Published online Feb 26, 2021. doi: 10.12998/wjcc.v9.i6.1304
Peer-review started: September 10, 2020
First decision: November 30, 2020
Revised: December 14, 2020
Accepted: December 23, 2020
Article in press: December 23, 2020
Published online: February 26, 2021
Processing time: 149 Days and 7.6 Hours
Previous studies using voxel-based morphometry (VBM) revealed changes in gray matter volume (GMV) of patients with depression, but the differences between patients with bipolar disorder (BD) and unipolar depression (UD) are less known.
To analyze the whole-brain GMV data of patients with untreated UD and BD compared with healthy controls.
Fourteen patients with BD and 20 with UD were recruited from the Mental Health Center of Shantou University between August 2014 and July 2015, and 20 non-depressive controls were recruited. After routine three-plane positioning, axial T2WI scanning was performed. The connecting line between the anterior and posterior commissures was used as the scanning baseline. The scanning range extended from the cranial apex to the foramen magnum. Categorical data are presented as frequencies and were analyzed using the Fisher exact test.
There were no significant intergroup differences in gender, age, or years of education. Disease course, age at the first episode, and Hamilton depression rating scale scores were similar between patients with UD and those with BD. Compared with the non-depressive controls, patients with BD showed smaller GMVs in the right inferior temporal gyrus, left middle temporal gyrus, right middle occipital gyrus, and right superior parietal gyrus and larger GMVs in the midbrain, left superior frontal gyrus, and right cerebellum. In contrast, UD patients showed smaller GMVs than the controls in the right fusiform gyrus, left inferior occipital gyrus, left paracentral lobule, right superior and inferior temporal gyri, and the right posterior lobe of the cerebellum, and larger GMVs than the controls in the left posterior central gyrus and left middle frontal gyrus. There was no difference in GMV between patients with BD and UD.
Using VBM, the present study revealed that patients with UD and BD have different patterns of changes in GMV when compared with healthy controls.
Core Tip: Disease course, age at the first episode, and Hamilton depression rating scale scores were similar between patients with unipolar depression (UD) and those with bipolar disorder (BD). UD patients showed smaller gray matter volumes (GMVs) than the controls in the right fusiform gyrus, left inferior occipital gyrus, left paracentral lobule, right superior and inferior temporal gyri, and the right posterior lobe of the cerebellum, and larger GMVs than the controls in the left posterior central gyrus and left middle frontal gyrus. There was no difference in GMV between patients with BD and UD.
- Citation: Zhang YN, Li H, Shen ZW, Xu C, Huang YJ, Wu RH. Healthy individuals vs patients with bipolar or unipolar depression in gray matter volume. World J Clin Cases 2021; 9(6): 1304-1317
- URL: https://www.wjgnet.com/2307-8960/full/v9/i6/1304.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i6.1304
Bipolar disorder (BD) is a severe mental illness featured by episodes of mania and depression in an alternating pattern, accompanied by changes in activity or energy and cognitive, physical, and behavioral symptoms[1,2]. The overall incidence of BD worldwide is approximately 2%, with the disease's subthreshold forms affecting another 2% of the global population[3]. The estimated lifetime prevalence of BD based on data from 11 countries is 0.4%-1.4%[4]. According to data from the World Health Organization, the disability rate associated with BD ranks sixth in the youth population, causing a heavy financial burden on families and society[5].
In clinical practice, BD type I is characterized by at least one episode of mania. In comparison, BD type II is characterized by at least one episode of depression and at least one hypomanic episode, without mania episodes[1,2]. However, since the disease episodes must be clinically monitored to determine the diagnosis, the two types are challenging to identify in the disease's early stages. As a result, diagnosis may be delayed by up to 10 years or even longer in one-third of patients with BD[6], and 60% of patients with BD who sought treatment for depressive symptoms were initially misdiagnosed as having unipolar depression (UD)[7]. Thus, the similarities in the clinical symptoms of BD and UD cause difficulties in a timely and accurate diagnosis of the two conditions[8].
Several clinical studies have attempted to identify biological indicators that can distinguish UD from BD[9-11]. Elevated plasma uric acid levels may be associated with emotional instability in patients with BD, while low uric acid levels may be related to UD patients’ depressive mood[10]. Similarly, patients with BD show high plasma levels of nerve growth factor (NT)-3 and NT-4/5[11]. Likewise, neuroelectrophysiological studies have suggested that the auditory steady-state response is a potential electrophysiological marker for distinguishing between BD and UD[9]. While patients with BD and UD show some differences in electrophysiological and biochemical indicators, the evidence for using these indicators in differential diagnosis remains inadequate.
Voxel-based morphometry (VBM) is an automatic, comprehensive, and objective technique for the analysis of brain structural magnetic resonance (MR) images[12]. In VBM, gray and white matter lesions in the brain are evaluated via quantitative analysis of the changes in brain gray and white matter volumes of each voxel of MR images. VBM is widely used in research to study a variety of neuropsychiatric diseases such as Alzheimer’s disease[13], depression[14,15], bipolar disorder[15,16], and schizophrenia[17], providing reliable imaging data regarding the changes in brain morphology in those diseases.
Previous VBM studies have reported gray matter structural abnormalities in patients with BD and depression. In patients with UD, smaller gray matter volume (GMV) are observed in the anterior cingulate gyrus[18], hippocampus[19], amygdala[18,19], caudate nucleus[14], frontal lobe[20], parietal lobe[20], temporal lobe[21], and cerebellum[21]. In contrast, the brain regions reported to show smaller GMVs in patients with BD include the frontal lobe[22] and the temporal lobe[23]. Since very few studies have directly compared the VBM findings in the two diseases, the exact differences in GMV changes between patients with BD and UD remain unclear.
The results of the available VBM studies also lacked consistency because of methodological differences[24,25]. For example, De Azevedo-Marques Perico et al[24] showed that the GMV of the right lateral anterior cingulate cortex in BD patients was larger than that in normal controls. In comparison, the GMV of the bilateral dorsolateral prefrontal cortex in patients with UD was smaller than that in controls. Patients with UD and BD showed significant GMV differences at the level of the right dorsolateral prefrontal lobe. In contrast, Wise et al[25] performed a meta-analysis of the VBM results in patients with BD and UD and showed that the GMVs of the bilateral insular lobe and the dorsomedial and ventromedial frontal cortices are smaller in both groups in comparison with normal controls, while the GMVs of the right frontal gyrus, left hippocampus and parahippocampal gyrus, right inferior temporal gyrus and fusiform gyrus, left inferior parietal lobule, and right cerebellar vermis are smaller in patients with UD than in those with BD[25]. Redlich et al[26] showed reduced GMV in the anterior cingulate gyrus in American and German patients with UD compared with BD and suggested a pattern classification (based on a multivariable analysis of GMV, white matter volume, and structural abnormalities) that had 79% accuracy. From Singapore, Cai et al[27] showed that patients with BP1 and UD have lower GMV in the right frontal gyrus, but that lower GMV in the right middle cingulate gyrus might be specific to BP1.
Nevertheless, although differences in the GMV distributions in patients with UD and BD have been reported across multiple studies, only rare studies[26,27] have attempted to determine whether these differences can be used to distinguish between BD and UD. Because of the possible cultural, language, ethnic, and technical [magnetic resonance imaging (MRI) sequences] differences in VBM results, this study aimed to use VBM to analyze the whole-brain GMV data of patients with untreated UD and BD compared with healthy controls.
This study included 14 patients with BD and 20 patients with UD admitted to the outpatient department of the Mental Health Center of Shantou University between August 2014 and July 2015. The study protocol was approved by the ethics committee of Shantou University Medical College ([2017]0301). All patients voluntarily participated in the study and signed an informed consent form.
All patients were diagnosed according to the diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV[28]. Intelligence quotient (IQ) was tested using the Wechsler Adult Intelligence Scale-Revised in China[29,30]. Depression was evaluated using the Hamilton depression rating scale (HDRS)[31], validated in Chinese[32].
For the UD group, the inclusion criteria were: (1) Meeting the diagnostic criteria for recurrent depression in DSM-IV (code 32.4); (2) 18-60 years of age; (3) HDRS score ≥ 17; (4) Right-handedness; (5) IQ > 70; and (6) No history of drug consumption for at least 2 wk. For the BD group, the inclusion criteria were as follows: (1) Meeting the diagnostic criteria for bipolar disorder depressive episodes in DSM-IV (codes 31.5 and 31.6); (2) 18-60 years of age; (3) HDRS score ≥ 17; (4) Right-handedness; (5) IQ > 70; and (6) No history of drug consumption for at least 2 wk. The exclusion criteria were: (1) Schizophrenia or schizoaffective psychosis; (2) Substance dependence; (3) Severe physical and Neurological diseases; (4) Pregnancy or lactation; (5) Intrauterine contraceptive ring usage; (6) Presence of a cardiac pacemaker or other implantable metal devices; and (7) Left-handedness.
Twenty non-depressive individuals were recruited as controls, based on the following criteria: (1) No history of significant physical or mental illness; (2) 18-60 years of age; (3) Right-handedness; and (4) IQ > 70.
After routine three-plane positioning, axial T2WI scanning was performed. The connecting line between the anterior and posterior commissures was used as the scanning baseline. The scanning range extended from the cranial apex to the foramen magnum. The scanning parameters were as follows: Repetition time (TR), 7000 ms; Echo time (TE), 107.3 ms; Layer thickness, 5 mm; Layer spacing, 1 mm; Matrix, 384 × 384; Field of view (FOV), 240 × 240 mm2; Bandwidth, 62.5; And number of excitations (NEX), 2. T1 fluid-attenuated inversion recovery scanning was performed with the following parameters: TR, 1750 ms; TE, 24 ms; Matrix, 320 × 224; and FOV, 240 × 240 mm2. Axial scanning was performed using the 3D-BRAVO sequence (T1WI) with the following parameters: TR, 6 ms; TE, 1.8 ms; Flip angle, 15°; Layer thickness, l mm; Continuous scanning with no intervals; FOV, 256 mm × 256 mm; Matrix, 256 × 256; NEX, l; Bandwidth, 41.67 Hz; number of scanning layers, 168; Voxel size, 1 mm × 1 mm × 1 mm; And scanning time, 2 m 59 s.
Information about demographics (gender, age, income, employment status, and years of education) and clinical characteristics (age at the first episode, disease course, and HDRS scores) of the subjects were collected.
The VBM8 and DARTEL toolkits in the SPM8 software package (Statistical Parametric Mapping, http://www.fil.ion.ucl.ac.uk/spm) were used to process structural image data, as described previously[33]. Post-processing was performed using the DARTEL algorithm. The specific processing steps were: (1) Format conversion: The original data in DICOM format was converted to the NIfTI format; (2) Segmentation: Images of the individual brain structures were segmented to obtain white matter, gray matter, and cerebrospinal fluid images; (3) Template establishment: Three groups of subjects (BD, UD, and controls) were used to generate the template, and the DARTEL algorithm was used to estimate the optimal registration template for each participant; (4) Volume modulation map generation: Space was normalized to the Montreal Neurological Institute (MNI) space[34] to generate the volume modulation map; (5) Data smoothing: A Gaussian kernel with a full-width at half maximum of 8 mm was used for smoothing the data; and (6) Statistical analysis: Statistical analysis was performed on the data by using a two-sample t test in SPM8. The Alpha Sim application was used for correction for multiple comparisons. Age, sex, and total intracranial volume were used as covariates. After false discovery rate correction, P < 0.05 and a voxel threshold of > 100 were considered significant, at least statistically. The voxels with statistical significance were superimposed on 3D MNI standard templates to generate pseudo-color maps. The characteristics of GMV changes in the whole brains of the three groups were analyzed.
Categorical data are presented as frequencies and were analyzed using the Fisher exact test. Continuous data are presented as the mean ± SD and were analyzed using one-way analysis of variance (ANOVA) with the SNK post hoc test. SPSS 21.0 (IBM, Armonk, NY, United States) was used for statistical analysis. P values < 0.05 were considered statistically significant.
MRI was performed in 14 patients with BD, 20 patients with UD, and 20 non-depressive volunteers. One patient with BD who showed a subarachnoid cyst was excluded. There were no significant intergroup differences in gender, age, or years of education (P > 0.05 for all three characteristics, ANOVA). Disease course, age at the first episode, and HDRS scores were similar between patients with UD and those with BD (Table 1).
| BD (n = 13) | UD (n = 20) | Controls (n = 20) | P value | |
| Age (yr) | 31.0 ± 7.6 | 28.0 ± 9.1 | 31.7 ± 11.4 | 0.698 |
| Gender (male/female) | 6/7 | 7/13 | 10/10 | 0.616 |
| Education (yr) | 11.7 ± 4.0 | 10.6 ± 3.5 | 13.1 ± 3.9 | 0.083 |
| First-episode age (yr) | 22.0 ± 8.0 | 23.3 ± 8.4 | -- | 0.442 |
| Diseasecourse (mo) | 108.0 ± 55.0 | 56.4 ± 29.4 | -- | 0.064 |
| HDRS score | 24.8 ± 5.6 | 26.5 ± 7.1 | -- | 0.572 |
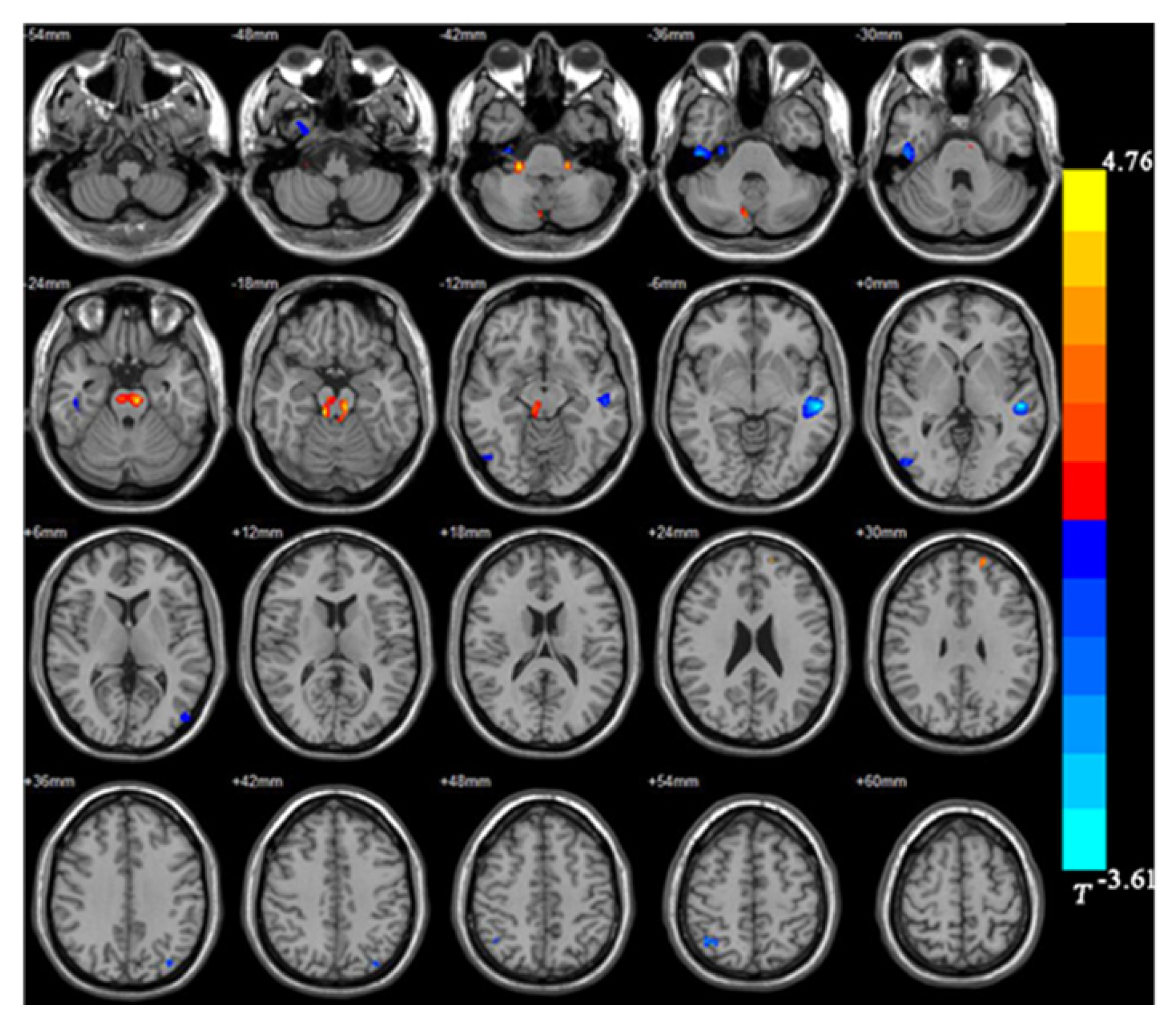
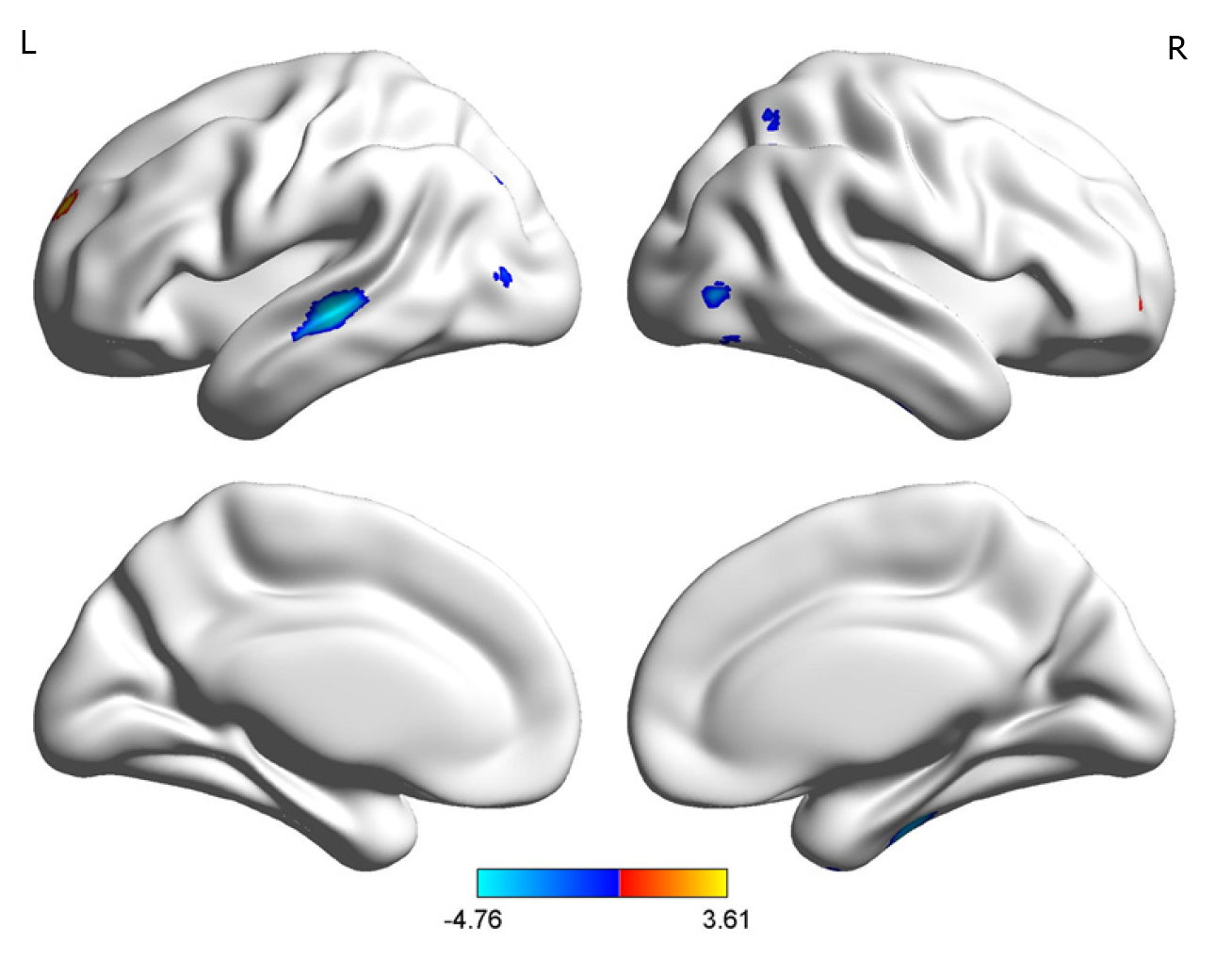
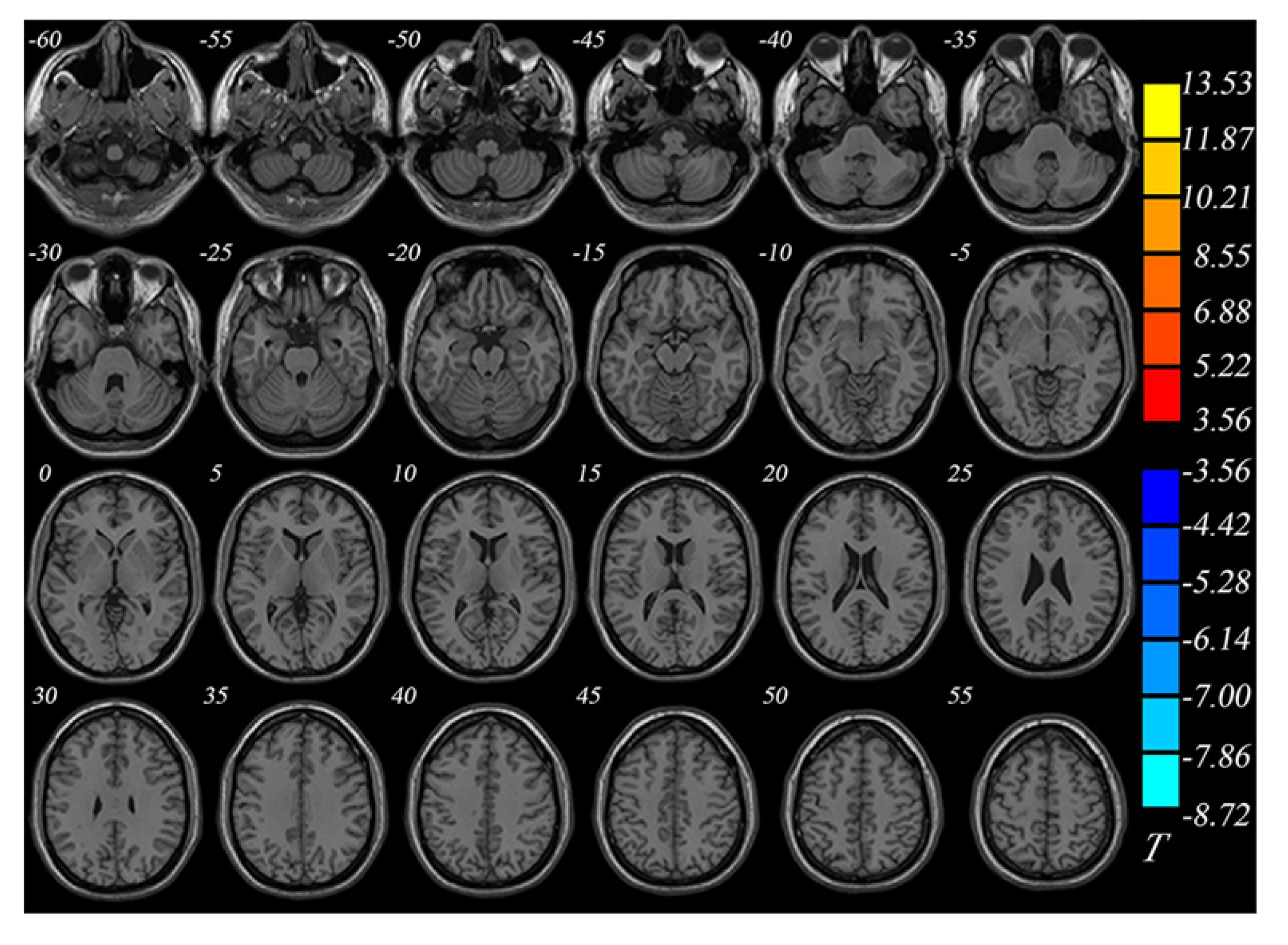
The BD group showed significantly smaller GMVs in the right inferior temporal gyrus, left middle temporal gyrus, right middle occipital gyrus, and right superior parietal gyrus compared with healthy controls. The GMVs in the midbrain, left superior frontal gyrus, and right cerebellum in the BD group were larger than those in the controls (Table 2 and Figures 1 and 2).
| MNI coordinate | |||||
| Brain region | Size | X | Y | Z | Ta |
| Right inferior temporal gyrus | 607 | 28.5 | -22.5 | -39 | -3.95 |
| Right middle occipital gyrus | 110 | 51 | 70.5 | -13.5 | -2.94 |
| Right superior parietal gyrus | 152 | 33 | -60 | 55.5 | -3.84 |
| Left middle temporal gyrus | 795 | -49.5 | -24 | -1.5 | -4.76 |
| Right cerebellum | 174 | 22.5 | -34.5 | -42 | 3.49 |
| Left superior frontal gyrus | 101 | 33 | 49.5 | -3 | 2.99 |
| Midbrain | 803 | -7.5 | -27 | -19.5 | 3.61 |
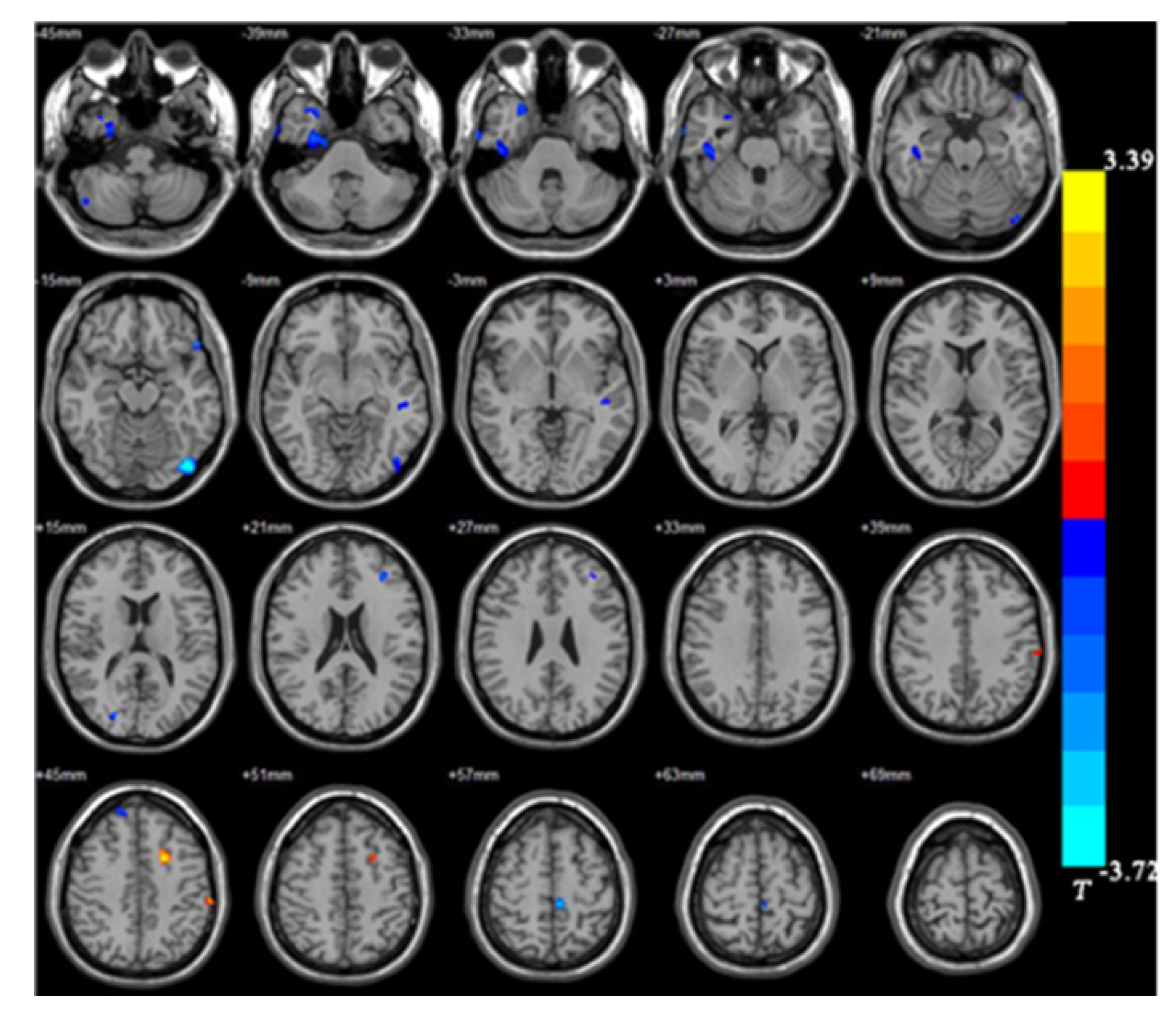
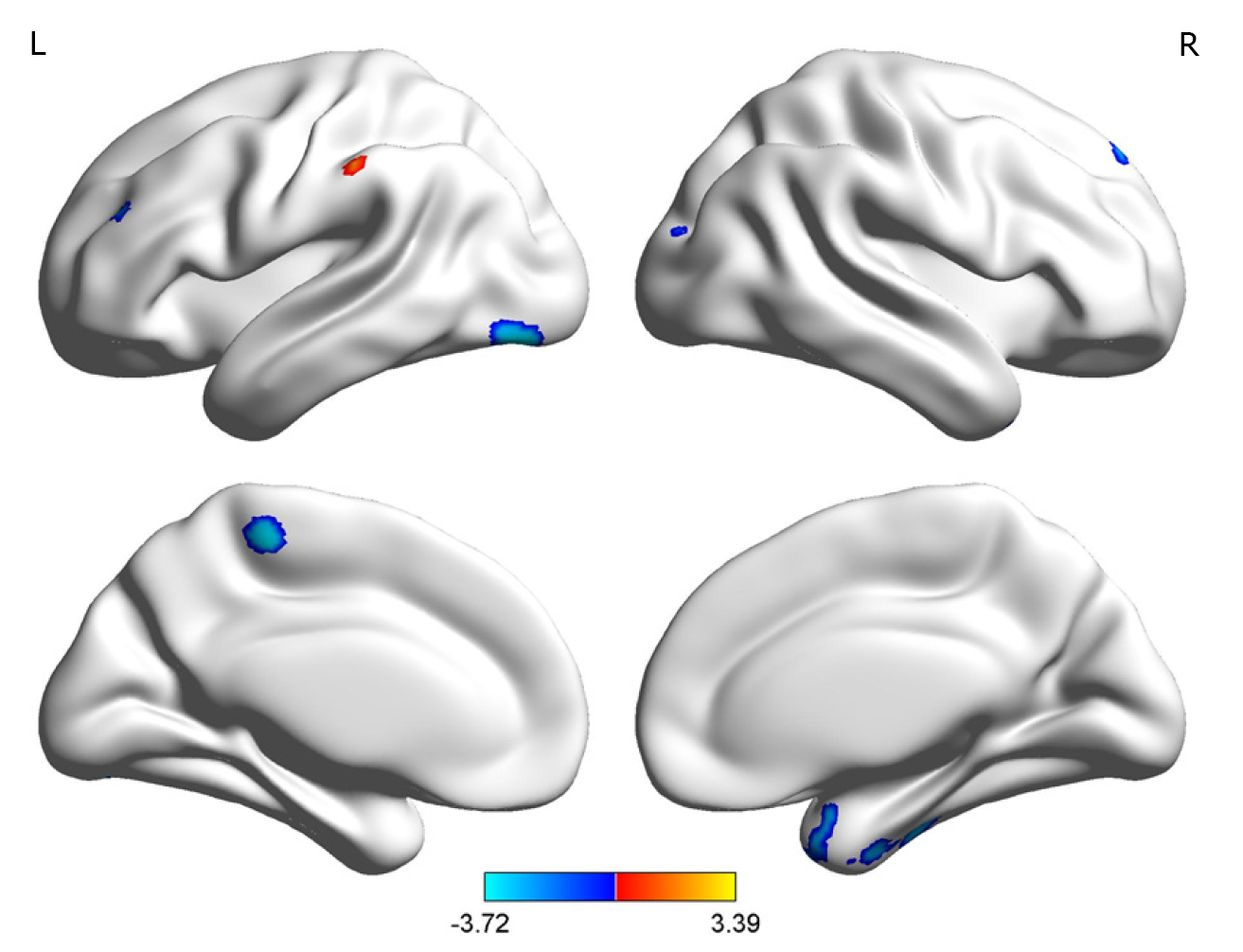
The UD group showed smaller GMVs in the right fusiform gyrus, left inferior occipital gyrus, left paracentral lobule, right superior temporal gyrus, right inferior temporal gyrus, and right posterior cerebellar lobe compared with healthy controls. The GMVs in the left post central gyrus and left middle frontal gyrus in the UD group were larger than those in the controls (Table 3 and Figures 3 and 4).
| MNI coordinate | |||||
| Brain region | Size | X | Y | Z | Ta |
| Right fusiform gyrus | 889 | 33 | 3 | -52.5 | -3.45 |
| Right posterior cerebellar lobe | 138 | 49.5 | -66 | -46.5 | -2.87 |
| Right inferior temporal gyrus | 449 | 45 | -21 | -30 | -2.74 |
| Right superior temporal gyrus | 262 | 25.5 | 12 | -37.5 | -2.74 |
| Left inferior occipital gyrus | 537 | -43.5 | -82.5 | -15 | -3.72 |
| Left paracentral lobule | 126 | -6 | -31.5 | 58.5 | -3.45 |
| Left medial frontal gyrus | 216 | -19.5 | 7.5 | 43.5 | 3.39 |
| Left postcentral gyrus | 113 | -61.5 | -28.5 | 45 | 3.10 |
Between patients with UD and those with BD, the GMVs in the superior frontal gyrus (orbital part; 963 mm3 vs 958 mm3), middle frontal gyrus (3294 mm3 vs 3301 mm3), insula (1770 mm3 vs 1761 mm3), cuneus (1488 mm3 vs 1495 mm3), amygdala (114 mm3 vs 109 mm3), thalamus (1667 mm3 vs 1678 mm3), caudate (994 mm3 vs 989 mm3), and supramarginal gyrus (1973 mm3 vs 1981 mm3) were not significantly different (Table 4 and Figure 5; P > 0.05 for all).
| Brain region | UD (mm3) | BD (mm3) | P value |
| Superior frontal gyrus, orbital part | 963 × 8 | 958 × 8 | > 0.05 |
| Middle frontal gyrus | 3294 × 8 | 3301 × 8 | > 0.05 |
| Insula | 1770 × 8 | 1761 × 8 | > 0.05 |
| Cuneus | 1488 × 8 | 1495 × 8 | > 0.05 |
| Amygdala | 114 × 8 | 109 × 8 | > 0.05 |
| Thalamus | 1667 × 8 | 1678 × 8 | > 0.05 |
| Caudate | 994 × 8 | 989 × 8 | > 0.05 |
| Supramarginal gyrus | 1973 × 8 | 1981 × 8 | > 0.05 |
BD is often misdiagnosed as UD[8], and none of the existing biochemical or neuroelectrophysiological markers have been validated for the differential diagnosis of these two diseases[9-11], and most functional imaging studies did not directly compare the two conditions or yielded inconsistent findings[24-27,35]. Therefore, this study examined whether the GMV changes identified in VBM could distinguish UD from BD. The results show that both UD and BD patients showed significant changes in GMV when compared with healthy controls and that the patterns of changes were different. Still, the differences did not reach statistical significance between the UD and BD groups, probably because of study limitations. Although different patterns of change were observed, the present study does not suggest VBM as a supplementary technique for the differential diagnosis of UD and BD. Additional studies with a larger sample size is necessary to confirm this result.
The study by Redlich et al[26] was conducted in parallel at two centers (one in the United States and one in Germany). They showed that UD and BD could be distinguished using a multivariable model that includes GMVs (anterior cingulate gyrus), and consistent results were obtained between the two centers. Of note, the two centers shared similar patients, i.e, Caucasians of European ancestry and languages sharing the same root. Cai et al[27] showed significant differences in GMVs between BP1 and UD patients in the right middle cingulate gyrus, and that this region could be used to differentiate the two diseases. Chen et al[35], after adjusting for age, sex, body mass index, duration of illness, trivalent inactivated virus vaccine, lithium, and symptoms, showed that GMVs in the right orbitofrontal cortex and left lingual gyrus were significantly different between UD and BD patients. Besides, Li et al[36] and Tan et al[37] revealed differences in neuromatabolite levels between UD and BD, based on multi-voxel proton MR spectroscopy. Here, the results showed no significant differences between UD and BD for any part of the brain, which contradicts the studies above. Some factors could be responsible for those discrepancies. Chinese patients without drugs for at least 2 wk were recruited in the present study. Cultural and ethnic differences can be observed in the functional MRI data[38,39] and could account for the discrepancies. Of course, drugs used in psychiatry affect brain functions[40]. In addition, the MRI sequences are known to affect the brain function results[41,42]. The present study used the 3D-BRAVO protocol, while Cai et al[27] and Redlich et al[26] used T1W images, and Chen et al[35] used the BRAVO sequence. The discrepancies can also be attributed to the small number of participants. Another limitation was the lack of follow-up and re-imaging examinations after treatment, precluding comparisons of patients before and after treatment. Additional studies are still necessary to determine whether VBM can distinguish between UD and BD, especially in the context of inter-population variability. As suggested by Redlich et al[26], the answer may lie in multivariable models or, as suggested by Chen et al[35], in a combination of VBM and biochemical parameters.
The present study revealed two different patterns of changes in GMV between healthy controls and UD and BD. Still, no statistically significant difference was observed in GMV between UD and BD patients. The two groups showed the involvement of different brain regions compared to the normal control group, which possibly supports the differences in the pathophysiological mechanisms between the two groups. In addition, even though the patients did not receive treatment for at least 2 wk before undergoing functional imaging, they were not newly diagnosed patients. Some previously administered drugs may have had mid- and long-term effects on the brain's functional areas. Additional studies are essential to determine the exact mechanisms involved in the pathogenesis of BD and UD. Most studies reported that BD and UD patients had smaller cortical volumes in some brain regions; Some studies have also reported larger cortical volumes of local brain regions in depressive patients. For example, Qiu et al[43] studied patients with first-episode depression without medication and found that the GMVs of the right orbitofrontal cortex, right middle frontal gyrus, and right superior margin gyrus were larger than those in the controls. Similarly, Papmeyer et al[44] reported that patients with depression had larger left inferior frontal gyrus volume compared with the controls. Since compensatory changes in the GMV in different regions have been previously reported in patients with vestibular neuritis[45], the larger GMV in the left superior frontal gyrus in patients with BD and the left middle frontal gyrus in patients with UD might be compensatory responses to the reduction in other regions’ GMV. Still, this hypothesis has to be confirmed in future studies.
Compared with controls, the present study showed that patients with UD had a smaller GMV in the right cerebellum posterior lobe, while patients with BD had a larger GMV in the right cerebellum. The cerebellum is located behind the cerebral hemisphere and consists of the bilateral cerebellar hemispheres and the cerebellar vermis in the middle. Although the cerebellum was previously thought to mainly regulate the movement and balance functions of the human body, the cerebellum is also involved in higher brain function regulation, including cognition and emotions through multiple afferent and efferent neural pathways[46]. The cerebellum is involved in the pathogenesis of BD and UD[47-49]. Here, the results suggest that the pathogenesis of BD and UD involves the cerebellum, but the specific mechanisms need to be elucidated in future studies.
The temporal lobe is located below the frontal and parietal lobes, in front of the occipital lobe, and below the lateral fissure. It is divided into the superior temporal gyrus, middle temporal gyrus, inferior temporal gyrus, and superior temporal sulcus and inferior temporal sulcus[50,51]. The temporal lobe is responsible for processing auditory information and is also related to memory and emotions. The temporal and parietal lobes are crucially involved in regulating emotion by the neural circuits connected with the nerve fiber bundle and frontal lobe[52,53]. Here, patients with BD had smaller GMV of the right temporal lobe, as supported by Chen et al[54], who suggested that the temporal lobe was damaged and that the extensive damage to the temporal lobe structure might be involved in the pathogenesis of BD.
Based on the combination of anatomical nerve connections and functional regions, Koenigs et al[55] proposed dividing the prefrontal cortex (PFC) into the dorsolateral PFC (dlPFC) and the ventromedial PFC. The dlPFC mainly includes the middle frontal gyrus and the superior frontal gyrus and mainly receives specific signals from the sensory cortex. It is closely associated with the premotor and oculomotor areas of the frontal lobe and the lateral parietal cortex. Therefore, it is mainly responsible for cognitive and executive functions, including operational working memory, purposeful behavior, abstract thinking, and attention control[56]. Thus, the frontal lobe plays an important role in the pathogenesis of BD and UD[57].
The midbrain is located above the pons and is the midpoint of the whole brain. It is the reflection center for vision and hearing[58]. Lauterbach et al[59] reported that midbrain lesions could cause BD, while Wang et al[60] found that reduced functional connections in the midbrain were associated with a polygenic genetic risk in patients with BD[60]. Another study[61] also reported abnormal connections between the striatum and midbrain in patients with BD and bipolar mania. These results support the involvement of the midbrain in the pathogenesis of BD. The present study showed a larger GMV in the midbrain of patients with BD compared to the controls, which might also be a compensatory midbrain response to the smaller GMV in the primary center. However, this hypothesis has to be validated in future studies as well.
This study has limitations. The small sample size is, of course, a significant limitation, especially when using a whole-brain approach (instead of a priori regions of interest to reduce risk of type II error) and performing a comparison (BD vs UD) where large effect sizes are not expected[62]. This could have resulted in a lack of statistical power, especially when comparing UD vs BD. In addition, sex representation was unbalanced in the UD group.
In conclusion, UD patients showed smaller GMVs than the controls in the right fusiform gyrus, left inferior occipital gyrus, left paracentral lobule, right superior and inferior temporal gyri, and right posterior lobe of the cerebellum, and larger GMVs than the controls in the left posterior central gyrus and left middle frontal gyrus. There were no differences in GMV between patients with BD and those with UD. Although different patterns of change were observed, the present study does not suggest VBM as a supplementary technique for the differential diagnosis of UD and BD, and additional studies with a larger sample size are necessary. Classification techniques based on machine learning could be explored[26].
Previous studies using voxel-based morphometry (VBM) revealed changes in gray matter volume (GMV) patients with depression.
The differences of GMV using VBM between bipolar disorder (BD) and unipolar depression (UD) are less known.
To analyze the whole-brain GMV data of patients with untreated UD and BD compared with healthy controls.
Patients with BD, those with UD, and non-depressive controls were enrolled to further analyze the brain images.
There were differences in GMV between UD patients and controls, as well as between BD patients and controls. There were no differences in GMV between UD and BD patients.
BM might have a low value for differentiating between UD and BD. However, patients with UD and BD had different patterns of changes in GMV when compared with healthy controls.
Classification techniques based on machine learning could be explored.
Manuscript source: Unsolicited manuscript
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ballini A S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Wang LYT
| 1. | Anderson IM, Haddad PM, Scott J. Bipolar disorder. BMJ. 2012;345:e8508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 2. | Price AL, Marzani-Nissen GR. Bipolar disorders: a review. Am Fam Physician. 2012;85:483-493. [PubMed] |
| 3. | Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381:1672-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 483] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 4. | Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, Viana MC, Andrade LH, Hu C, Karam EG, Ladea M, Medina-Mora ME, Ono Y, Posada-Villa J, Sagar R, Wells JE, Zarkov Z. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68:241-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1693] [Cited by in RCA: 1756] [Article Influence: 125.4] [Reference Citation Analysis (0)] |
| 5. | Mathers CD, Fat DM, Boerma J. The global burden of disease: 2004 update. Geneva: World Health Organization, 2008. |
| 6. | Baldessarini RJ, Tondo L, Baethge CJ, Lepri B, Bratti IM. Effects of treatment latency on response to maintenance treatment in manic-depressive disorders. Bipolar Disord. 2007;9:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Hirschfeld RM, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: how far have we really come? J Clin Psychiatry. 2003;64:161-174. [PubMed] |
| 8. | Hirschfeld RM, Cass AR, Holt DC, Carlson CA. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Pract. 2005;18:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Isomura S, Onitsuka T, Tsuchimoto R, Nakamura I, Hirano S, Oda Y, Oribe N, Hirano Y, Ueno T, Kanba S. Differentiation between major depressive disorder and bipolar disorder by auditory steady-state responses. J Affect Disord. 2016;190:800-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Kesebir S, Tatlıdil Yaylacı E, Süner O, Gültekin BK. Uric acid levels may be a biological marker for the differentiation of unipolar and bipolar disorder: the role of affective temperament. J Affect Disord. 2014;165:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Loch AA, Zanetti MV, de Sousa RT, Chaim TM, Serpa MH, Gattaz WF, Teixeira AL, Machado-Vieira R. Elevated neurotrophin-3 and neurotrophin 4/5 levels in unmedicated bipolar depression and the effects of lithium. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:243-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Woermann FG, Free SL, Koepp MJ, Sisodiya SM, Duncan JS. Abnormal cerebral structure in juvenile myoclonic epilepsy demonstrated with voxel-based analysis of MRI. Brain. 1999;122:2101-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 228] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Chaim TM, Duran FL, Uchida RR, Périco CA, de Castro CC, Busatto GF. Volumetric reduction of the corpus callosum in Alzheimer's disease in vivo as assessed with voxel-based morphometry. Psychiatry Res. 2007;154:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Kim MJ, Hamilton JP, Gotlib IH. Reduced caudate gray matter volume in women with major depressive disorder. Psychiatry Res. 2008;164:114-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Chen L, Wang Y, Niu C, Zhong S, Hu H, Chen P, Zhang S, Chen G, Deng F, Lai S, Wang J, Huang L, Huang R. Common and distinct abnormal frontal-limbic system structural and functional patterns in patients with major depression and bipolar disorder. Neuroimage Clin. 2018;20:42-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 16. | Adler CM, Levine AD, DelBello MP, Strakowski SM. Changes in gray matter volume in patients with bipolar disorder. Biol Psychiatry. 2005;58:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, Jolesz FA, McCarley RW. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage. 2002;17:1711-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 279] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 18. | Tang Y, Wang F, Xie G, Liu J, Li L, Su L, Liu Y, Hu X, He Z, Blumberg HP. Reduced ventral anterior cingulate and amygdala volumes in medication-naïve females with major depressive disorder: A voxel-based morphometric magnetic resonance imaging study. Psychiatry Res. 2007;156:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Chen VC, Shen CY, Liang SH, Li ZH, Tyan YS, Liao YT, Huang YC, Lee Y, McIntyre RS, Weng JC. Assessment of abnormal brain structures and networks in major depressive disorder using morphometric and connectome analyses. J Affect Disord. 2016;205:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Salvadore G, Nugent AC, Lemaitre H, Luckenbaugh DA, Tinsley R, Cannon DM, Neumeister A, Zarate CA Jr, Drevets WC. Prefrontal cortical abnormalities in currently depressed versus currently remitted patients with major depressive disorder. Neuroimage. 2011;54:2643-2651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Peng J, Liu J, Nie B, Li Y, Shan B, Wang G, Li K. Cerebral and cerebellar gray matter reduction in first-episode patients with major depressive disorder: a voxel-based morphometry study. Eur J Radiol. 2011;80:395-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 22. | Almeida JR, Akkal D, Hassel S, Travis MJ, Banihashemi L, Kerr N, Kupfer DJ, Phillips ML. Reduced gray matter volume in ventral prefrontal cortex but not amygdala in bipolar disorder: significant effects of gender and trait anxiety. Psychiatry Res. 2009;171:54-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Lochhead RA, Parsey RV, Oquendo MA, Mann JJ. Regional brain gray matter volume differences in patients with bipolar disorder as assessed by optimized voxel-based morphometry. Biol Psychiatry. 2004;55:1154-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | de Azevedo-Marques Périco C, Duran FL, Zanetti MV, Santos LC, Murray RM, Scazufca M, Menezes PR, Busatto GF, Schaufelberger MS. A population-based morphometric MRI study in patients with first-episode psychotic bipolar disorder: comparison with geographically matched healthy controls and major depressive disorder subjects. Bipolar Disord. 2011;13:28-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Wise T, Radua J, Via E, Cardoner N, Abe O, Adams TM, Amico F, Cheng Y, Cole JH, de Azevedo Marques Périco C, Dickstein DP, Farrow TFD, Frodl T, Wagner G, Gotlib IH, Gruber O, Ham BJ, Job DE, Kempton MJ, Kim MJ, Koolschijn PCMP, Malhi GS, Mataix-Cols D, McIntosh AM, Nugent AC, O'Brien JT, Pezzoli S, Phillips ML, Sachdev PS, Salvadore G, Selvaraj S, Stanfield AC, Thomas AJ, van Tol MJ, van der Wee NJA, Veltman DJ, Young AH, Fu CH, Cleare AJ, Arnone D. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol Psychiatry. 2017;22:1455-1463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 421] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 26. | Redlich R, Almeida JJ, Grotegerd D, Opel N, Kugel H, Heindel W, Arolt V, Phillips ML, Dannlowski U. Brain morphometric biomarkers distinguishing unipolar and bipolar depression. A voxel-based morphometry-pattern classification approach. JAMA Psychiatry. 2014;71:1222-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 211] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 27. | Cai Y, Liu J, Zhang L, Liao M, Zhang Y, Wang L, Peng H, He Z, Li Z, Li W, Lu S, Ding Y, Li L. Grey matter volume abnormalities in patients with bipolar I depressive disorder and unipolar depressive disorder: a voxel-based morphometry study. Neurosci Bull. 2015;31:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edition, text revision. Washington, D.C. , 2000. |
| 29. | Dai XY, Gong YX. [A comparative study on the analysis of the Chinese revision of the Wechsler Adult Intelligence Scale and the original scale (WAIS and WAIS-R)]. Acta Psychol Sinica. 1987;19:72-80. |
| 30. | Dai XY, Ryan JJ, Paolo AM, Harrington RG. Factor analysis of the mainland Chinese version of the Wechsler Adult Intelligence Scale (WAIS-RC) in a brain-damaged sample. Int J Neurosci. 1990;55:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21041] [Cited by in RCA: 22872] [Article Influence: 351.9] [Reference Citation Analysis (0)] |
| 32. | Zheng YP, Zhao JP, Phillips M, Liu JB, Cai MF, Sun SQ, Huang MF. Validity and reliability of the Chinese Hamilton Depression Rating Scale. Br J Psychiatry. 1988;152:660-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 309] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 33. | Zhang Z, Wang Y, Shen Z, Yang Z, Li L, Chen D, Yan G, Cheng X, Shen Y, Tang X, Hu W, Wu R. The Neurochemical and Microstructural Changes in the Brain of Systemic Lupus Erythematosus Patients: A Multimodal MRI Study. Sci Rep. 2016;6:19026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Chau W, McIntosh AR. The Talairach coordinate of a point in the MNI space: how to interpret it. Neuroimage. 2005;25:408-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Chen MH, Chang WC, Hsu JW, Huang KL, Tu PC, Su TP, Li CT, Lin WC, Bai YM. Correlation of proinflammatory cytokines levels and reduced gray matter volumes between patients with bipolar disorder and unipolar depression. J Affect Disord. 2019;245:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Li H, Xu H, Zhang Y, Guan J, Zhang J, Xu C, Shen Z, Xiao B, Liang C, Chen K, Zhang J, Wu R. Differential neurometabolite alterations in brains of medication-free individuals with bipolar disorder and those with unipolar depression: a two-dimensional proton magnetic resonance spectroscopy study. Bipolar Disord. 2016;18:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Tan HZ, Li H, Liu CF, Guan JT, Guo XB, Wen CH, Ou SM, Zhang YN, Zhang J, Xu CT, Shen ZW, Wu RH, Wang XQ. Main Effects of Diagnoses, Brain Regions, and their Interaction Effects for Cerebral Metabolites in Bipolar and Unipolar Depressive Disorders. Sci Rep. 2016;6:37343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Paige LE, Ksander JC, Johndro HA, Gutchess AH. Cross-cultural differences in the neural correlates of specific and general recognition. Cortex. 2017;91:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | McCutcheon R, Bloomfield MAP, Dahoun T, Quinlan M, Terbeck S, Mehta M, Howes O. Amygdala reactivity in ethnic minorities and its relationship to the social environment: an fMRI study. Psychol Med. 2018;48:1985-1992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Wandschneider B, Koepp MJ. Pharmaco fMRI: Determining the functional anatomy of the effects of medication. Neuroimage Clin. 2016;12:691-697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Symms M, Jäger HR, Schmierer K, Yousry TA. A review of structural magnetic resonance neuroimaging. J Neurol Neurosurg Psychiatry. 2004;75:1235-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Binder JR, Swanson SJ, Hammeke TA, Sabsevitz DS. A comparison of five fMRI protocols for mapping speech comprehension systems. Epilepsia. 2008;49:1980-1997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 43. | Qiu L, Lui S, Kuang W, Huang X, Li J, Li J, Zhang J, Chen H, Sweeney JA, Gong Q. Regional increases of cortical thickness in untreated, first-episode major depressive disorder. Transl Psychiatry. 2014;4:e378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 44. | Papmeyer M, Giles S, Sussmann JE, Kielty S, Stewart T, Lawrie SM, Whalley HC, McIntosh AM. Cortical Thickness in Individuals at High Familial Risk of Mood Disorders as They Develop Major Depressive Disorder. Biol Psychiatry. 2015;78:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 45. | Hong SK, Kim JH, Kim HJ, Lee HJ. Changes in the gray matter volume during compensation after vestibular neuritis: a longitudinal VBM study. Restor Neurol Neurosci. 2014;32:663-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Sullivan EV. Cognitive functions of the cerebellum. Neuropsychol Rev. 2010;20:227-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Depping MS, Nolte HM, Hirjak D, Palm E, Hofer S, Stieltjes B, Maier-Hein K, Sambataro F, Wolf RC, Thomann PA. Cerebellar volume change in response to electroconvulsive therapy in patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2017;73:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 48. | Mills NP, Delbello MP, Adler CM, Strakowski SM. MRI analysis of cerebellar vermal abnormalities in bipolar disorder. Am J Psychiatry. 2005;162:1530-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 49. | Zhang J, Zu CT, Wu RH. Progress in imaging studies of cerebellum in mental illness. J Int Psych. 2011;2011:234-237. |
| 50. | Carr VA, Rissman J, Wagner AD. Imaging the human medial temporal lobe with high-resolution fMRI. Neuron. 2010;65:298-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 51. | Khashper A, Chankowsky J, Del Carpio-O'Donovan R. Magnetic resonance imaging of the temporal lobe: normal anatomy and diseases. Can Assoc Radiol J. 2014;65:148-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Koelsch S, Skouras S, Lohmann G. The auditory cortex hosts network nodes influential for emotion processing: An fMRI study on music-evoked fear and joy. PLoS One. 2018;13:e0190057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 53. | Munoz-Lopez MM, Mohedano-Moriano A, Insausti R. Anatomical pathways for auditory memory in primates. Front Neuroanat. 2010;4:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 54. | Chen X, Wen W, Malhi GS, Ivanovski B, Sachdev PS. Regional gray matter changes in bipolar disorder: a voxel-based morphometric study. Aust N Z J Psychiatry. 2007;41:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Koenigs M, Huey ED, Calamia M, Raymont V, Tranel D, Grafman J. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. J Neurosci. 2008;28:12341-12348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 180] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 56. | Forbes CE, Poore JC, Krueger F, Barbey AK, Solomon J, Grafman J. The role of executive function and the dorsolateral prefrontal cortex in the expression of neuroticism and conscientiousness. Soc Neurosci. 2014;9:139-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 57. | Gałecki P, Talarowska M, Anderson G, Berk M, Maes M. Mechanisms underlying neurocognitive dysfunctions in recurrent major depression. Med Sci Monit. 2015;21:1535-1547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 58. | Presacco A, Simon JZ, Anderson S. Evidence of degraded representation of speech in noise, in the aging midbrain and cortex. J Neurophysiol. 2016;116:2346-2355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 59. | Lauterbach EC. Bipolar disorders, dystonia, and compulsion after dysfunction of the cerebellum, dentatorubrothalamic tract, and substantia nigra. Biol Psychiatry. 1996;40:726-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 60. | Wang T, Zhang X, Li A, Zhu M, Liu S, Qin W, Li J, Yu C, Jiang T, Liu B. Polygenic risk for five psychiatric disorders and cross-disorder and disorder-specific neural connectivity in two independent populations. Neuroimage Clin. 2017;14:441-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 61. | Altinay MI, Hulvershorn LA, Karne H, Beall EB, Anand A. Differential Resting-State Functional Connectivity of Striatal Subregions in Bipolar Depression and Hypomania. Brain Connect. 2016;6:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 62. | Carter CS, Bearden CE, Bullmore ET, Geschwind DH, Glahn DC, Gur RE, Meyer-Lindenberg A, Weinberger DR. Enhancing the Informativeness and Replicability of Imaging Genomics Studies. Biol Psychiatry. 2017;82:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |