Traugott A, Moley JF. Medullary thyroid cancer: medical management and follow-up. Curr Treat Options Oncol. 2005;6:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Gambardella C, Offi C, Clarizia G, Romano RM, Cozzolino I, Montella M, Di Crescenzo RM, Mascolo M, Cangiano A, Di Martino S, Candela G, Docimo G. Medullary thyroid carcinoma with double negative calcitonin and CEA: a case report and update of literature review. BMC Endocr Disord. 2019;19:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Wells SA Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, Raue F, Frank-Raue K, Robinson B, Rosenthal MS, Santoro M, Schlumberger M, Shah M, Waguespack SG; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25:567-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 1467] [Article Influence: 146.7] [Reference Citation Analysis (0)] |
| 25. | Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10769] [Cited by in RCA: 9681] [Article Influence: 1075.7] [Reference Citation Analysis (1)] |