Published online Feb 16, 2021. doi: 10.12998/wjcc.v9.i5.1237
Peer-review started: December 3, 2020
First decision: December 13, 2020
Revised: December 14, 2020
Accepted: December 22, 2020
Article in press: December 22, 2020
Published online: February 16, 2021
Processing time: 58 Days and 4.5 Hours
Isolated musculocutaneous nerve injury is a rare condition. Herein, we report the first case of bilateral musculocutaneous neuropathy after vigorous stretching of both upper extremities with normal results of sensory nerve action potential. Clinicians should be aware of this rare condition that can appear bilaterally. In addition, the interpretation of the aberrant electrodiagnostic study results of this case was discussed.
A 29-year-old male complaining of bilateral forearm tingling and upper extremity weakness visited the outpatient clinic. The symptoms began 6 mo prior, and he visited another hospital before visiting our department. The diagnosis was not made even after cervical spine magnetic resonance imaging, electrodiagnostic study, brain magnetic resonance imaging, and arteriography were conducted. The patient performed unique exercises that stretched the pectoralis minor and coracobrachialis muscles. On the follow-up electrodiagnostic study, abnormal spontaneous activities in the bilateral biceps and brachialis muscles were observed. The patient was diagnosed with bilateral musculocutaneous neuropathy. Steroid pulse therapy was administered for approximately 6 wk. After treatment, his muscle strength returned to the predisease condition.
Clinicians should be aware of this condition, have adequate understanding of anatomy, and advise to correct inappropriate exercises.
Core Tip: We report the first case of bilateral musculocutaneous neuropathy after vigorous stretching of both upper extremities with normal results of sensory nerve action potential. We recommend clinicians be aware of this rare condition that can occur bilaterally. In addition, the interpretation of aberrant electrodiagnostic study results of this case is discussed.
- Citation: Jung JW, Park YC, Lee JY, Park JH, Jang SH. Bilateral musculocutaneous neuropathy: A case report. World J Clin Cases 2021; 9(5): 1237-1246
- URL: https://www.wjgnet.com/2307-8960/full/v9/i5/1237.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i5.1237
Isolated musculocutaneous nerve injury is a rare condition and generally associated with brachial plexopathy[1]. In previous reports, isolated musculocutaneous injury occurred after intense physical activity, repeated strenuous upper extremity activity[2,3], after quick and strong movements[4], trauma[5], surgery[6], wrestling, or incorrect pitching by a pitcher[7,8]. Musculocutaneous lesions usually occur proximal to the biceps and brachialis muscles[9]. The most common lesion site is the coracobra-chialis muscle[9]. Due to hypertrophy or powerful contraction of the muscle, mechanical and ischemic nerve injury can occur[9]. In previous reports, patients presented with unilateral arm symptoms.
Electrodiagnostic evaluation is important for diagnosing peripheral neuropathy. Appropriate electrodiagnostic evaluation is needed for differential diagnosis[10]. Peripheral nerves respond to injury in various ways, and test findings are different depending on the timing of the test[11]. Furthermore, peripheral neuropathy has neurapraxic nature and is often self-resolving[5]. Therefore, performing and interpreting electrodiagnostic studies properly can be difficult during diagnosing neuropathy.
Herein, we report a case of bilateral musculocutaneous neuropathy after vigorous stretching of both upper extremities with normal results of sensory nerve action potential (SNAP). Because the importance of various stretching and exercise is emphasized, we recommend clinicians be aware of this rare condition that can occur bilaterally. In addition, the interpretation of the aberrant electrodiagnostic study results in this case is discussed.
A 29-year-old male complaining of bilateral forearm tingling and bilateral upper extremity weakness visited the outpatient clinic of our department.
His primary symptoms were bilateral forearm tingling and pain. These symptoms began 6 mo prior to the visit. Within a few days, bilateral upper extremity weakness also began. There was no improvement in symptoms for several weeks. Thus, he visited the neurology department of another hospital. Under suspicion of radiculopathy, cervical spine magnetic resonance imaging (MRI) with enhancement and electrodiagnostic study were performed. However, abnormalities were not found. During the follow-up period at that hospital, brain MRI, computed tomography, and arteriography of the left upper extremity were performed, but a diagnosis could not be made.
Later, when the patient visited the hospital, he complained of bilateral forearm tingling, weakness, and bilateral arm muscle spasms.
The patient was not taking any medications and had no previous diagnoses. The patient did not have any history of hospitalization or surgery.
He had no familial history of congenital, allergic, or systemic disease. The patient smoked approximately half a pack of cigarettes a day for 5 years and did not drink alcohol.
On physical examination, no external wound was observed. The circumference of both arms was not different and distinct atrophy of biceps or deltoid muscles was not present. The range of motion for both shoulders and elbow joints was not limited. The pain was not aggravated by movement.
Paresthesia was present in both forearms, but the symptom site did not match with peripheral nerve distribution or cervical root dermatome. Manual muscle testing was grade 5 throughout the right and left upper extremities except in bilateral elbow and shoulder flexion. Manual muscle test of bilateral elbow and shoulder flexion showed grade 4. The patient could flex both his elbows with a 2 kg dumbbell but not with a 5 kg. The deep tendon reflex of both bicep muscles was symmetrically decreased.
Before the symptoms began, he started working out at a fitness center. He did weight training, which is commonly practiced. However, he extensively stretched his pectoralis minor muscles. Figure 1 shows the stretching exercise of the pectoralis minor that the patient described. Both shoulder joints were mildly extended, 90° externally rotated, and 45° abducted. Both scapulae were retracted, and the elbow joints were bent approximately 90°. The patient placed his elbows on the wall right next to the door and pushed his trunk forward.
There were no abnormalities in laboratory tests including complete blood count, electro profile, liver function test, kidney function test, routine urine analysis, blood coagulation test, and thyroid function test. Acetylcholine receptor antibody, erythrocyte sedimentation rate, C-reactive protein, vitamin B12, and folate were within normal limits. In addition, blood calcium, ionized calcium, creatine kinase, and phosphate were within normal limits.
Tables 1 and 2 show the electrodiagnostic study of the patient. Bilateral axillary, musculocutaneous, median, and ulnar compound motor action potential (CMAP) were within normal limits. Bilateral median, ulnar, and bilateral lateral antebrachial cutaneous SNAP were within normal limits. Bilateral median F-waves were within normal limits. Electromyography of the bilateral upper extremities showed abnormal spontaneous activities in the bilateral biceps and brachialis muscles with reduced recruitment and interference pattern. These electrophysiologic findings were indicative of bilateral musculocutaneous neuropathy.
| Nerve conduction (sensory) | Recording site | Stimulation site | Peak latency (ms) | Amplitude (μV) | Distance (cm) | Conduction velocity (m/s) | Nerve conduction (motor) | Recording site | Stimulation site | Onset latency (ms) | Amplitude (mV) | Distance (cm) | Conduction velocity (m/s) |
| Right median | Index finger | Palm | 1.65 | 49.9 | 7 | 60.9 | Right median | APB | Wrist | 2.80 | 8.4 | ||
| Wrist | 2.90 | 54.8 | 7 | 63.6 | Antecubital | 6.25 | 8.3 | 22 | 63.8 | ||||
| Left median | Index finger | Palm | 1.60 | 59.8 | 7 | 63.6 | Left median | APB | Wrist | 2.95 | 8.3 | ||
| Wrist | 2.80 | 59.7 | 7 | 66.7 | Antecubital | 6.50 | 8.3 | 22 | 62.0 | ||||
| Right ulnar | Small finger | Wrist | 2.70 | 44.0 | 13 | 63.4 | Right ulnar | ADM | Wrist | 2.05 | 8.3 | ||
| Left ulnar | Small finger | Wrist | 2.90 | 37.0 | 14 | 59.6 | Below elbow | 5.85 | 8.8 | 23 | 60.5 | ||
| Right lateral antebrachial | Lateral forearm | 12 cm proximal | 2.40 | 13.5 | 11 | 57.9 | Left ulnar | ADM | Wrist | 2.15 | 5.8 | ||
| Left lateral antebrachial | Lateral forearm | 12 cm proximal | 2.60 | 18.8 | 13 | 63.4 | Below elbow | 6.00 | 7.4 | 22 | 57.1 | ||
| Right medial antebrachial | Medial forearm | 12 cm proximal | 2.30 | 12.6 | 11 | 66.7 | Right ulnar | ADM | Wrist | 2.25 | 8.2 | ||
| Left medial antebrachial | Medial forearm | 12 cm proximal | 2.40 | 19.9 | 12 | 64.9 | (Post-exercise) | Below elbow | 5.95 | 8.4 | 23 | 62.2 | |
| Left ulnar | ADM | Wrist | 2.45 | 8.1 | |||||||||
| (Post-exercise) | Below elbow | 6.15 | 9.6 | 23 | 62.2 | ||||||||
| Right axillary | Deltoid | Erb’s point | 3.65 | 8.6 | |||||||||
| Left axillary | Deltoid | Erb’s point | 4.05 | 7.2 | |||||||||
| Right musculocutaneous | Biceps | Erb’s point | 4.25 | 2.4 | |||||||||
| Left musculocutaneous | Biceps | Erb’s point | 4.05 | 1.3 |
| Muscle | Spontaneous | MUAP | Recruitment pattern | Interference pattern | Muscle | Spontaneous | MUAP | Recruitment pattern | Interference pattern |
| (Right) | Positive sharp wave | (Left) | Positive sharp wave | ||||||
| APB | None | Normal | Normal | Full | APB | None | Normal | Normal | Full |
| First DI | None | Normal | Normal | Full | First DI | None | Normal | Normal | Full |
| ECRL | None | Normal | Normal | Full | ECRL | None | Normal | Normal | Full |
| FCR | None | Normal | Normal | Full | FCR | None | Normal | Normal | Full |
| Biceps | 2+ | Normal | Reduced | Reduced | Biceps | 2+ | Normal | Reduced | Reduced |
| Brachialis | 1+ | Normal | Reduced | Full | Brachialis | 1+ | Normal | Reduced | Full |
| Infraspinatus | None | Normal | Normal | Full | Infraspinatus | None | Normal | Normal | Full |
| Deltoid | None | Normal | Normal | Full | Deltoid | None | Normal | Normal | Full |
| Triceps | None | Normal | Normal | Full | Triceps | None | Normal | Normal | Full |
| Cervical Paraspinal | None | NA | NA | NA | Cervical Paraspinal | None | NA | NA | NA |
Tables 3 and 4 show the patient’s electrodiagnostic study performed at a previous hospital approximately 4 mo before the follow-up study. Compared with Table 3, Table 1 shows that amplitude of each musculocutaneous CMAP was decreased.
| Nerve conduction (sensory) | Recording site | Recording site | Stimulation site | Onset latency (ms)1 | Amplitude (μV) | Nerve conduction (motor) | Recording site | Stimulation site | Onset latency (ms) | Amplitude (mV) | Distance (cm) | Conduction velocity (m/s) |
| Left median | Index finger | Index finger | Wrist | 2.29 | 71.8 | Left median | APB | Wrist | 3.18 | 12.1 | ||
| Left ulnar | Small finger | Small finger | Wrist | 2.34 | 49.8 | Antecubital | 6.77 | 12.4 | 19 | 52.9 | ||
| Left radial | Thumb | Thumb | 12 cm proximal | 1.82 | 36.3 | Left ulnar | ADM | Wrist | 2.24 | 11.0 | ||
| Right medial antebrachial | Medial forearm | Medial forearm | 12 cm proximal | 1.93 | 15.9 | Below elbow | 5.73 | 11.6 | 19 | 54.4 | ||
| Left medial antebrachial | Medial forearm | Medial forearm | 12 cm proximal | 1.61 | 15.8 | Right axillary | Deltoid | Erb’s point | 3.70 | 3.6 | ||
| Left axillary | Deltoid | Erb’s point | 4.38 | 3.2 | ||||||||
| Right musculocutaneous | Biceps | Erb’s point | 5.31 | 3.8 | ||||||||
| Left musculocutaneous | Biceps | Erb’s point | 4.95 | 5.1 |
| Muscle (Left) | Spontaneous | MUAP analysis | Recruitment pattern | Interference pattern |
| Positive sharp wave | ||||
| First DI | None | Normal | Normal | Full |
| ADM | None | Normal | Normal | Full |
| FCU | None | Normal | Normal | Full |
As mentioned above, electrophysiologic findings were indicative of bilateral musculocutaneous neuropathy. To confirm the diagnosis, MRI of both arms was performed.
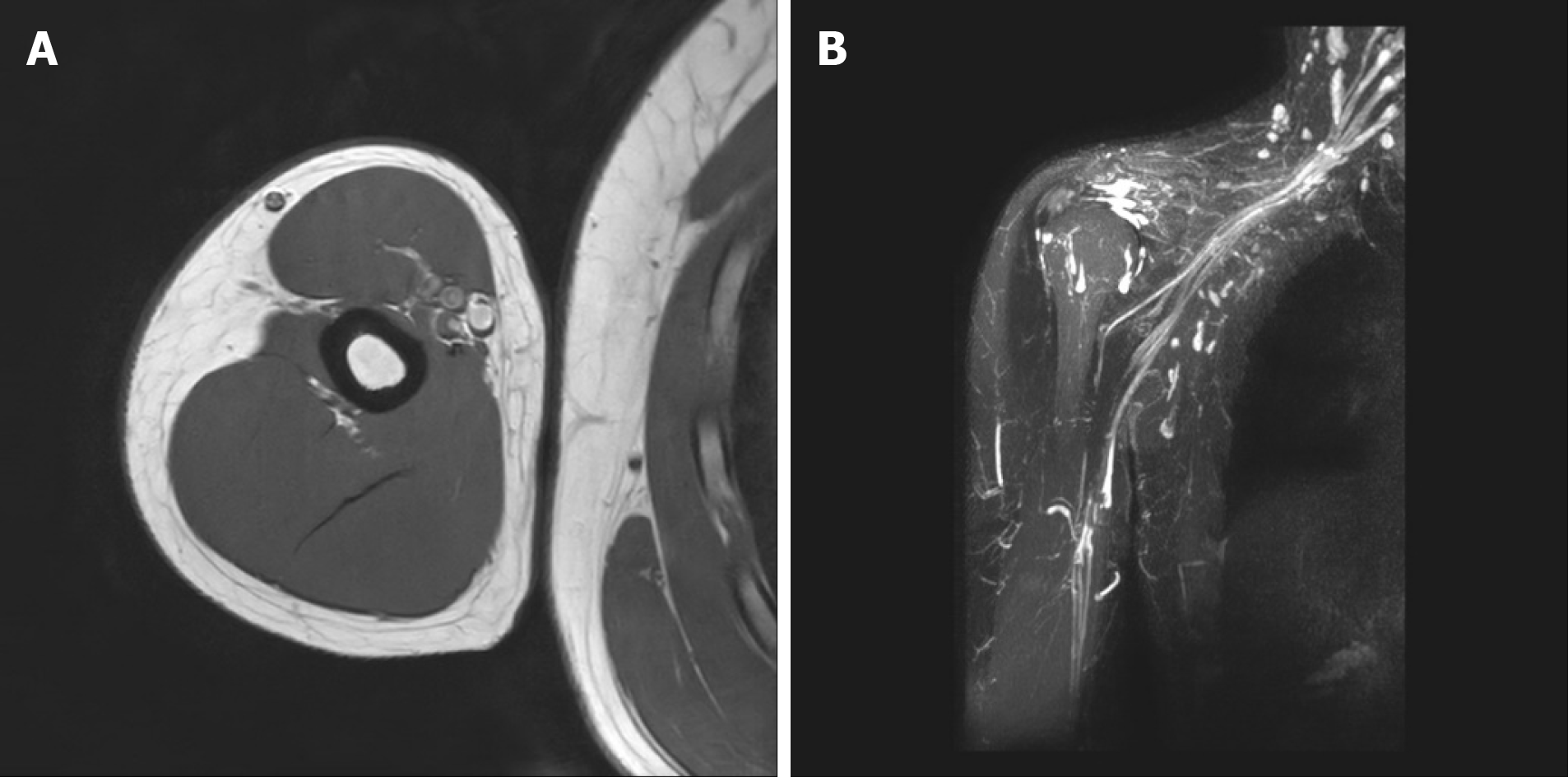
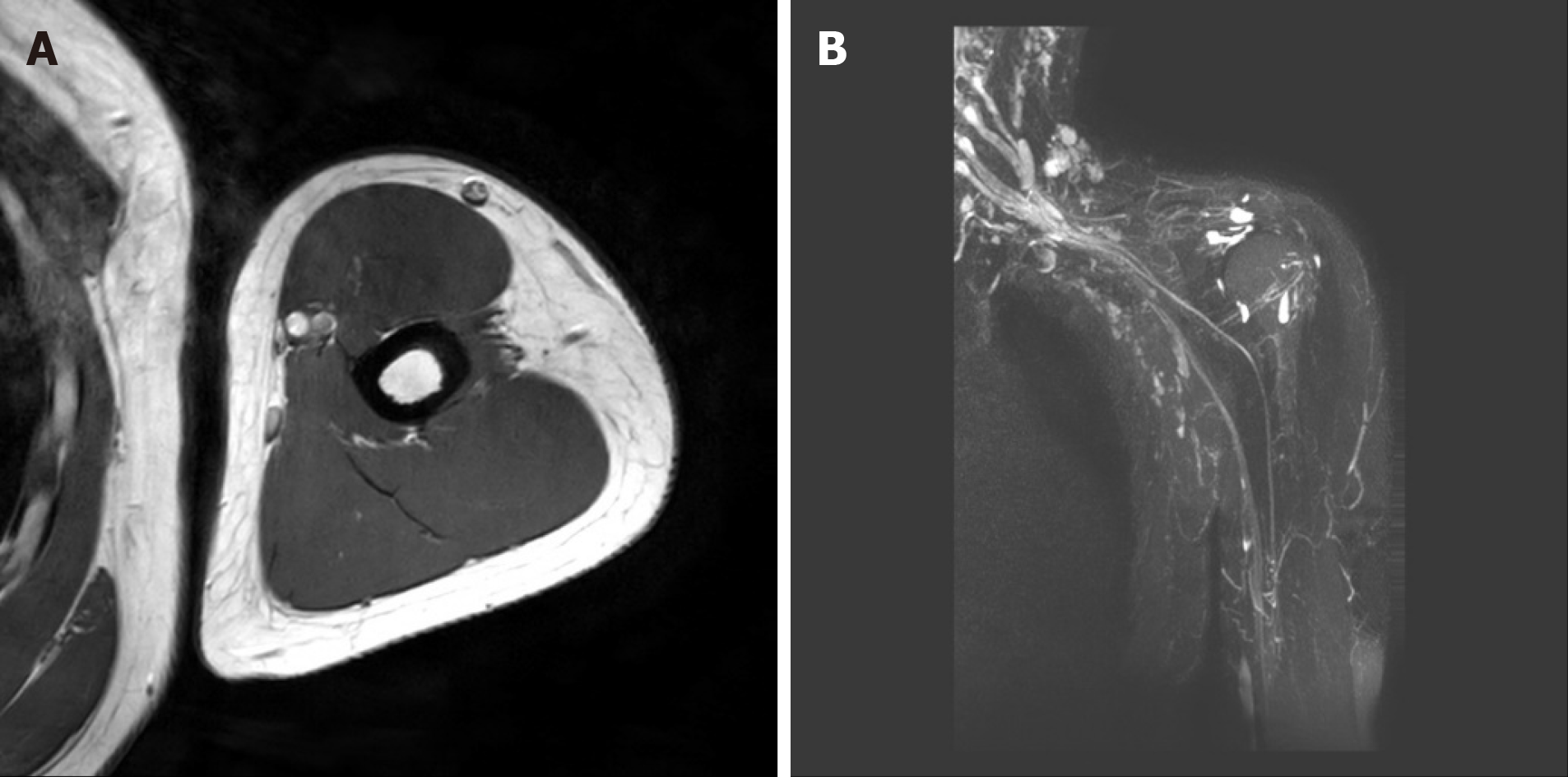
Figures 2 and 3 showed the MRI of both arms and brachial plexus of the patient. Significant abnormality was not observed in either brachial plexus and distal peripheral nerves.
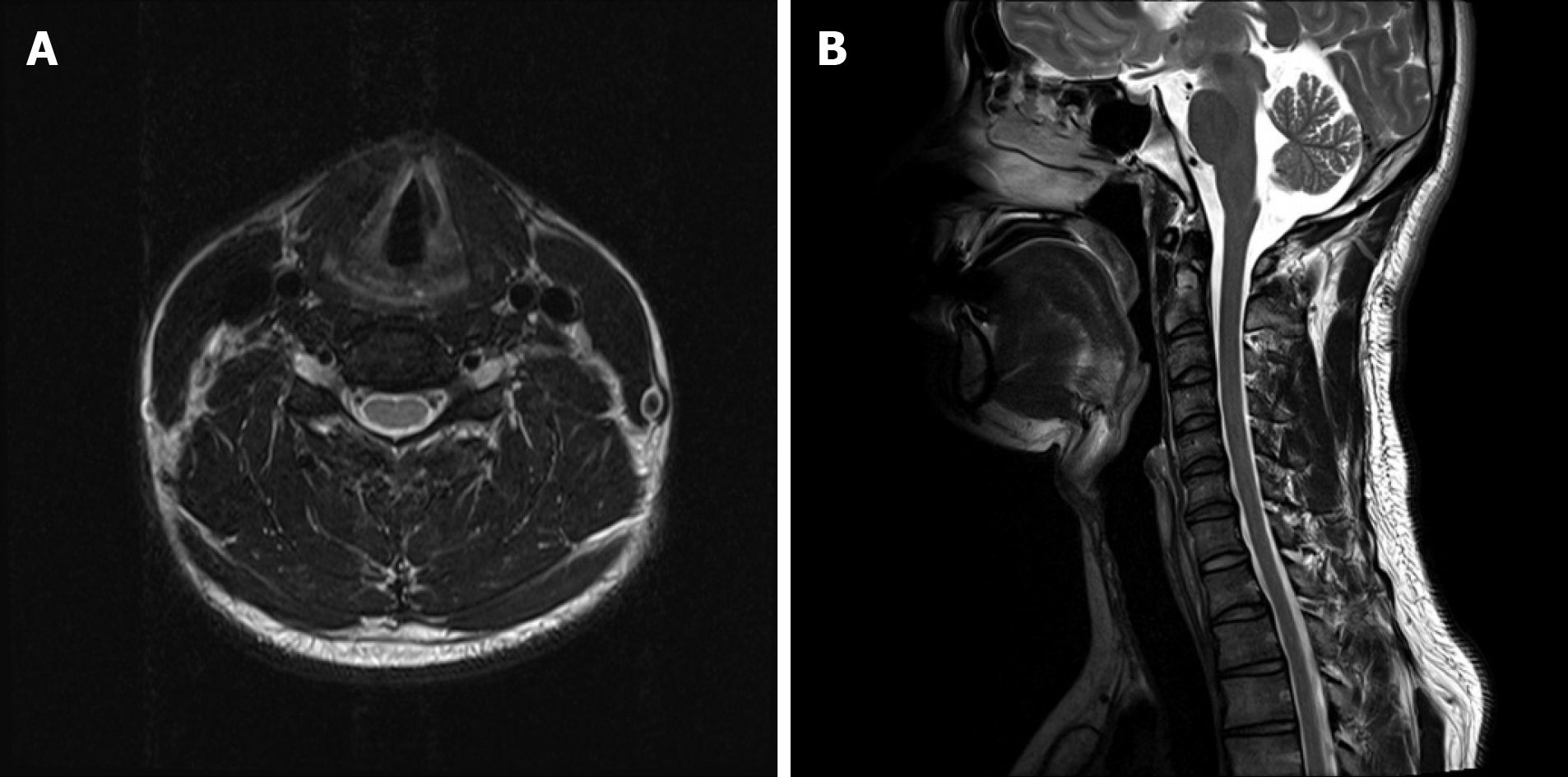
Figure 4 showed the patient’s cervical spine MRI performed at a previous hospital approximately 6 mo before the visit to our clinic. Specific abnormalities were not observed on cervical spine MRI.
Final diagnosis of the presented case was bilateral musculocutaneous neuropathy with active denervation.
Steroid pulse therapy was administered for treatment. For the first 2 d, the patient was prescribed 64 mg of triamcinolone (Ledercort®). The following 2 d, 48 mg of triamcinolone was prescribed and 32 mg for the following 3 d. During the treatment period, 30 mg of lansoprazole (Lanston LFTD®), a proton-pump inhibitor, was prescribed daily.
A week after diagnosis, the patient visited the outpatient clinic. His strength improved sufficiently to perform elbow flexion with a 5 kg dumbbell. Because his strength did not recover to the predisease level, he was prescribed an additional 24 mg of triamcinolone for 2 d, 16 mg for 2 d, 8 mg for 3 d, and 4 mg for 4 d.
On the follow-up visit after 11 d, the patient stated he was slightly improving and had started stretching exercise again. He was instructed not to perform the stretching, and 5 mg intramuscular injection of hydroxocobalamin (Lanobin®) was administered. Another 4 mg of triamcinolone and 30 mg of duloxetine hydrochloride (Cymbalta®) was prescribed for an additional 4 wk.
On the last follow-up visit after 4 wk, his muscle strength returned to the predisease condition. Informed written consent was obtained from the patient for publication of this report and any accompanying images.
In the present case report, the diagnosis of a patient with bilateral musculocutaneous neuropathy was described. We would like to discuss the anatomy, etiology, diagnostic consideration, and differential diagnosis of this rare case.
The musculocutaneous nerve originates from the brachial plexus lateral cord and consists of fibers from the fifth, sixth, and seventh roots of the cervical spinal nerve. Furthermore, the components of the fifth and sixth cervical spinal nerve roots contribute primarily to the musculocutaneous nerve[12,13]. It passes through the coracobrachialis muscle and travels between the biceps brachii and brachialis muscles that it innervates[12]. The nerve becomes the lateral antebrachial cutaneous nerve between these muscles near the lateral margin of the bicipital aponeurosis[14]. The nerve is usually injured at the level of the coracobrachialis muscle and generally patients with this type of injury present with pain and weakness of the biceps brachii and paresthesia of the forearm[13,15].
Various injury mechanisms for musculocutaneous neuropathy have been described. Prolonged positioning of the arm during surgery, direct injury to the nerve during surgery, repetitive vigorous upper extremity activity such as lifting, throwing, or carrying may be the etiology of musculocutaneous neuropathy[9]. In addition, a single event of forceful extension of the arm could also be a causative factor[4].
A case of musculocutaneous nerve injury after repeated sessions of skydiving simulation in a wind-tunnel was also reported[1]. Similar to our patient, the subject placed her arms in an abducted, extended, and externally rotated position while experiencing freefall. Our patient repetitively stretched his pectoralis minor muscles with both shoulder joints mildly extended, 90° externally rotated, and 45° abducted. In addition, during the follow-up period when the patient performed the stretching exercise again, his muscle strength recovery was slow. After he was advised to not perform the stretching exercise, the muscle strength recovery was noticeably faster. Therefore, we postulated his stretching exercise was the etiology of the bilateral musculocutaneous neuropathy. The coracobrachialis muscle originates from the coracoid process of the scapula and inserts into the anteromedial surface of the humerus[16]. The stretching exercise shown in Figure 1 may have lengthened his coracobrachialis. Consequently, the musculocutaneous nerve passing through the coracobrachialis muscle might be squeezed. Furthermore, repetitive stretching may have caused the pathological insult and the resulting neuropathy.
Electrodiagnostic studies play an important role in diagnosing peripheral neuropathy[17]. The electrodiagnostic findings in the present case report showed that amplitude of each musculocutaneous CMAP was decreased compared with a previous study. In addition, we performed a nerve conduction study of the bilateral lateral antebrachial cutaneous nerve and needle electromyography of the biceps brachii, brachialis, and deltoid muscles. Proper evaluation of muscles and nerves based on presenting symptoms is important.
In general, electrodiagnostic findings of musculocutaneous neuropathy include abnormalities of lateral antebrachial cutaneous SNAP, musculocutaneous CMAP, and electromyography abnormalities of the biceps brachii and brachialis muscles[9]. However, in our case, lateral antebrachial cutaneous SNAP was within normal limits. There are two probable explanations for the test results.
First, because SNAP is detectable later than CMAP after peripheral nerve injury[11], the SNAP amplitude possibly decreased but might have not yet reached the upper normal limit at that time point. In a previous case report, Kissel et al[9] reported an electrodiagnostic study should be performed 10-21 d after peripheral nerve injury. At this time, the specific lesion extent and amount of axonal loss can be assessed[9]. However, the mechanism of injury in the present case was not due to single insult. Because the nerve damage occurred due to numerous repetitive stretching, the injury might have acute-on-chronic trait. Therefore, conducting an electrodiagnostic study at the appropriate time was difficult.
Second, the lateral antebrachial cutaneous SNAP values can be interpreted as abnormal. In general, electrodiagnostic studies are conducted with left-right comparison. However, in some cases when left-right comparison cannot be performed, the results are interpreted by comparison with other intact nerves[18]. The reference values of lateral and medial antebrachial cutaneous SNAP are shown in Table 5[19]. The upper normal limit of lateral antebrachial cutaneous SNAP is approximately 60% greater than of medial antebrachial cutaneous SNAP. In the current case, the amplitude of right and left lateral antebrachial cutaneous SNAP (13.5 μV and 12.6 μV, respectively) was proportionally smaller than of medial antebrachial cutaneous SNAP (12.6 μV and 19.9 μV, respectively). However, lateral antebrachial cutaneous SNAP has never been compared with medial antebrachial cutaneous SNAP, and appropriate comparison criteria for diagnosis are needed. In addition, the examiner should always remember to correlate the test results in a clinical aspect.
| Nerve conduction (sensory) | Onset latency (ms) | Peak latency (ms) | Amplitude (μV) |
| Lateral antebrachial cutaneous | 1.6-2.1 | 2.2-2.6 | 12-50 |
| Medial antebrachial cutaneous | 1.7-2.6 | 10-30 |
When confirming the diagnosis, excluding other possible differential diagnoses is also necessary. In the present case report, biceps tendon injury or rupture and strain or tear of the biceps or brachialis muscle should have also been considered as differential diagnoses[9]. In case of biceps tendon injury or rupture or strain or tear of the biceps or brachialis muscles, sensory changes would not be observed. In the present case, the patient had sensory symptoms in both forearms. Furthermore, hematoma or muscle injury was not observed on the MRI of both arms[9]. In addition, cervical radiculopathy involving the fifth or sixth spinal nerve root, should have been considered as a differential diagnosis[9]. If the patient had cervical radiculopathy, sensory change would have matched the dermatome of the fifth or sixth spinal nerve root. Furthermore, other muscles supplied by the fifth or sixth spinal nerve root, such as deltoids and supraspinatus muscles, would also have been weak[20]. In addition, the patient did not show specific abnormalities on cervical spine MRI. Brachial plexus injury should also have been excluded for definite diagnosis. In general, patients with brachial plexus injury present with broader distributions of sensory change and muscle weakness in upper extremities. Therefore, the differential diagnoses described above were less likely to be applicable to our patient.
The present case report had several limitations. First, the radiologist reported no abnormalities on MRI of the bilateral arm and brachial plexus. MRI is a static diagnostic tool and performing proper nerve tracing based on the patient’s movement is difficult. For a more accurate diagnosis, the nerve route could have been traced to detect any entrapment. Second, there is a previous report of a recreational parachutist presenting with bilateral arm weakness, who was later found to have hereditary neuropathy with predisposition to pressure palsies[21]. The patient in the present case report was also likely to have hereditary neuropathy with predisposition to pressure palsies. Because the pressure applied during recreational parachuting is greater than the compression during the stretching exercise, we could have recommended testing the PMP22 gene.
To the best of our knowledge, this is the first reported case of bilateral musculocutaneous neuropathy. Although rare, clinicians should take this disease into consideration when conducting electrodiagnostic studies and be aware the condition may appear bilaterally. Furthermore, people can usually access various exercises and stretching methods through the internet. However, some exercises can cause complications. Clinicians should have adequate understanding of anatomy and give appropriate advice.
Manuscript source: Unsolicited manuscript
Specialty type: Clinical neurology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Melo FF, Fu T S-Editor: Zhang H L-Editor: Filipodia P-Editor: Li JH
| 1. | Mautner K, Keel JC. Musculocutaneous nerve injury after simulated freefall in a vertical wind-tunnel: a case report. Arch Phys Med Rehabil. 2007;88:391-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Pećina M, Bojanić I. Musculocutaneous nerve entrapment in the upper arm. Int Orthop. 1993;17:232-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Papanikolaou A, Maris J, Tsampazis K. Isolated musculocutaneous nerve palsy after heavy physical activity. Injury Extra. 2005;36:486-488. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Yilmaz C, Eskandari MM, Colak M. Traumatic musculocutaneous neuropathy: a case report. Arch Orthop Trauma Surg. 2005;125:414-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Merrell CA, Merrell KL. A variation of musculocutaneous neuropathy: implications for electromyographers. PM R. 2010;2:780-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Besleaga D, Castellano V, Lutz C, Feinberg JH. Musculocutaneous neuropathy: case report and discussion. HSS J. 2010;6:112-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Henry D, Bonthius DJ. Isolated musculocutaneous neuropathy in an adolescent baseball pitcher. J Child Neurol. 2011;26:1567-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. |
Chabra T, Case report on isolated musculocutaneous nerve injury following a wrestling match.
|
| 9. | Kissel JA, Leonardelli C. Isolated musculocutaneous neuropathy: a case report. J Can Chiropr Assoc. 2019;63:162-170. [PubMed] |
| 10. | Daniel D, Machiel JZ. Focal Peripheral Neuropathies. In: Daneil D, Anthony AA, Machiel Z. Electrodiagnostic Medicine. 2nd. Philadelphia: Hanley & Belfus, Cop, 2002: 1043. |
| 11. | Daniel D, Machiel JZ, Anthony AA. Peripheral Nervous System’s Reaction to Injury. In: Daneil D, Anthony AA, Machiel Z. Electrodiagnostic Medicine. 2nd. Philadelphia: Hanley & Belfus, Cop, 2002: 126-129. |
| 12. | Mastaglia FL. Musculocutaneous neuropathy after strenuous physical activity. Med J Aust. 1986;145:153-154. [PubMed] |
| 13. | Lorei MP, Hershman EB. Peripheral nerve injuries in athletes. Treatment and prevention. Sports Med. 1993;16:130-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 81] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | McCarty EC, Tsairis P, Warren RF. Brachial neuritis. Clin Orthop Relat Res. 1999: 37-43. [PubMed] |
| 15. | Bassett FH 3rd, Nunley JA. Compression of the musculocutaneous nerve at the elbow. J Bone Joint Surg Am. 1982;64:1050-1052. [PubMed] |
| 16. | Ilayperuma I, Nanayakkara BG, Hasan R, Uluwitiya SM, Palahepitiya KN. Coracobrachialis muscle: morphology, morphometry and gender differences. Surg Radiol Anat. 2016;38:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Quan D, Bird S. Nerve Conduction Studies and Electromyography in the Evaluation of Peripheral Nerve Injuries. The University of Pennsylvania Orthopaedic Journal. 1999;12:45-51. |
| 18. | Werner RA, Albers JW. Relation between needle electromyography and nerve conduction studies in patients with carpal tunnel syndrome. Arch Phys Med Rehabil. 1995;76:246-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Daniel D, Anthony AA, Macheil JZ. Nerve conduction studies. In: Daneil D, Anthony AA, Machiel Z. Electrodiagnostic Medicine. 2nd. Philadelphia: Hanley & Belfus, Cop, 2002: 206-207. |
| 20. | Abbed KM, Coumans JV. Cervical radiculopathy: pathophysiology, presentation, and clinical evaluation. Neurosurgery. 2007;60:S28-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Marriott M, Macdonell R, McCrory P. Flail arms in a parachutist: an unusual presentation of hereditary neuropathy with liability to pressure palsies. Br J Sports Med. 2002;36:465-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |