Published online Feb 26, 2021. doi: 10.12998/wjcc.v9.i6.1284
Peer-review started: November 3, 2020
First decision: November 20, 2020
Revised: December 2, 2020
Accepted: December 16, 2020
Article in press: December 16, 2020
Published online: February 26, 2021
Processing time: 95 Days and 10.4 Hours
Acute kidney injury (AKI) is a sudden or rapid decline in the filtration function of the kidneys which is marked by increased serum creatinine or blood urea nitrogen.
To examine the value of alprostadil-assisted continuous venous-venous hemofiltration (CVVH) in the treatment of severe AKI in severely ill patients.
This was a retrospective study and the inclusion criteria were as follows: (1) Age of patients (≥ 18 years); (2) Admission to intensive care unit due to non-renal primary disease, APACHE II score (≥ 18 points); (3) The diagnostic criteria of AKI guidelines were formulated with reference to the Global Organization for the Improvement of Prognosis in Kidney Diseases, with AKI grades of II-III; (4) All patients were treated with CVVH; and (5) Complete basic data were obtained for all patients.
The clinical effect of alprostadil administered in the treatment group was better than that observed in the control group (P < 0.05). The urine output of patients in the alprostadil group returned to normal time (9.1 ± 2.0 d) and was lower than that in the control group (10.6 ± 2.5 d), the difference was statistically significant (P < 0.05); adverse reactions occurred in the alprostadil group compared with the control group, but the difference was not statistically significant (P > 0.05).
Alprostadil-assisted CVVH in the treatment of severely ill patients with AKI can effectively improve the renal resistance index and partial pressure of urine oxygen, and has a positive effect on improving renal function.
Core Tip: Alprostadil-assisted continuous venous-venous hemofiltration in the treatment of severely ill patients with acute kidney injury may provide guidance and the basis for clinical practice.
- Citation: Jia Y, Liu LL, Su JL, Meng XH, Wang WX, Tian C. Effect of alprostadil in the treatment of intensive care unit patients with acute renal injury. World J Clin Cases 2021; 9(6): 1284-1292
- URL: https://www.wjgnet.com/2307-8960/full/v9/i6/1284.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i6.1284
Acute kidney injury (AKI) is a sudden or rapid decline in the filtration function of the kidneys which is marked by increased serum creatinine (Scr) or blood urea nitrogen (BUN). Treatment of severe AKI is usually relatively complicated.
Continuous venous-venous hemofiltration (CVVH) is an important method for the clinical treatment of kidney diseases, which uses convection and diffusion to remove solutes by mimicking the filtration principle of glomeruli. It introduces arterial or venous blood into semi-permeable membrane filters with good permeability, and ultimately removes water and solute. However, kidney function damage is a common complication of CVVH, which increases the cost of clinical treatment and has a serious impact on the quality of life of patients[1]. Alprostadil has been proved to have the effects of dilating vascular smooth muscle, anti-platelet agglutination, protecting cells and inhibiting inflammation. It has positive effects on organ protection in critically ill patients, but relatively few clinical studies have reported its use in CVVH to avoid kidney injury[2].
In order to improve the effect of CVVH in patients with AKI and reduce treatment complications, this study was conducted to observe the effect of alprostadil-assisted CVVH in the treatment of severely ill patients with AKI to provide guidance and the basis for clinical practice.
This retrospective study was conducted to select 100 intensive care unit (ICU) patients with AKI, of whom 50 patients received CVVH and basic treatment measures (control group), and the remaining 50 patients were also given alprostadil simultaneously (alprostadil group). The study subjects were admitted from January 2017 to March 2019, and the inclusion criteria were as follows: (1) Age of patients (≥ 18 years); (2) Admission to ICU due to non-renal primary disease, Acute Physiological Function and Chronic Health Score II (APACHE II) score (≥ 18 points); (3) The diagnostic criteria of AKI guidelines were formulated with reference to the Global Organization for the Improvement of Prognosis in Kidney Diseases, with AKI grades of II-III; (4) All patients were treated with CVVH; and (5) Complete basic data were obtained for all patients. The exclusion criteria were as follows: (1) Patients with primary kidney disease and renal dysfunction; (2) Patients with advanced malignant tumors; (3) Patients after kidney transplantation and liver transplantation; (4) Contraindications to CVVH treatment; and (5) Patients who were transferred for treatment.
In the alprostadil group, the age of patients ranged from 42 to 79 years, with an average of 61.7 ± 8.0 years, including 29 males and 21 females, all of whom had basic diseases, including 13 patients admitted to hospital due to trauma, 10 patients with cardiovascular diseases, 7 patients with diabetes, and 20 patients with cerebrovascular diseases. The APACHE II score was 23.6 ± 2.7 points, while the AKI grade was grade II in 29 cases and grade III in 21 cases. In the control group, the age of patients ranged from 38 to 79 years, with an average of 60.4 ± 10.3 years, including 32 males and 18 females, who had basic diseases, including 15 patients admitted to hospital due to trauma, 6 patients with cardiovascular diseases, 5 patients with diabetes, and 23 patients with cerebrovascular diseases. The APACHE II score was 24.0 ± 2.5 points, while AKI grade was grade II in 33 cases and grade III in 17 cases. There was no significant difference in age, gender and other baseline data between the two groups
Treatment methods: Control group: Jinbao Prismaflex continuous blood purification equipment was used for treatment, and 4000 mL/bag of replacement fluid was selected to filter the basic replacement fluid. The blood flow rate was set to 150 mL/min, and citrate was used for anticoagulation. The initial pump speed was 2.0%-2.5% of the blood flow rate, and 10% calcium gluconate solution was pumped into the venous end of the treatment pipeline to maintain the whole-body free calcium ion level of 1.0-1.2 mmol/L, and fluid resuscitation, anti-infection and glucocorticoid support were given at the same time for symptomatic treatment.
Alprostadil group: In addition to the treatment given to the control group, patients were also treated with alprostadil (produced by Harbin Pharmaceutical Group Bioengineering Co., Ltd., drug approval No: National drug approval No. H20059787, drug batch No. 20170516), and intravenous injection of 10 UG of alprostadil and 10 mL of normal saline was performed, once every 12 h for 1 wk.
Fluid infusion rate was controlled and vital signs of the patients were strictly monitored during treatment in both groups.
Data collection: Comparisons between the two groups of patients, included the APACHE II score, Sequential Organ Failure Score (SOFA), Scr, BUN, urinary kidney injury molecule-1 (KIM-1), urinary neutrophil gelatinase-associated lipocalin (NAGL), urinary interleukin-8 (IL-8), renal resistance index, urinary partial pressure of oxygen and therapeutic effect before and after treatment for 7 d [criteria for the improvement of renal function: (1) Cure: After treatment, the patient’s urine volume is more than 1000 mL/24 h, Scr is less than 176.8 mmol/L, and BUN is less than 7.14 mmol/L; (2) Improvement: After treatment, the patient’s urine volume is more than 1000 mL/24 h, Scr and urea nitrogen levels significantly decreased, but did not reach the normal level (trouble to confirm the normal range of creatinine, should not be 176.8); and (3) Ineffective: Did not meet the above criteria or patient died]. The fasting venous blood of the patients before and after treatment was sampled and centrifuged at a speed of 3000/rpm for 10 min to separate the serum. Then, using the kit provided by BIO SWAMP Company, and operating according to the kit instructions, an enzyme-linked immunosorbent assay was used to determine the concentration changes in urine and KIM-1 in patients.
SPSS 21.0 software was used for statistical analysis, while Scr, BUN, KIM-1 and other measurement indicators in the two groups were expressed by mean ± SD. Independent sample t-test was used for comparison between the two groups, and χ2 or non-parametric test was used for comparison of enumeration data such as gender and clinical efficacy, with P < 0.05 considered statistically significant.
Before treatment, there was no significant difference in APACHE II score and SOFA score between the alprostadil group and the control group (P > 0.05); while after 7 d of treatment, the APACHE II score and SOFA score of the alprostadil group were lower than those of the control group (P < 0.05), as shown in Table 1.
| Group | n | Year | Sex | Basic diseases | APACHE ІІ | AKI | |||||
| Men | Women | Trauma | Cardiovascular disease | Cerebrovascular disease | Other | ІІ | Ш | ||||
| Alprostadil group | 50 | 61.7 ± 8.0 | 29 | 21 | 13 | 10 | 20 | 7 | 23.6 ± 2.7 | 29 | 21 |
| Control group | 50 | 60.4 ± 10.3 | 32 | 18 | 15 | 6 | 23 | 5 | 24.0 ± 2.5 | 33 | 17 |
| t/χ2 | 0.705 | 0.378 | 1.676 | -0.769 | 0.679 | ||||||
| P | 0.483 | 0.539 | 0.642 | 0.444 | 0.410 | ||||||
Before treatment, there were no significant differences in the levels of Scr, BUN, KIM-1, NAGL and IL-8 between the alprostadil group and the control group (P > 0.05), while after 7 d of treatment, the levels of Scr, BUN, KIM-1, NAGL and IL-8 in the alprostadil group were lower than those in the control group (P < 0.05), as shown in Table 2.
| Group | n | Scr (μmol/L) | BUN (mmol/L) | KIM-1 (pg/mL) | |||
| Before treatment | 7 d after treatment | Before treatment | 7 d after treatment | Before treatment | 7 d after treatment | ||
| Alprostadil group | 50 | 448.1 ± 95.2 | 180.6 ± 34.7ab | 17.8 ± 3.9 | 8.1 ± 1.8ab | 1338.2 ± 202.1 | 787.4 ± 144.2ab |
| Control group | 50 | 420.8 ± 81.7 | 207.1 ± 42.4a | 17.3 ± 3.4 | 9.3 ± 2.0a | 1310.3 ± 231.8 | 855.8 ± 153.9a |
| T value | 1.539 | -3.420 | 0.683 | -3.154 | 0.642 | -2.293 | |
| P value | 0.127 | 0.001 | 0.496 | 0.002 | 0.523 | 0.024 | |
| Group | n | NAGL (ng/mL) | IL-8 (ng/mL) | ||||
| Before treatment | 7 d after treatment | Before treatment | 7 d after treatment | ||||
| Alprostadil group | 50 | 126.9 ± 16.4 | 89.5 ± 11.0ab | 58.3 ± 9.1 | 36.8 ± 7.0ab | ||
| Control group | 50 | 130.0 ± 20.1 | 96.7 ± 12.4a | 56.8 ± 8.3 | 41.0 ± 8.3a | ||
| T value | -0.845 | -3.071 | 0.861 | -2.735 | |||
| P value | 0.400 | 0.003 | 0.391 | 0.007 | |||
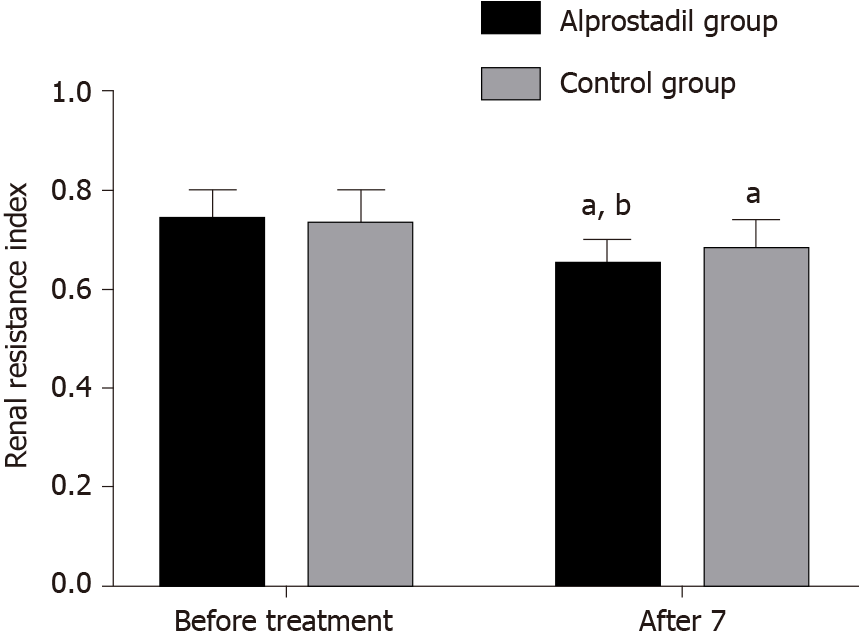
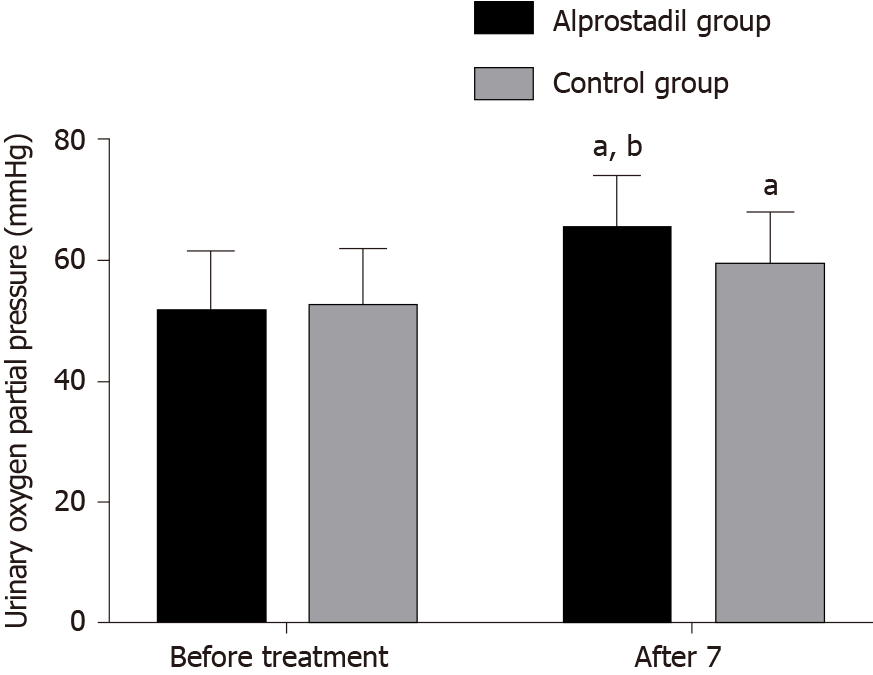
Before treatment, there was no significant difference in renal resistance index and partial pressure of oxygen in urine between the alprostadil group and control group (P > 0.05), while after 7 d of treatment, the renal resistance index in the alprostadil group was lower than that in the control group (P < 0.05), and the partial pressure of oxygen in urine was higher than that in the control group (P < 0.05), as shown in Table 3, Figures 1 and 2.
| Group | n | Renal resistance index | Partial pressure of oxygen in urine (mmHg) | ||
| Before treatment | 7 d after treatment | Before treatment | 7 d after treatment | ||
| Alprostadil group | 50 | 0.74 ± 0.06 | 0.65 ± 0.05ab | 51.32 ± 10.32 | 65.06 ± 9.04ab |
| Control group | 50 | 0.73 ± 0.07 | 0.68 ± 0.06a | 52.40 ± 9.64 | 59.25 ± 8.75a |
| T value | 0.767 | -2.716 | -0.541 | 3.265 | |
| P value | 0.445 | 0.008 | 0.590 | 0.002 | |
After treatment, the cure rate, improvement rate and ineffectiveness in the alprostadil group were 64.00%, 28.00% and 8.00%, respectively, while those in the control group were 42.00%, 44.00% and 14.00%, respectively. The clinical effect in the alprostadil group was superior to that in the control group (P < 0.05), as shown in Table 4.
| Group | n | Cure | Improve | Ineffective |
| Alprostadil group | 50 | 32 (64.00) | 14 (28.00) | 4 (8.00) |
| Control group | 50 | 21 (42.00) | 22 (44.00) | 7 (14.00) |
| Z value | -2.154 | |||
| P value | 0.031 | |||
The recovery time of urine volume in the alprostadil group was 9.1 ± 2.0 d, which was lower than that in the control group (10.6 ± 2.5 d), and the difference was statistically significant (P < 0.05), while the incidence of adverse reactions in the alprostadil group was not significantly different to that in the control group (P > 0.05), as shown in Table 5.
| Group | n | Injection spot ache | Abdominal distention | Incidence of adverse reactions (%) |
| Alprostadil group | 50 | 2 | 4 | 6 (12.00) |
| Control group | 50 | 0 | 2 | 2 (4.00) |
| χ2 value | 2.174 | |||
| P value | 0.14 |
The ICU is a place to rescue severely ill patients, and most patients suffer from multiple organ failure. It has been reported that the mortality rate of patients with more than 2 failed organs is more than 50%, and that of patients with more than 4 failed organs is 100%, which seriously affects the quality of life and safety of patients[3,4]. Studies have shown that the release of proinflammatory mediators is increased in severely ill patients, and then tissue replacement downregulates the release of proinflammatory mediators. Generally, the concentration of local inflammatory mediators is higher than the concentration in the circulation and does not lead to destruction of the body’s inflammatory response. However, with aggravation of the disease, the local proinflammatory response cannot resist the invasion and will mobilize the systemic defense mechanism, and the excessive and long-lasting release of the proinflammatory response may destroy the homeostasis of the human body and form a systemic inflammatory cascade reaction, eventually causing cell damage, endothelial cell dysfunction, increased microvascular permeability, microcirculation disorder, and changes in the body’s coagulation system, which cannot restore the stability of the body’s internal environment and have a serious impact on the safety of patients[5,6]. Therefore, in the treatment of severely ill patients, it is necessary not only to provide effective nutritional support to avoid aggravating the burden of organ function, but also to carry out active treatment of anti-inflammatory mediators in the body. Animal experiments have confirmed that the uncontrolled inflammatory response causes the occurrence of MODS; therefore, it is of great significance to improve the prognosis of patients by effectively removing toxins and inflammatory mediators from the body[7,8].
At present, hemodialysis is an important treatment method in severely ill patients, which can remove endotoxin substances, filter excess water, and prolong the survival time of patients. CVVH treatment has become a commonly used method in clinical practice, which uses a variety of physical techniques to displace inflammatory factors from the body, and improve the lung microcirculation and oxygen carrying capacity of parenchymal cells, as well as tissue oxygen utilization, but can also clear inflammatory mediators, improve endothelial cell function in patients, and have a stabilizing effect on disorders of coagulation and fibrinolysis[9]. However, it was found that AKI can occur during the course of CVVH treatment, which is due to abnormal renal microcirculation, reduced peripheral vascular resistance, uneven distribution of blood flow in organs, and reduced capillary blood volume. AKI generally occurs mainly in the renal cortex and medulla, and the reduction of renal blood flow causes kidney injury, as well as the swelling of endothelial cells which causes renal vascular occlusion[10,11]. On the other hand, due to the expansion of endothelial cells, the reduction of vascular substances and the enhancement of vasoconstrictor substances after injury, the redistribution of renal blood flow leads to the oxidative stress reaction in vivo, and renal tubular cell damage can also cause AKI due to the uneven distribution of renal blood flow caused by microcirculation disorder[12,13].
This study combined alprostadil with CVVH in the treatment of patients complicated by AKI, which is a prostaglandin substance containing high vasoactive substances, and can improve blood viscosity in patients, and at the same time inhibit the function of monocytes, neutrophils and reduce the platelet adhesion rate, so as to achieve anti-inflammatory and other effects[14,15]. This study found that after 7 d of treatment, the levels of Scr, BUN and KIM-1 in the alprostadil group were lower than those in the control group, which indicated that the application of alprostadil improved renal function in patients and reduced the degree of renal injury. This is mainly because alprostadil is not easily inactivated as it is wrapped by lipid microspheres, and contains prostatic E1, which can target and be dispersed to injured blood vessels, and has the effects of dilating blood vessels and anti-platelet aggregation. Alprostadil can also lead to increased intracellular levels of cyclic adenosine monophosphate, inhibit thromboxane formation, reduce calcium influx, and play a role in dilating blood vessels[16,17]. In addition, this study also found that after 7 d of treatment, the measured values of renal resistance index in the alprostadil group were lower than those in the control group, and the measured values of partial pressure of oxygen in urine were higher than those in the control group, which indicated that the application of alprostadil reduced renal resistance. This is mainly due to the effect of alprostadil on stabilizing lysosomal membrane, weakening vasoconstrictive response, enhancing erythrocyte deformability, stabilizing renal hemodynamics, and enhancing renal glomerular filtration; thus, renal function in patients can be protected by increasing renal blood flow to reduce the pressure in the glomerulus[18,19]. Animal experiments have also demonstrated that alprostadil could alleviate pathological damage in renal tissue and promote the regeneration and recovery of renal tubular epithelial cells in rats with acute renal tubular injury, with a good renal protective effect[20].
This study also found that after 7 d of treatment, the APACHE II score and SOFA score in the alprostadil group were lower than those in the control group, indicating that the application of alprostadil in patients undergoing CVVH complicated by AKI can improve the clinical signs and health status of patients. The therapeutic effect and clinical effect in the alprostadil group were better than those in the control group, which indicated that the application of alprostadil in patients undergoing CVVH complicated by AKI could improve the clinical effect. The recovery time of urine volume in the alprostadil group was 9.1 ± 2.0 d, which was short and did not increase adverse reactions, indicating that the application of alprostadil was relatively safe. The advantage of this study was the identification of the therapeutic effect of alprostadil in patients undergoing CVVH complicated with AKI, further confirming its role in protecting renal function in these patients, which is similar to the results of previous studies, and provides a basis for rational clinical treatment. In addition, this study analyzed changes in the renal resistance index and renal blood flow index, which were rarely measured in previous studies. However, due to the short follow-up time and the small number of patients enrolled in this study, it is necessary to expand the sample size and carry out long-term follow-up for further validation of these findings.
In conclusion, alprostadil combined with CVVH in the treatment of severely ill patients complicated by AKI can effectively improve renal resistance index, partial pressure of oxygen in urine, and has a positive effect on improving renal function.
Acute kidney injury is a serious disease.
To explore new methods for the treatment of acute kidney injury.
To examine the effect of alprostadil on continuous venous-venous hemofiltration (CVVH) in patients with acute renal injury (AKI).
This was a retrospective study of 100 intensive care unit patients with AKI.
The clinical effect in the alprostadil group was better than that in the control group (P < 0.05); The urine output of patients in the alprostadil group returned to normal (9.1 ± 2.0 d) quicker than in the control group (10.6 ± 2.5 d), the difference was statistically significant (P < 0.05); adverse reactions occurred in the alprostadil group compared with the control group, but the difference was not statistically significant (P > 0.05).
Alprostadil combined with CVVH in the treatment of severely ill patients complicated with AKI can effectively improve renal resistance index, partial pressure of oxygen in urine, and has a positive effect on improving renal function.
New treatment methods are required for patients with acute kidney injury.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dediulia T, Lo G, Morgan E S-Editor: Huang P L-Editor: Webster JR P-Editor: Liu JH
| 1. | Yang Y, Meng L, Wu S, Li Y, Zhong Y, Xu F, Zhou XC, Li GQ, Xu GL, Peng KF. LIGHT deficiency aggravates cisplatin-induced acute kidney injury by upregulating mitochondrial apoptosis. Int Immunopharmacol. 2020;89:106999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Manou E, Thodis E, Arsos G, Pasadakis P, Panagoutsos S, Papadopoulou D, Papagianni A. Fibroblast Growth Factor 23 and α-Klotho Protein Are Associated with Adverse Clinical Outcomes in Non-Dialysis CKD Patients. Kidney Blood Press Res. 2020;45:900-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Cui R, Li F, Shao J, Wang Y, Yue C, Zheng Y, Li X. Postoperative anemia is a risk factor for acute kidney injury after open aorta and vena cava surgeries. PLoS One. 2020;15:e0240243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Dunaevich A, Chen H, Musseri D, Kuzi S, Mazaki-Tovi M, Aroch I, Segev G. Acute on chronic kidney disease in dogs: Etiology, clinical and clinicopathologic findings, prognostic markers, and survival. J Vet Intern Med. 2020;34:2507-2515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Liu D, Shu G, Jin F, Qi J, Xu X, Du Y, Yu H, Wang J, Sun M, You Y, Zhu M, Chen M, Zhu L, Shen Q, Ying X, Lou X, Jiang S, Du Y. ROS-responsive chitosan-SS31 prodrug for AKI therapy via rapid distribution in the kidney and long-term retention in the renal tubule. Sci Adv. 2020;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 6. | Sako K, Furuichi K, Yamamura Y, Oshima M, Toyama T, Kaneko S, Wada T. Association between the recurrence period of acute kidney injury and mortality: a single-centre retrospective observational study in Japan. BMJ Open. 2019;9:e023259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Gadalean F, Simu M, Parv F, Vorovenci R, Tudor R, Schiller A, Timar R, Petrica L, Velciov S, Gluhovschi C, Bob F, Mihaescu A, Timar B, Spasovski G, Ivan V. The impact of acute kidney injury on in-hospital mortality in acute ischemic stroke patients undergoing intravenous thrombolysis. PLoS One. 2017;12:e0185589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Conroy AL, Hawkes MT, Elphinstone R, Opoka RO, Namasopo S, Miller C, John CC, Kain KC. Chitinase-3-like 1 is a biomarker of acute kidney injury and mortality in paediatric severe malaria. Malar J. 2018;17:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Wen Y, Parikh CR. The Aftermath of AKI: Recurrent AKI, Acute Kidney Disease, and CKD Progression. J Am Soc Nephrol. 2021;32:2-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Leballo G, Chakane PM. Cardiac surgery-associated acute kidney injury: pathophysiology and diagnostic modalities and management. Cardiovasc J Afr. 2020;31:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Oweis AO, Alshelleh SA. Incidence and outcomes of acute kidney injury in octogenarians in Jordan. BMC Res Notes. 2018;11:279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Nayak SL, Kumar M, Bihari C, Rastogi A. Bile Cast Nephropathy in Patients with Acute Kidney Injury Due to Hepatorenal Syndrome: A Postmortem Kidney Biopsy Study. J Clin Transl Hepatol. 2017;5:92-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 13. | Vanmassenhove J, Kielstein J, Jörres A, Biesen WV. Management of patients at risk of acute kidney injury. Lancet. 2017;389:2139-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 14. | Sun P, Xu L, Zhang Q, Li Q. Impact of Toll-like receptor 4 deficiency on cerebrocardiac syndrome. J Huazhong Univ Sci Technolog Med Sci. 2014;34:161-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Bellomo R, Ronco C, Mehta RL, Asfar P, Boisramé-Helms J, Darmon M, Diehl JL, Duranteau J, Hoste EAJ, Olivier JB, Legrand M, Lerolle N, Malbrain MLNG, Mårtensson J, Oudemans-van Straaten HM, Parienti JJ, Payen D, Perinel S, Peters E, Pickkers P, Rondeau E, Schetz M, Vinsonneau C, Wendon J, Zhang L, Laterre PF. Acute kidney injury in the ICU: from injury to recovery: reports from the 5th Paris International Conference. Ann Intensive Care. 2017;7:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 16. | Yu F, Zhu J, Lei M, Wang CJ, Xie K, Xu F, Lin SH. Exploring the metabolic phenotypes associated with different host inflammation of acute respiratory distress syndrome (ARDS) from lung metabolomics in mice. Rapid Commun Mass Spectrom. 2021;35:e8971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Liu G, Liu F, Xiao L, Kuang Q, He X, Wang Y, Yu Y. Treatment of hyperlipidemic acute pancreatitis with modified Dachengqi decoction combining with conventional therapy based on "six-hollow-organs to be unblocked" theory. Ann Palliat Med. 2020;9:2045-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Blinder JJ, Asaro LA, Wypij D, Selewski DT, Agus MSD, Gaies M, Ferguson MA. Acute Kidney Injury After Pediatric Cardiac Surgery: A Secondary Analysis of the Safe Pediatric Euglycemia After Cardiac Surgery Trial. Pediatr Crit Care Med. 2017;18:638-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Mavrakanas TA, Aurian-Blajeni DE, Charytan DM. Early versus late initiation of renal replacement therapy in patients with acute kidney injury: a meta-analysis of randomised clinical trials. Swiss Med Wkly. 2017;147:w14507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Chen Z, Hua S. Transcription factor-mediated signaling pathways' contribution to the pathology of acute lung injury and acute respiratory distress syndrome. Am J Transl Res. 2020;12:5608-5618. [PubMed] |