Published online Feb 16, 2021. doi: 10.12998/wjcc.v9.i5.1215
Peer-review started: December 2, 2020
First decision: December 21, 2020
Revised: December 23, 2020
Accepted: January 7, 2021
Article in press: January 7, 2021
Published online: February 16, 2021
Processing time: 58 Days and 20.3 Hours
Fournier's gangrene (FG) is a serious, aggressive and often deadly polymicrobial infection of the soft tissues of the perineum, the rectum and the external genital organs. It is an anatomical subcategory of necrotizing fasciitis, which has a similar etiology and treatment strategy.
A 60-year-old man was admitted to the hospital during severe acute respiratory syndrome coronavirus 2 pandemic with complaints of fever up to 38.9 °C, abdominal pain, and edema of the scrotum, the penis, the perineum, and the right gluteal region for 2 d. Computed tomography of the abdomen and the pelvis revealed extensive inflammatory infiltrations of the subcutaneous tissue of the hypogastrium, and the penis; along with liquefaction and presence of gas in the subcutaneous tissues of the scrotum, the perineum, and the right gluteal region. The patient was diagnosed with FG, and was urgently qualified to undergo surgery in the Department of Urology. After performing the necessary examinations, a resection of the necrotic tissues with bilateral orchiectomy and excision of the penile and scrotal skin was performed. After surgery, he was transferred to the intensive care unit for further management.
Early management prevents the resection of the other organs by inhibiting the contiguous spread of infection.
Core Tip: Fournier's gangrene (FG) is a serious and often deadly polymicrobial infection of the soft tissues of the perineum, the rectum and the external genital organs. We present herein, a case of patient diagnosed with FG, urgently qualified to undergo surgery. After performing the examinations, a resection of the necrotic tissues with bilateral orchiectomy and excision of the penile and scrotal skin was performed. This case highlights that the mortality rate of FG can be reduced if a patient is presented early to the hospital. Early management prevents the resection of the other organs by inhibiting the contiguous spread of infection.
- Citation: Grabińska A, Michalczyk Ł, Banaczyk B, Syryło T, Ząbkowski T. Management protocol for Fournier’s gangrene in sanitary regime caused by SARS-CoV-2 pandemic: A case report. World J Clin Cases 2021; 9(5): 1215-1220
- URL: https://www.wjgnet.com/2307-8960/full/v9/i5/1215.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i5.1215
Fournier's gangrene (FG) is a serious, aggressive, and often deadly polymicrobial infection of the soft tissue of the perineum, the rectum, and the external genital organs. It is an anatomical subcategory of necrotizing fasciitis, which has similar etiology and treatment strategy. This disease was first reported by Jean Alfred Fournier, who described five cases in young males. FG is ten times more common in males than in females and can occur at any age[1]. It usually causes a painful scrotal or perineal swelling with sepsis. On physical examination, small areas of necrotic skin with erythema and edema are visible. Crepitus with a malodorous discharge occur in more advanced stage of the disease. Mortality increases due to certain risk factors like immunosuppression, diabetes, alcoholism, artherosclerosis, malnutrition, recent urethral or perineal surgery, HIV infection, liver disease, leukemia, and obesity[2]. The insidious onset of the disease is present in 40% of the cases, and undiagnosed pain results in the treatment delay. FG can be caused by trauma, insect bite, or unsafe sexual practices[3]. Computed tomography (CT) or magnetic resonance imaging (MRI) may contribute to the assessment of the degree of rectal involvement[3]. The degree of internal necrosis is usually higher, as is suggested by the external symptoms. Therefore, repeated debridement of the surgical wound with urinary catheterization is necessary to decrease the mortality rate[1]. Surgical wound debridement should be performed thoroughly within 24 h because a delayed or an inappropriate surgery may result in a higher mortality rate. It is recommended to immediately start empirical broad-spectrum antibiotic therapy parenterally which provides appropriate coverage against all the probably microbes. The suggested scheme usually includes broad-spectrum penicillin or a third-generation cephalosporin (combined with a beta lactamase inhibitor), along with gentamicin, and either metronidazole, or clindamycin. The scheme could be modified according to the microbiological culture and sensitivity findings[3].
A 60-year-old man was admitted to the hospital during severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic with complaints of fever up to 38.9 °C, abdominal pain and edema of the scrotum, the penis, the perineum, and the right gluteal region for 2 d.
The patient had the above-mentioned symptoms for 48 h.
The patient had a medical history of hypertension, osteoporosis, and hemorrhoids.
No personal and family history was identified.
His blood pressure was 103/62 mmHg, heart rate was 135/min, and oxygen saturation was 88%.
Blood tests indicated a high degree of inflammation with a white blood cell count of 13.11/μL and C-reactive protein level of 61.4 mg/dL. Biochemical parameters were as follows: serum creatinine 4.3 mg/dL; blood urea 157 mg/dL; blood sugar 142 mg/dL, and procalcitonin 8.53 ng/mL.
Every patient should be classified as either being suspected of having SARS-CoV-2 infection or having a confirmed infection during the pandemic. The epidemiological interview, immunological examination, reverse transcriptase-polymerase chain reaction (RT-PCR), and CT/X-ray of the chest are necessary to assess the risk of infection. If a CT scan of the abdomen was performed in the Emergency Department, it should be extended to perform CT of chest as well. Due to the high risk of SARS-CoV-2 infection, it is recommended to use personal protective equipment (PPE) of at least the third grade (according to the four grades of the American National Standards Institute (ANSI) classification. The PPE in the third grade of the ANSI classification includes surgical mask and cap for the patient, N95/FFP3 mask with a surgical mask, cap, protective goggles, face shield, single-use biological protective suit, surgical barrier apron, surgical gloves (three pairs), and safety shoes. In addition, RT-PCR test for SARS-CoV-2 was performed, and the result was negative.
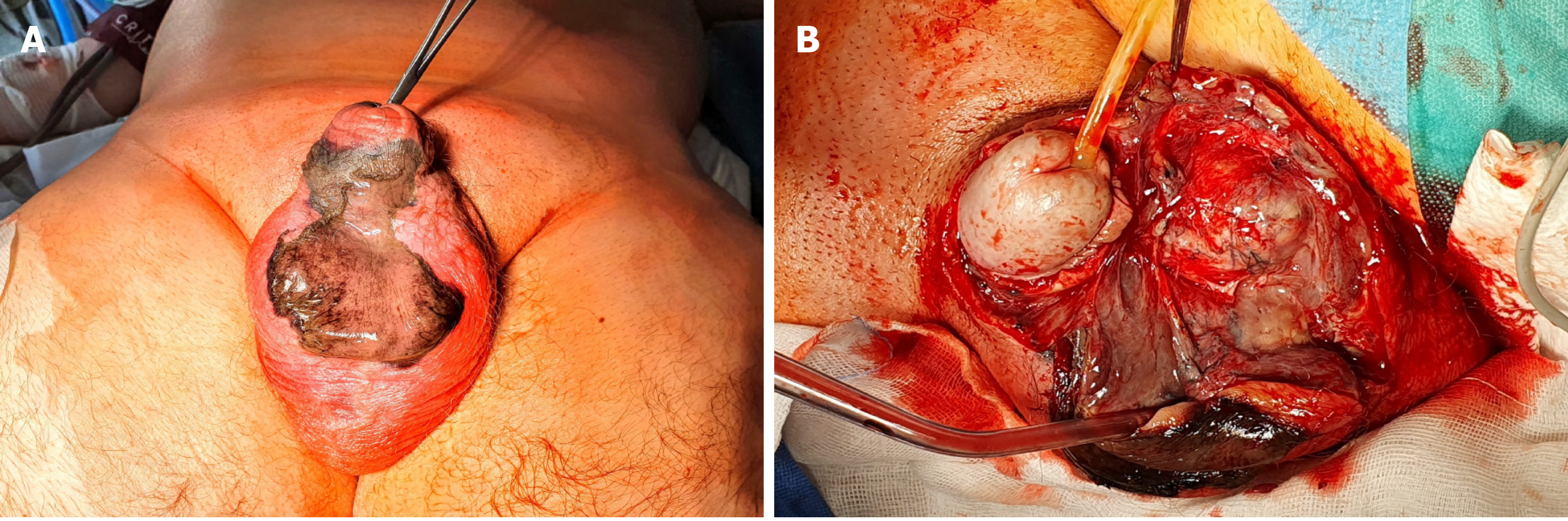
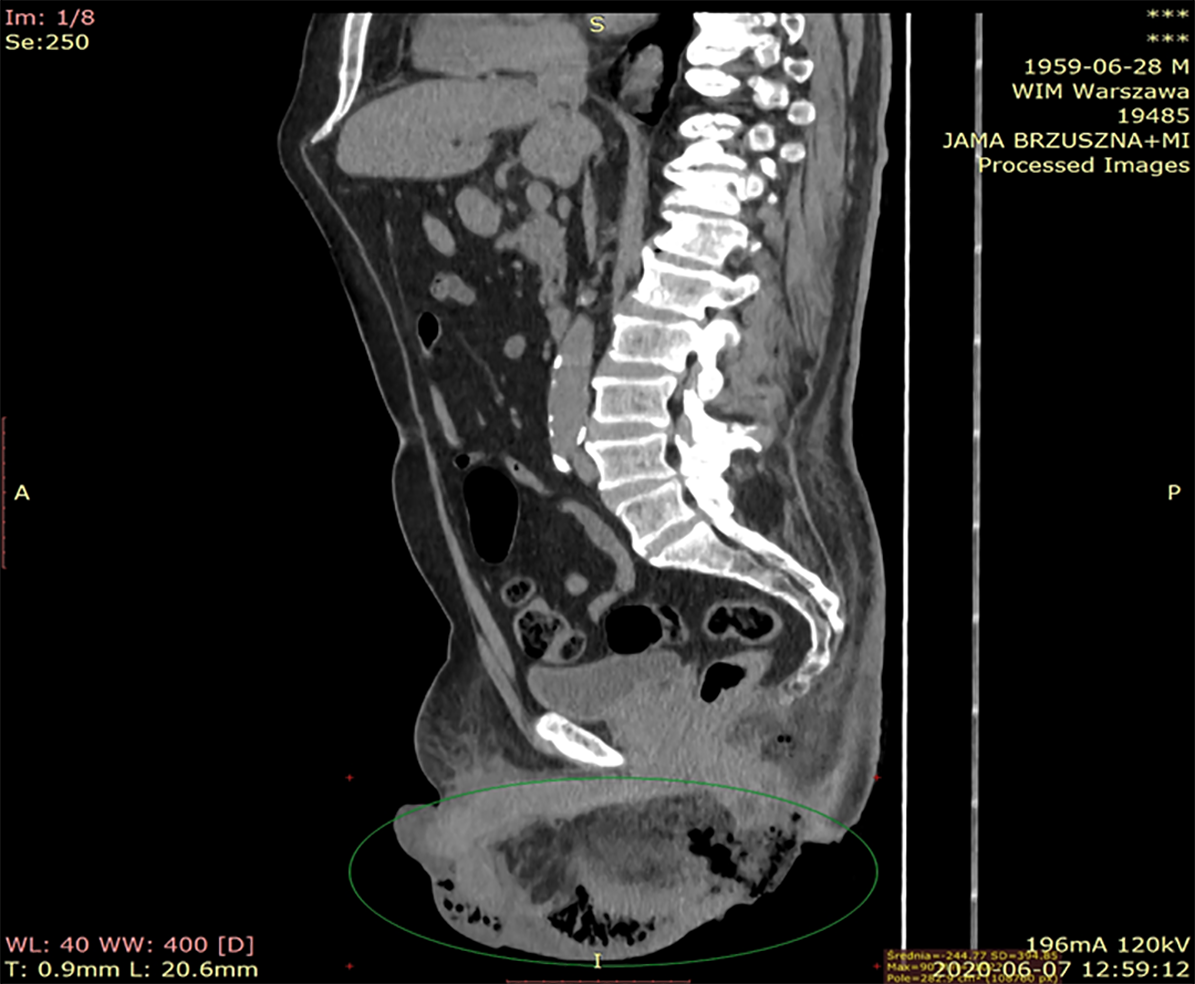
CT of the abdomen and the pelvis revealed extensive inflammatory infiltration of the subcutaneous tissues of the hypogastrium and the penis, and liquefaction, and presence of gas in the subcutaneous tissues of the scrotum, the perineum, and the right gluteal region (Figures 1 and 2).
The patient was diagnosed with FG, and was urgently qualified to undergo surgery in the Department of Urology.
Antibiotic therapy using meropenem at a dose 1 g thrice daily, metronidazole at a dose of 500 mg thrice daily, and linezolid 600 mg twice daily were started intravenously. This antibiotic therapy lasted for 18 d.
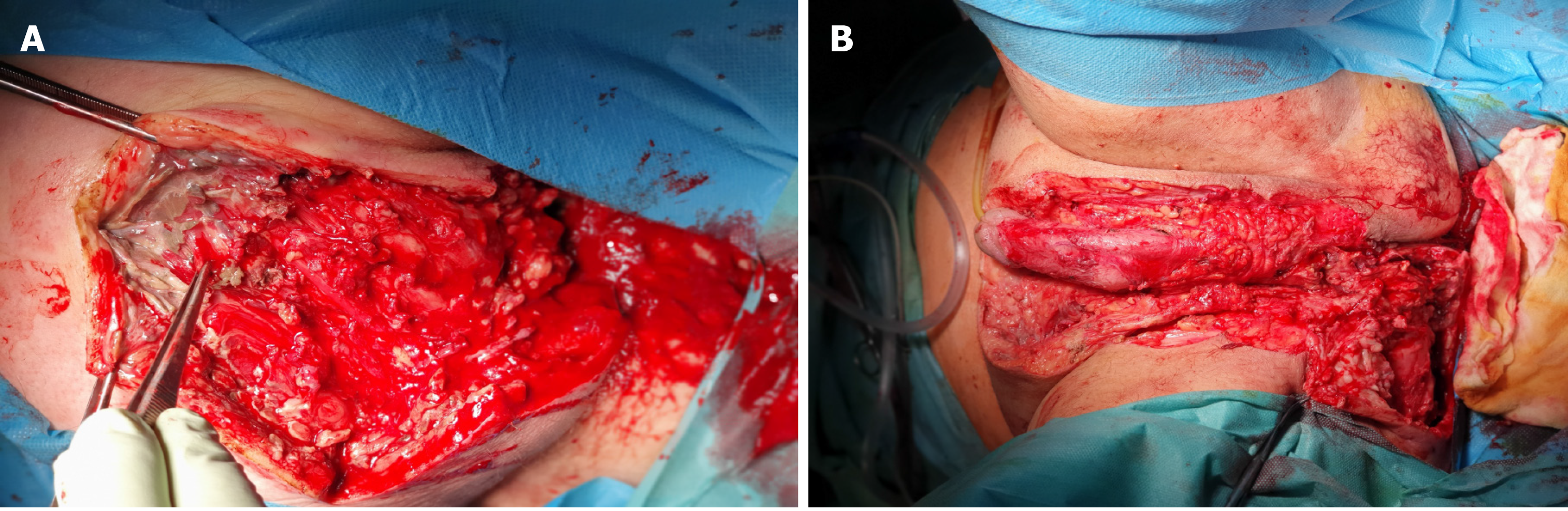
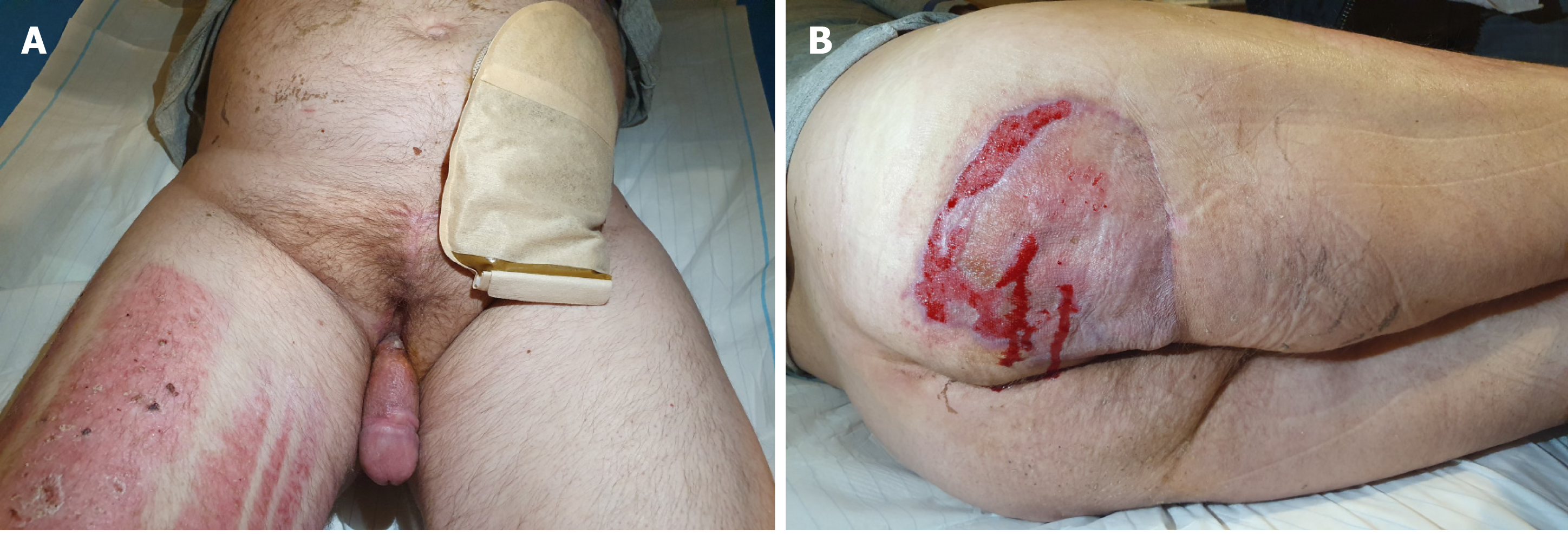
After performing the necessary examinations, a resection of the necrotic tissues with bilateral orchiectomy, and the excision of the penile and scrotal skin were performed (Figure 3).
After surgery, he was transferred to the intensive care unit (ICU) for further management.
In the ICU, the patient was in a very serious condition and required intensive therapy — it was used a respiratory therapy — which included mechanical ventilation, broad-spectrum antibiotics, and supportive and nutritional therapies.
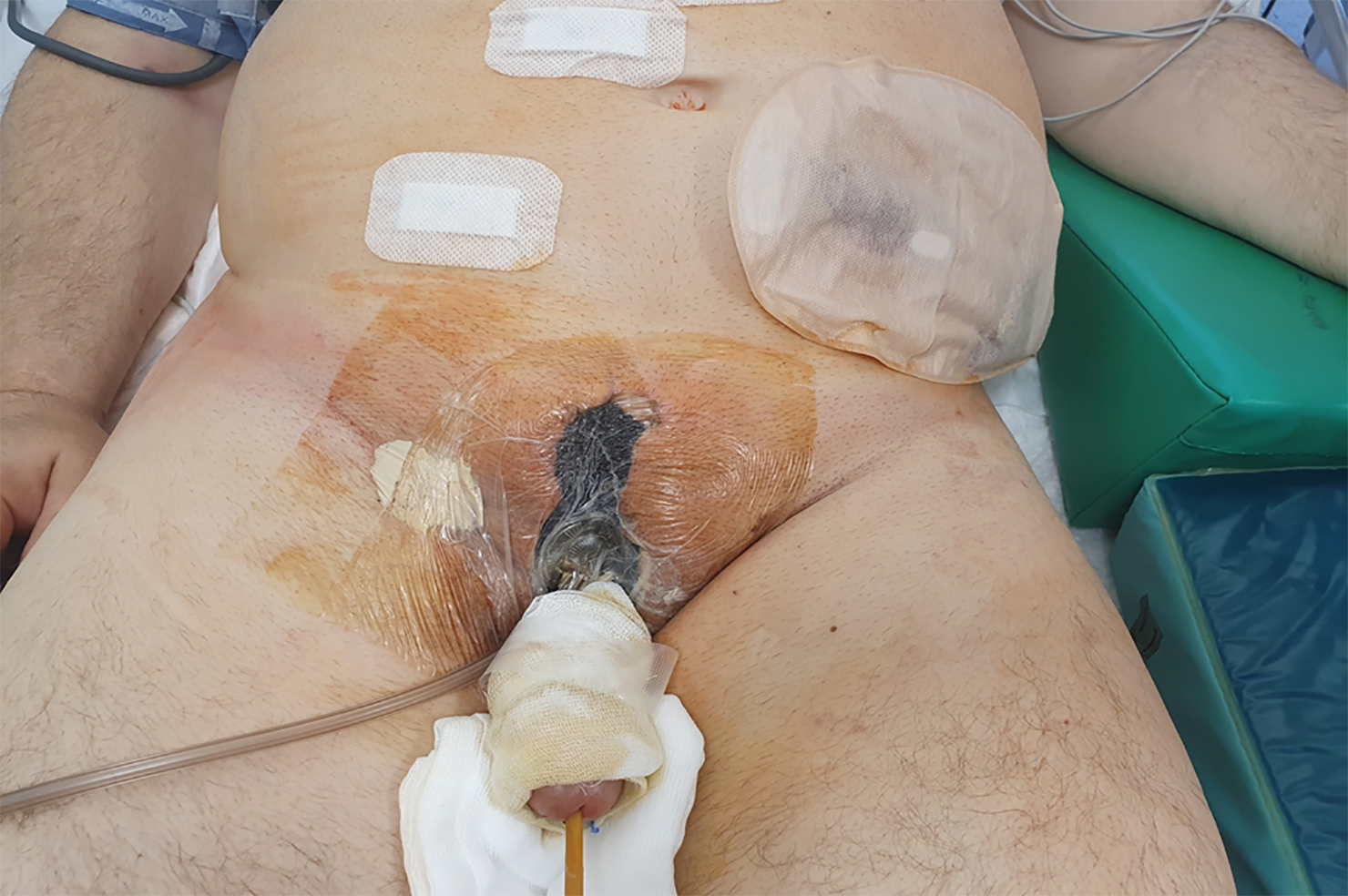
Colostomy was performed on the patient; wound was debrided several times; and negative pressure wound therapy (NPWT) was applied. As a result of this therapy, the patient’s condition started improving. After sedation was discontinued, the patient recovered consciousness, was extubated, and was able to breathe on his own with oxygen on low flow. He was hemodynamically stable, and diuresis was stimulated using a small dose of furosemide. Postoperatively, the inflammatory markers decreased significantly. The culture of the pus material showed Escherichia coli and Pseudomonas aeruginosa; therefore, the antibiotic therapy was modified to include cephazolin dose of 1g twice daily, intravenously, for 24 d. NPWT was also discontinued. The patient was transferred to the Department of Plastic Surgery so that free-skin grafts could be applied to the debrided areas (Figure 4). After 46 d the patient was discharged.
Currently, the patient is living in a nursing home where he has received continous nursing care, free-skin graft care, and regular dressing changes. The patient is in good physical and mental condition but requires regular physiotherapy, under the care of a urological and surgical clinic. The patient receives testosterone supplementation at a dose of 80 mg twice daily. After complete wound closure following the application of the free-skin graft to the right gluteal area, the patient will be qualified for colostomy reversal resume the continuity and proper functioning of the gastrointestinal tract (Figure 5).
FG is a rare infectious disease with high morbidity and mortality. Despite the advancements made in understanding the etiology and the pathophysiology of FG, mortality rates remain high[4]. The mortality rate ranges from 3% to 45%. In addition, diabetes plays a crucial role because about 60% of patients with FG have diabetes[5].
Many conditions predispose to FG, such as diabetes, alcoholism, immunodeficiency, trauma, and genitourinary infections[4].
A systematic literature review from 1980 to 2017, which included randomized clinical trials, reviews, and prospective and retrospective studies, showed mortality rates of 5%-10%[6-8]. The higher risk of mortality correlates with old age, obesity, and diabetes. A prospective study by Roghmann et al[9] analyzing the FG severity score showed that the disease-specific severity score predicted the results; however, it was not better than the standard scoring system employed in critical care.
Lauerman et al[10] performed a retrospective study on the antibiotic duration and outcomes in FG. The study did not show a significant difference in mortality in patients who were administered ≤ 10 d of parenteral antibiotics (n = 80) vs patients who were administered > 10 d of parenteral antibiotics (n = 88).
A systematic literature review of wound closure showed a low data quality of 16 case series that included 425 male patients. This review recommended primary or secondary wound closure when the scrotal defect was ≤ 50%, and flap or skin grafting for scrotal defects that were either > 50% or extended outside the scrotum[11].
The comparative case series showed a beneficial aspect of the use of hyperbaric oxygen therapy in 16 patients vs 12 patients in whom this therapy was not used: a decreased mortality and need for a fewer debridements were also noticed in these patients[11].
A randomized clinical trial of low quality, which included 30 cases, assessed the treatment results for the honey dressing. This trial showed a shorter hospital stay (28 d) with the honey dressing in comparison to the Edinburgh solution of lime dressing (32 d)[12].
FG is a urological emergency with a high mortality rate. The multidisciplinary approach plays a key role in the management of FG. The mortality rate of FG can be reduced if a patient presents to the hospital early, the broad-spectrum antibiotic therapy is administered immediately, and a wide surgical debridement of the necrotic tissues is performed urgently. Early management prevents the resection of the other organs by inhibiting the contiguous spread of infection. This case study also showed a high effectiveness of the NPWT.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Georgiev T, Lee SS, Rahman MM S-Editor: Gao CC L-Editor: A P-Editor: Xing YX
| 1. | Chennamsetty A, Khourdaji I, Burks F, Killinger KA. Contemporary diagnosis and management of Fournier's gangrene. Ther Adv Urol. 2015;7:203-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 2. | Taylor GM, Hess DV. Fournier gangrene: a rare case of necrotizing fasciitis of the entire right hemi-pelvis in a diabetic female. Oxf Med Case Reports. 2018;2018:omx094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Krishna Gowtham V, Vaishnavi A, Bhargav Narendra J. Case Report on Fournier’s Gangrene. World J Curr Med Pharm Res. 2020;2:191-193. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Hota PK. Fournier's Gangrene: Report of 2 Cases. Case Rep Emerg Med. 2012;2012:984195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Puvanendran R, Huey JC, Pasupathy S. Necrotizing fasciitis. Can Fam Physician. 2009;55:981-987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Furr J, Watts T, Street R, Cross B, Slobodov G, Patel S. Contemporary Trends in the Inpatient Management of Fournier's Gangrene: Predictors of Length of Stay and Mortality Based on Population-based Sample. Urology. 2017;102:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Kim SY, Dupree JM, Le BV, Kim DY, Zhao LC, Kundu SD. A contemporary analysis of Fournier gangrene using the National Surgical Quality Improvement Program. Urology. 2015;85:1052-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Sorensen MD, Krieger JN. Fournier's Gangrene: Epidemiology and Outcomes in the General US Population. Urol Int. 2016;97:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 9. | Roghmann F, von Bodman C, Löppenberg B, Hinkel A, Palisaar J, Noldus J. Is there a need for the Fournier's gangrene severity index? BJU Int. 2012;110:1359-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Lauerman MH, Kolesnik O, Sethuraman K, Rabinowitz R, Joshi M, Clark E, Stein D, Scalea T, Henry S. Less is more? J Trauma Acute Care Surg. 2017;83:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Li C, Zhou X, Liu LF, Qi F, Chen JB, Zu XB. Hyperbaric Oxygen Therapy as an Adjuvant Therapy for Comprehensive Treatment of Fournier's Gangrene. Urol Int. 2015;94:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Subrahmanyam M, Ugane SP. Krishna Gowtham V, Vaishnavi A, Bhargav Narendra J. Case Report on Fournier’s Gangrene. Indian J Surg. 2004;66:75-77. |