Published online Feb 16, 2021. doi: 10.12998/wjcc.v9.i5.1184
Peer-review started: October 29, 2020
First decision: November 20, 2020
Revised: December 2, 2020
Accepted: December 16, 2020
Article in press: December 16, 2020
Published online: February 16, 2021
Processing time: 92 Days and 21 Hours
The diagnosis and etiology of multiple primary malignant neoplasms (MPMNs) are difficult to establish. Here, we report a case of heterochronic triple primary malignancies with gastric cancer, nasopharyngeal squamous cell cancer, and then rectal cancer.
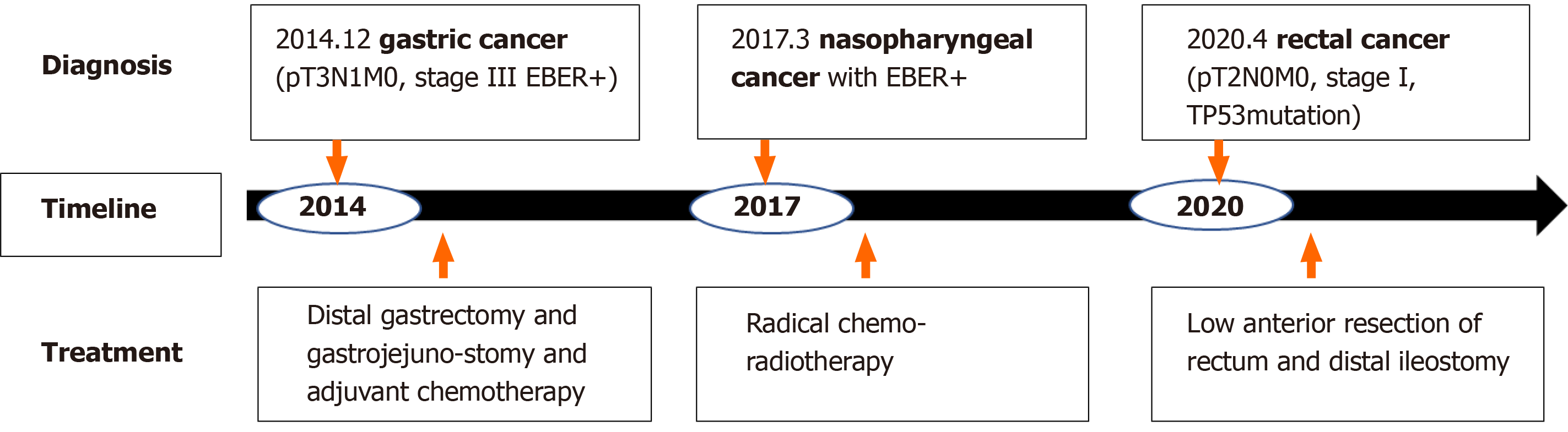
The patient was first diagnosed with gastric cancer at the age of 33 in 2014 and underwent distal gastrectomy and gastrojejunostomy and six cycles of adjuvant chemotherapy. Three years later, he was diagnosed with nasopharyngeal cancer and treated with radical chemoradiotherapy in 2017. Recently, a mass in the middle of the rectum was resected and reported as ulcerative, moderately to poorly differentiated adenocarcinoma. Research on the etiology of MPMNs showed that Epstein-Barr virus (EBV) infection may be the cause of gastric cancer and nasopharyngeal squamous cell cancer since these two primary lesions were positive for transcripts of EBV-encoded ribonucleic acid using an in situ hybridization EBV-encoded ribonucleic acid probe in formalin-fixed, paraffin-embedded tissue. The cause of rectal cancer may be due to a somatic mutation of tumor protein 53 gene in exon 8 (c.844C>T, p.Arg282Trp) through high-throughput sequencing for the rectal cancer. Appropriate standard therapy for each primary cancer was administered, and the patient has no evidence of cancer disease to date.
To our knowledge, this is the first report on heterochronic triple primary malignancies whose cause may be associated with EBV infection and tumor protein 53 genetic mutations. The etiological research may not only elucidate the cause of MPMN but also has implications in clinical management.
Core Tip: The occurrence of triple heterochronic primary malignancies associated with Epstein-Barr virus (EBV) infection and genetic mutations is an extremely rare event. To the best of our knowledge, this is the first case of a 39-year-old male patient with triple heterochronic primary tumors including EBV-associated stomach cancer and nasopharyngeal cancer and tumor protein 53 mutant rectal carcinoma in the literature, and appropriate therapy for each primary cancer led to his good clinical outcome. This case further confirms the value of precision medicine; only a precise diagnosis can lead to appropriate treatment and good prognosis. Further research is needed to elucidate the role of EBV infection and the tumor protein 53 gene in the carcinogenesis of multiple primary malignant neoplasms.
- Citation: Peng WX, Liu X, Wang QF, Zhou XY, Luo ZG, Hu XC. Heterochronic triple primary malignancies with Epstein-Barr virus infection and tumor protein 53 gene mutation: A case report and review of literature. World J Clin Cases 2021; 9(5): 1184-1195
- URL: https://www.wjgnet.com/2307-8960/full/v9/i5/1184.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i5.1184
Multiple primary malignant neoplasms (MPMNs) refer to two or more primary malignant tumors simultaneously or successively occurring in different organs and/or tissues in the same patient, which is clinically rare and comprises only 0.52%-11.7% of all malignant cancers[1,2]. Heterochronic primary malignancy is defined as the second primary cancer, which is diagnosed more than 6 mo after the diagnosis of the first primary cancer. Herein, we reviewed cases of heterochronic triple primary malignant tumors and only found 19 prior cases with heterochronic triple primary malignancies in the English literature. The increasing number of MPMN clinical cases is due to improvements in diagnostic techniques, increasingly novel evidence-based medicine, and increased survival times of cancer patients, justifying that the establishment of a confirmative diagnosis and etiology of MPMNs still plays a pivotal role in extending the life span of cancer patients.
The causes of triple primary malignant tumors are unclear, and multiple factors may synergistically contribute to the occurrence and development of cancer. Epstein-Barr virus (EBV), a ubiquitous human oncovirus, is closely associated with malignant lymphoma, nasopharyngeal carcinoma, and gastric cancer in China and eastern Asia. Studies have shown that EBV genome integration in the human cancer genome is detected in 10% of all gastric cancers and more than 95% of nasopharyngeal squamous cell cancers[3,4]. EBV-positive tumors have a much poorer prognosis than EBV-negative tumors, and EBV-targeted therapies can significantly improve the prognosis of EBV-associated tumors[5,6]. In addition to viral factors, many gene mutations in the human genome have been implicated in the pathogenesis of MPMNs, such as p53, breast cancer gene 1/2, phosphatase and tensin homolog, adenomatous polyposis coli (APC), p16, etc. As is well known, tumor protein 53 (P53) gene mutations, carried by more than 50% of human tumors, can induce cell cycle disturbances and inhibit cell apoptosis that contribute to the generation of most malignant tumors[7,8]. In this study, we present a rare case of heterochronic triple primary malignancies with EBV infection-associated stomach cancer and nasopharyngeal cancer and TP53 mutation-associated rectal carcinoma.
A 39-year-old man who was 178 cm in height and 83 kg in weight came to our institute for consultation for rectal cancer resected more than 1 mo ago.
In February 2020, blood was found in his stool, and colonoscopy showed irregular protuberant lesions 7 cm above the anus. On April 3, 2020, he was pathologically diagnosed with moderately to poorly differentiated adenocarcinoma.
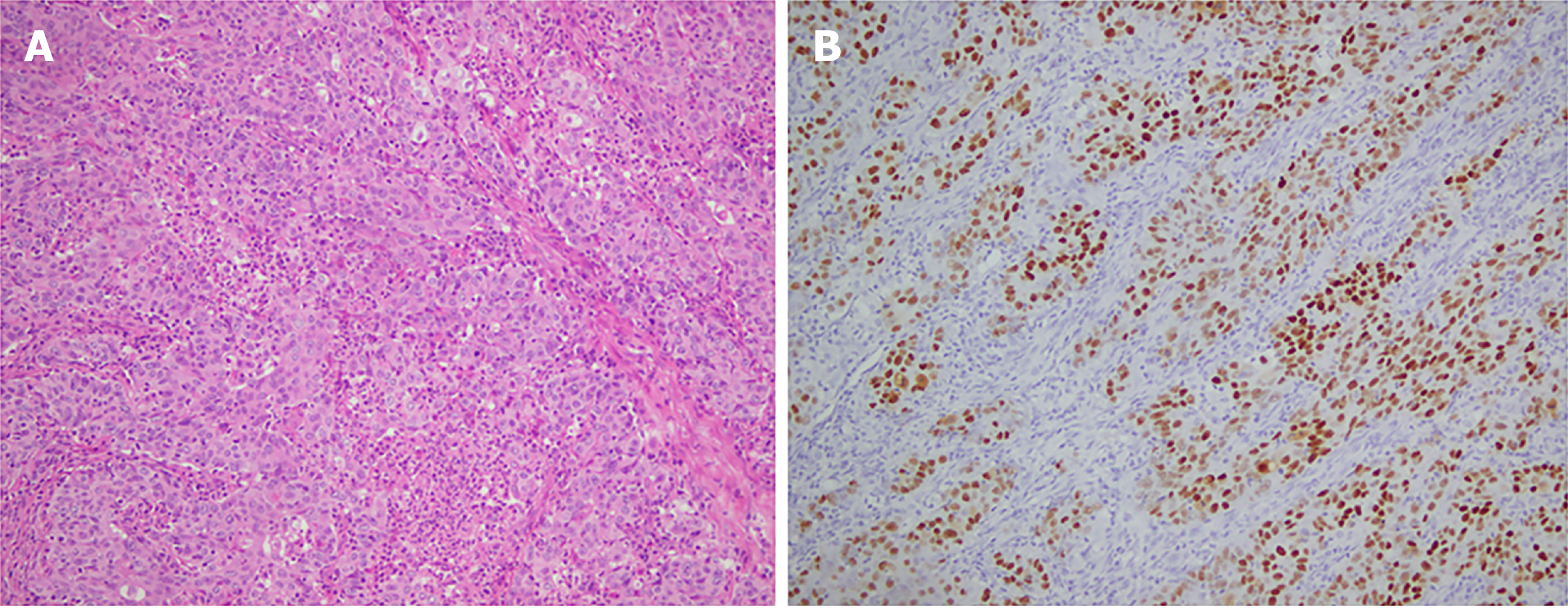
In December 2014, the patient was admitted to the First Affiliated Hospital of Nanjing Medical University for abdominal distention. On December 9, 2014, he was found to have mostly circular protuberant lesions in the gastric body and antrum by gastroscopy and then underwent distal gastrectomy and gastrojejunostomy. Histopathological examination of the surgical specimens revealed a 10 cm × 7 cm × 2 cm-sized mass, which invaded the entire layer of the gastric wall and reached the extracorporeal adipose fibrous connective tissue. This confirmed the diagnosis of stomach carcinoma with lymphoid stroma, diffuse type in the Lauren classification (Figure 1A). There was involvement of only one lymph node in the greater curvature with immunohistochemical staining including cytokeratin (CK)-L (++), Villin (+), P63 (-), P40 (-), CK5/6 (-), and EBV-encoded ribonucleic acid (RNA) (EBER) in situ hybridization (+) (Figure 1B). Adjuvant chemotherapy was administered after the operation. After two cycles of paclitaxel, cisplatin, and tegafur, the treatment regimen was changed to paclitaxel, oxaliplatin, and tegafur for four cycles because of Grade 4 myelosuppression.
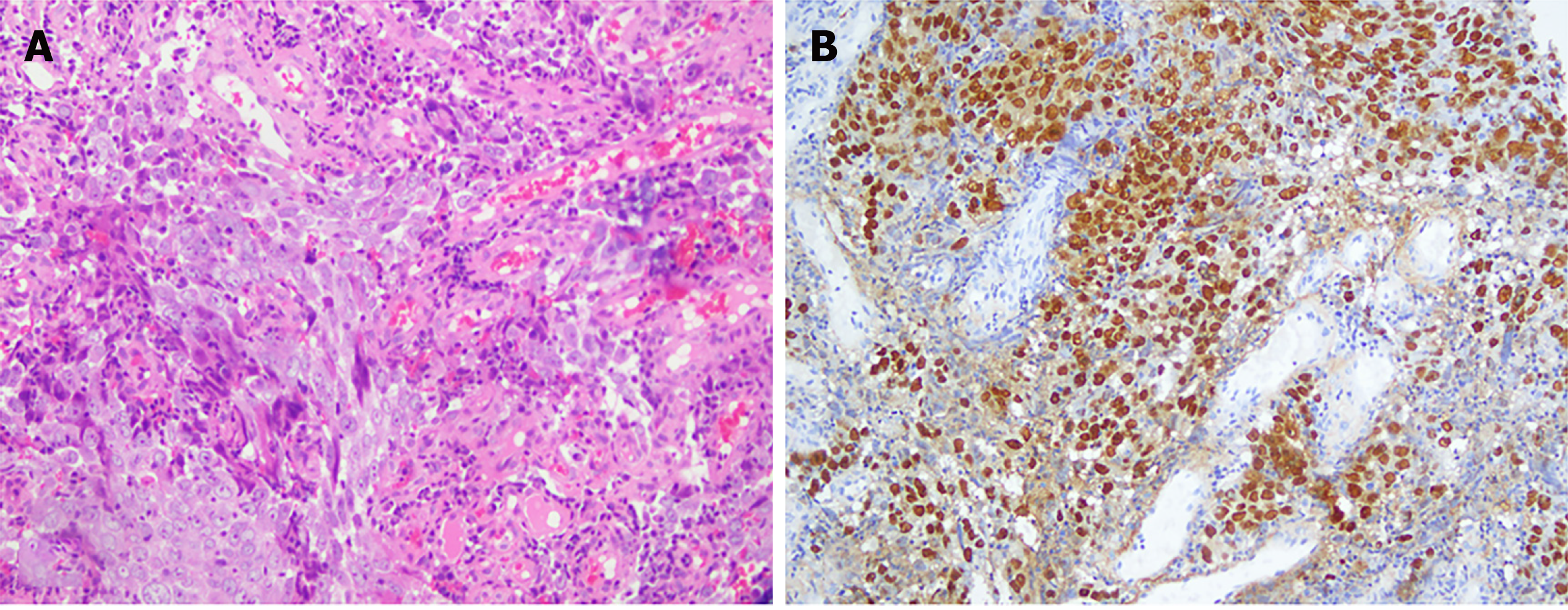
On March 13, 2017, the patient had epistaxis, and he was diagnosed with poorly differentiated squamous cell carcinoma of the nasopharynx with pathology of nasal endoscopic biopsy (Figure 2A). Immunohistochemical findings were as follows: CK-pan (-), CK5/6 (-), P63 (-), Melan A (-), human melanoma black 45 (-), Ki67 (+), EBER (+) (Figure 2B), CK7 (-), CK8/18 (+), CK-L (+), and P40 (focal+).
On March 27, 2017, his nasopharyngeal biopsy specimen was pathologically consulted by the Cancer Hospital of Sun Yat Sen University and Fudan University Shanghai Cancer Center, and both institutions found that there was great similarity between the gastric and nasopharyngeal lesions in terms of hemoxylin & eosin morphology and immunohistochemistry (IHC). Both of them were likely to be primary tumors, but nasopharyngeal metastasis of gastric cancer could not be completely ruled out. The patient was concurrently treated with radical radiotherapy and cisplatin chemotherapy, followed by nimotuzumab for six cycles.
The patient had no history of diabetes mellitus, hypertension, or tobacco or alcohol abuse, and his family history was unremarkable.
The Eastern Cooperative Oncology Group score of the patient was 1, and no superficial lymph nodes could be palpated.
His serum tumor markers and other laboratory values were all within normal range.
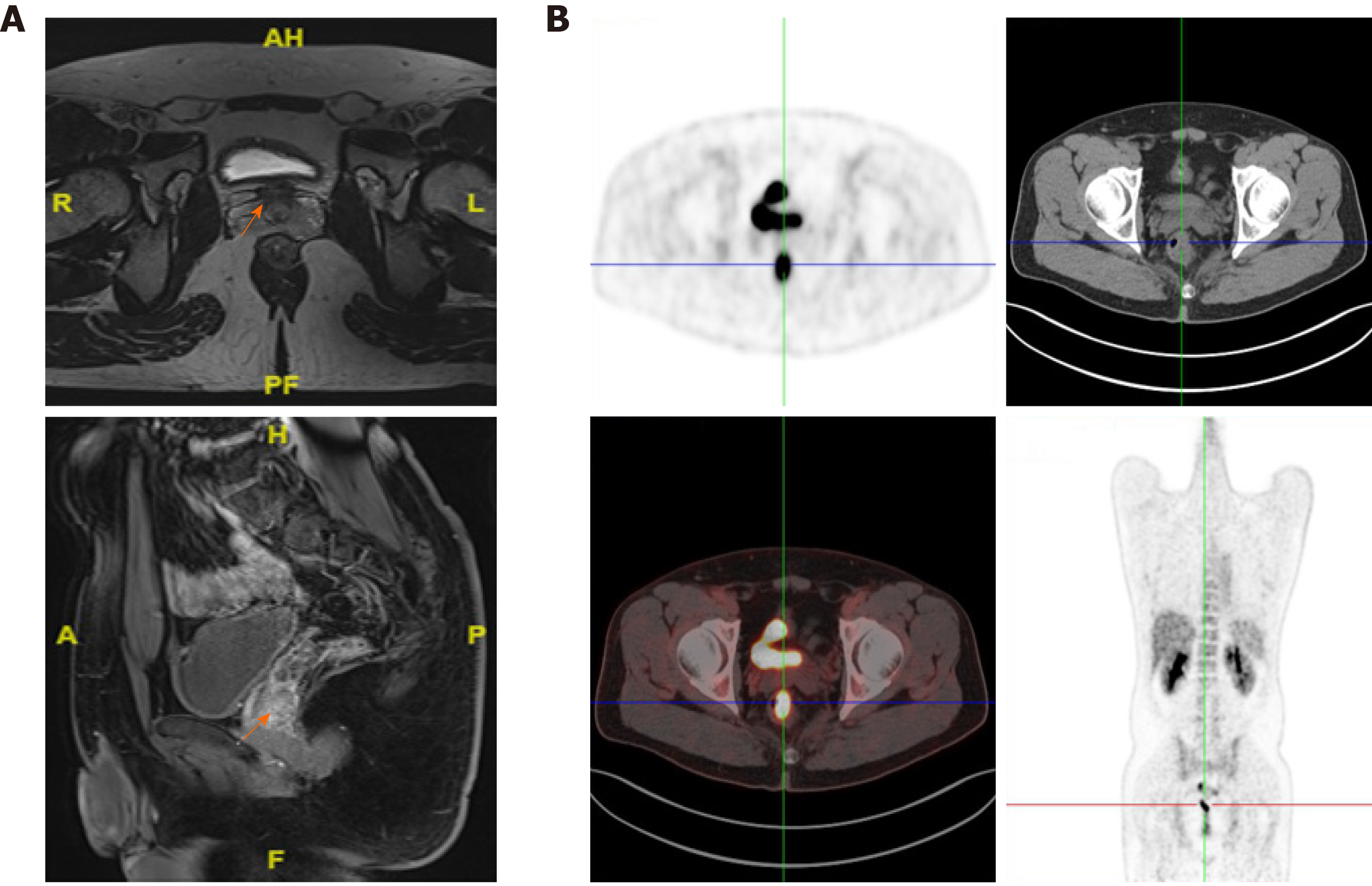
The patient came to our hospital. Magnetic resonance imaging indicated a rectal malignant tumor approximately 58 mm away above the anus (Figure 3A). On April 13, 2020, whole-body positron emission topography/computed tomography with 18-fluorodeoxy-glucose (FDG) scanning revealed thickening of the mid-rectal wall with high FDG uptake, suggesting a malignant tumor (Figure 3B), and increased FDG uptake at the junction of rectum and colon, which was likely to be inflammatory. No abnormal FDG-uptake was observed in the rest.
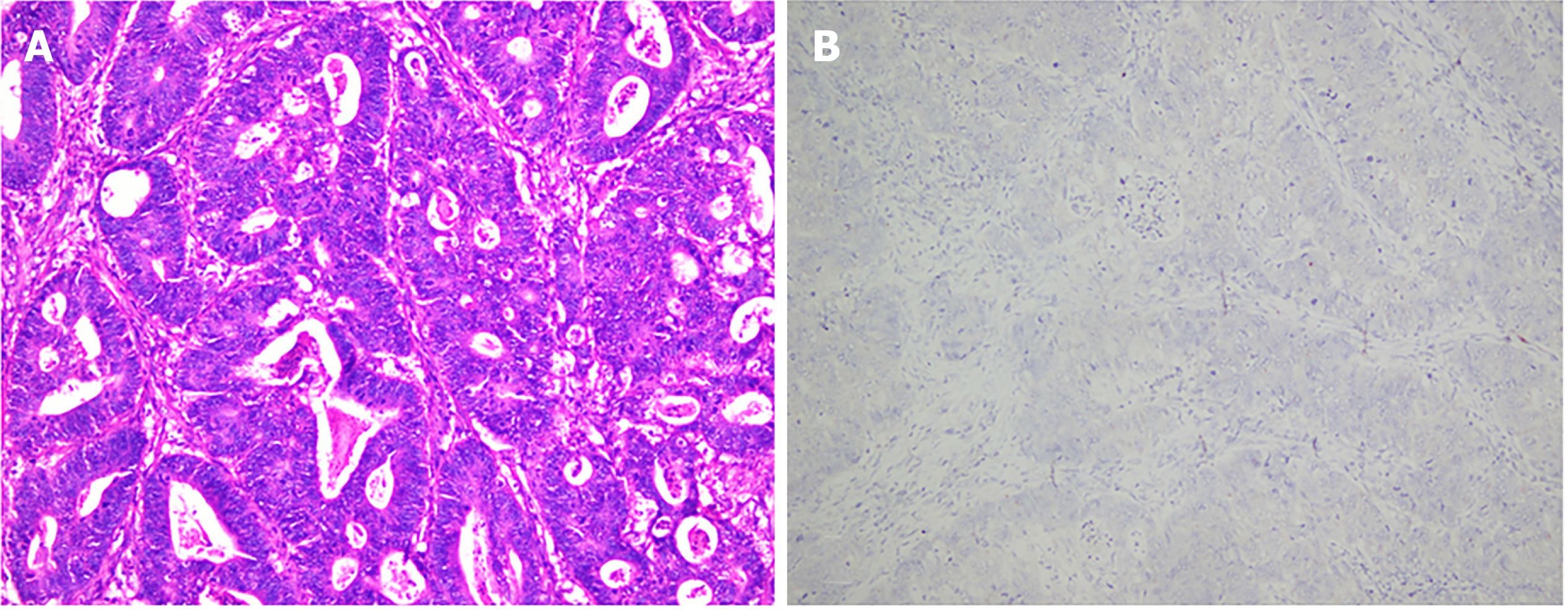
On April 26, 2020, he underwent low anterior resection of the rectum and distal ileostomy. During the operation, the tumor was located 3 cm above the dentate line, with a size of 3 cm × 3 cm and no infiltration into the whole intestinal wall. Postoperative pathology revealed that the tumor was ulcerative, moderately to poorly differentiated adenocarcinoma (Figure 4A), a tumor size of 2.5 cm × 1.5 cm × 1 cm, infiltration into the superficial muscle layer, and T2N0, mesorectal fascia (-), and extramural venous invasion (-). IHC indicated the following tumor cells: Special AT-rich sequence-binding protein 2 (+), caudal-type homeobox 2-88 (+), CK20 (+), Villin (+), human MutL homolog 1 (+), human melanocyte stimulating hormone 2 (+), human melanocyte stimulating hormone 6 (+), PMS1 homolog 1 (+), CD44 (focal+), E-cadherin (+), hair and enhancer of split-1 (+), Ki-67 (80%+), CK7 (-), human epidermal growth factor receptor 2 (-), BRAF (-), 5-hydroxytryptamine receptor 2B (-), zinc finger E-box binding homeobox 1 (-), and EBER (-) (Figure 4B).
On Apr 20, 2020, a consultation involving the whole Department of Pathology in our hospital occurred.
The pathology of the gastric lesion was performed, and based on the IHC results in the original hospital, poorly differentiated carcinoma in the distal stomach was observed, consistent with lymphoepithelioma-like carcinoma. The cancer invaded the whole gastric wall and reached the serosa without definite nerve invasion. No cancer was found in the upper or lower margins or anastomotic rings. One lymph node metastasis was found in 14 lymph nodes in the great curvature of the stomach, whereas there were no positive nodes in 37 Lymph nodes in the lesser curvature of the stomach. It is difficult to classify further into subtypes based on the Lauren classification. IHC of the original hospital was reevaluated with tumor cells of CK+++, EBER+.
The morphology of the nasopharyngeal biopsy was consistent with EBV-related undifferentiated carcinoma and p63-tumor cells. Based on the medical history, nasopharyngeal metastasis of stomach carcinoma could not be fully ruled out.
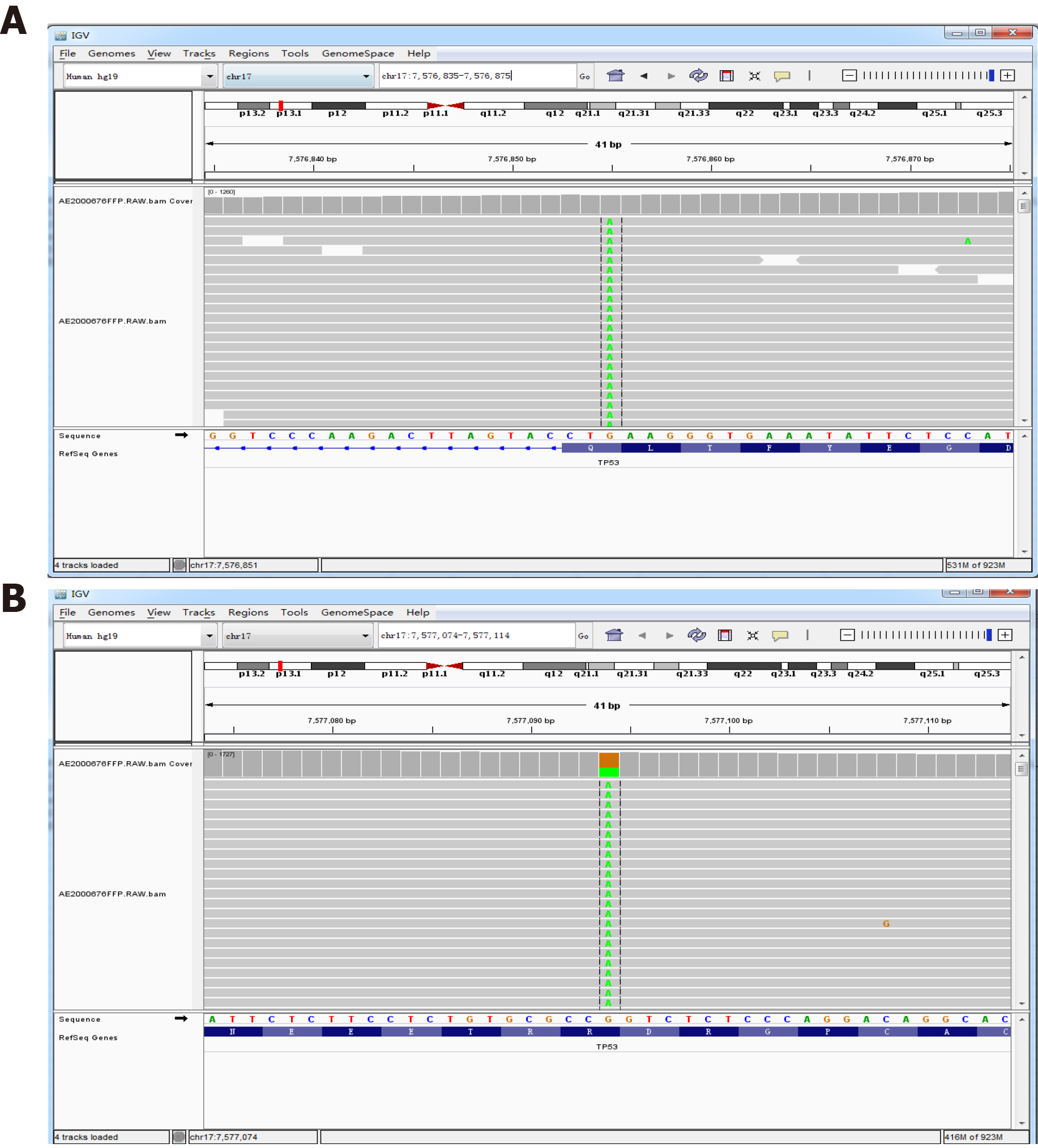
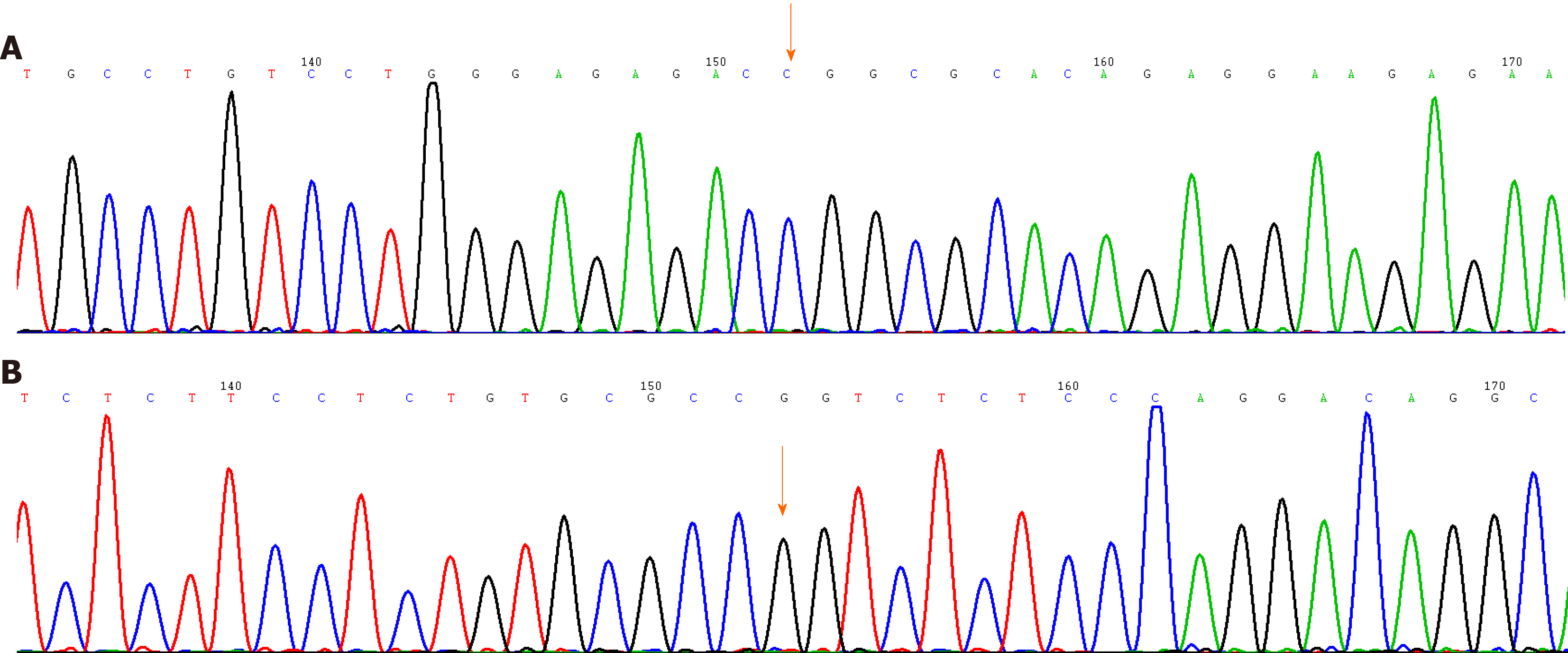
On May 14, 2020, the next-generation sequencing test for his rectal cancer specimens revealed the following: 24 microsatellite (MS) loci were evaluated, and 22 MSs were analyzed. The sample status was microsatellite stable (MSS). The following pathogenic/probably pathogenic variations were detected in the paraffin tissue deoxyribonucleic acid: (1) KRAS (NM_033360.3) missense mutation in exon 4 c.436G > A (p.Ala146Thr), AF 34.43%; (2) APC (NM_000038.5) nonsense mutation in exon 16 c.3340C>T (p.Arg1114*), AF 20.54%; (3) APC (NM_000038.5 code shift mutation in exon 16 c.4127_4128del (p.Tyr1376fs), AF 22.71%; (4) TP53 (NM_000546.5) nonsense mutation in exon 9 c.991C>T (p.Gln331*), AF 3.71% (Figure 5A); (5) TP53 (NM_000546.5) missense mutation in exon 8 c.844C>T (p.Arg282Trp), AF 37.89% (Figure 5B); and (6) single nucleotide polymorphisms of drug metabolism-related enzymes indicated that bilirubin UDP-glucuronosyltransferase 1A1*28, bilirubin UDP-glucuronosyltransferase 1A1*6, cytochrome P450 2D6*10, dihydropyrimidine dehydrogenase*2A, and dihydropyrimidine dehydrogenase*13 were wild type. In September 2020, gene sequencing revealed that the mutation in the TP53 gene was not present in the peripheral blood samples (Figure 6).
After combining clinical features and pathological findings, the patient was diagnosed with MPMN with rectal cancer (pT2N0M0, stage I) as the third primary, nasopharyngeal undifferentiated cancer as the second primary (EBER+), and gastric cancer as the first primary (pT3N1M0, stage III, EBER+). The timeline of diagnosis and treatment is shown in Figure 7.
There was no adjuvant chemotherapy or radiotherapy administered. The patient was regularly followed up.
The patient was followed up until September 30, 2020. The postoperative recovery of rectal cancer was satisfactory, and normal life was resumed. The blood tests for the tumor markers showed no findings, and the routine imaging examination showed no evidence of tumor occurrence or metastasis. The patient provided written informed consent for publication of this case report.
Currently, the diagnosis of MPMNs is mostly based on Warren et al[9]'s revised diagnostic criteria: (1) Each tumor is pathologically confirmed as malignant; (2) Each tumor must exist independently in the anatomy; and (3) Each tumor must be excluded as metastases of other tumors. According to the time interval between the two primaries, Moertel et al[10] divided MPMN into synchronous and metachronous MPMNs[10], which occur within 6 mo and more than 6 mo, respectively. In this case, the middle-aged male patient developed three distinct primary malignant tumors within three different organs (stomach, nasopharynx, and colorectum), and the time interval between them was longer than 6 mo; thus, the progression of the patient’s disease met the criteria of the diagnosis of metachronous MPMN.
Notably, there was a debate about the pathological diagnosis of primary nasopharyngeal carcinoma in the present case. This case and published studies have shown that EBV infection-related lymphoepithelioma-like gastric cancer and nasopharyngeal carcinoma share the same characteristics, except for different sites of occurrence[11]. However, 90% of nasopharyngeal squamous cell cancers in China are EBER positive, and patients with gastric cancer undergo radical treatments. Furthermore, the lesion in the nasopharynx was the only cancer lesion at that time and occurred 27 mo after the gastric cancer. Therefore, the tumor board of the multidisciplinary team for Cancer of Multiple and Unknown Primaries in Fudan University Shanghai Cancer Center agreed on nasopharyngeal carcinoma as the second primary carcinoma.
Possible causes of MPMN include abnormal activation of oncogenes, silencing of tumor suppressor genes, epigenetic alterations, chromosome instability, immune deficiency, exposure to environmental factors, and an unhealthy lifestyle[12-14]. Recently, EBV has become the focus of attention of many scholars. EBV, a double-stranded deoxyribonucleic acid virus with a length of approximately 172 kb, mainly attacks human B lymphocytes and epithelial cells through proliferative infection and latent infection, and the virus is mostly in the state of latent infection and does not replicate[15]. EBV has an infection rate of 1.09% in the general population and is linked to the development of two major epithelial malignancies, including gastric and nasopharyngeal carcinoma[16]. EBV-encoded small RNA (EBER, EBER1, and EBER2), used as a molecule to signal EBV infection in tissues by in situ hybridization, is highly expressed in latent EBV-infected cells to evade immune surveillance and maintain lifelong latent infection[17,18]. EBER1/2 could promote the growth of cells, regulate the innate immune system, and promote the formation of tumors via the following mechanisms: Adjusting the interleukin-6/signal transducer and activators of transcription-3/P21 and p27 pathways to promote the occurrence of gastric cancer, promoting the expression of focal adhesion kinase phosphorylation to promote cancer cell migration, and serving as an autocrine growth factor to promote the growth of cancer cells[19,20]. Several reports have shown that early diagnosis and early intervention of EBV infection are helpful to improve the prognosis of EBV virus-associated tumors. The patient denied smoking, drinking, and other bad living habits, which might exclude lifestyle carcinoma, and had no family history of cancer, which can exclude familiar and hereditary cancer; therefore, EBV infection may be the common cause for gastric and nasopharyngeal cancer.
However, the EBER transcript was not found in the colorectal cancer of the patient. The allele frequencies of the K-ras gene missense mutation, APC gene shift mutation, and TP53 gene missense mutation (exon 8) were 34.43%, 22.7%, and 37.89% by next-generation sequencing, respectively. Moreover, the mutation in the TP53 genes in the patient was a somatic heterozygous mutation, and no germline gene mutations were found, providing a direction for us to explore the etiologic factors of the third primary cancer.
Lv et al[2] reported that the most common organ of MPMN involved the digestive system with adenocarcinoma, including the stomach and the esophagus[2]. A literature review of metachronous triple primary neoplasms was performed after searching the PubMed database. Only 19 cases of clinical metachronous triple primary neoplasms have been reported between 1995 and 2020. Table 1 shows a summary of the previous studies describing metachronous triple primaries. Approximately half of the patients had primary colorectal cancer. There have been no reports of heterochronic triple primary malignancies with gastric cancer, nasopharyngeal cancer or rectal cancer. Kato et al[21] reported a high incidence of gastric cancer complicated with colorectal cancer[21]. Colorectal cancer is the fourth most common malignant tumor in China, posing a serious threat to human health. Obviously, there were different etiologic factors for gastric and rectal cancers in this patient.
| Ref. | Age in yr/gender | Sites | Tumor histology | Treatment | Outcome | Follow-up |
| Li et al[22], 2020 | 67/F | Uterine | Moderately differentiated endometrial cancer | Surgery RT CT ET | DFS | 19 mo |
| Colon | Ascending colon papillary adenocarcinoma and moderately differentiated tubular adenocarcinoma | |||||
| Breast | Invasive ductal carcinoma | |||||
| Feng et al[23], 2018 | 66/M | Prostate | Acinar adenocarcinoma | Surgery CT | DFS | 4 mo |
| Colon | Moderately differentiated adenocarcinoma | |||||
| Lung | Moderately differentiated adenocarcinoma | |||||
| Gheonea et al[24], 2017 | 75/M | Prostate | Prostate carcinoma | ET | NM | NM |
| Lung | Small lung cell carcinoma | |||||
| Skin | Basal cell carcinoma | |||||
| Kim et al[25], 2017 | 73/M | Lung | Small cell carcinoma | Surgery CT | DFS | 44 mo |
| Poorly differentiated adenocarcinoma | ||||||
| Moderately differentiated squamous cell carcinoma | ||||||
| Yoshikawa et al[26], 2017 | 60/M | Bile duct | Papillary adenocarcinoma | Surgery CT RT | DFS | NM |
| Poorly differentiated cholangio cellular carcinoma | ||||||
| A well-differentiated hilar cholangiocarcinoma | ||||||
| Pastore et al[27], 2015 | 70/M | Renal | Renal clear cell carcinoma | Surgery RT CT ET | NM | NM |
| Oropharynx | Poorly-differentiated (G3) squamous cell carcinoma | |||||
| Prostate | Adenocarcinoma | |||||
| Nishikawa et al[28], 2014 | 37/M | Hypopharynx | Squamous cell carcinoma | Surgery RT CT | DOD | 10 yr |
| Esophagus | ||||||
| Tongue | ||||||
| Okumura et al[29], 2013 | 73/M | Prostatic | Moderately differentiated adenocarcinoma | NM | NM | NM |
| Bladder | Grade 2 urothelial carcinoma | |||||
| Renal | Grade 2 clear renal cell carcinoma | |||||
| Zargar-Shoshtari et al[30], 2013 | 71/M | Renal | Clear renal cell carcinoma | Surgery ET | DOD | 1 yr |
| Prostate | Adenocarcinoma | |||||
| Breast | Poorly differentiated invasive ductal cell carcinoma | |||||
| Freeman[31], 2013 | 72/M | Colon | Moderately differentiated adenocarcinoma in the distal sigmoid colon | Surgery | DFS | 15 yr |
| Infiltrative moderately differentiated colonic | ||||||
| Adenocarcinoma in the descending colon moderately differentiated adenocarcinoma in the rectum | ||||||
| Chang et al[32], 2013 | 67/M | Maxillary sinus | Adenoid cystic carcinoma | Surgery RT | DFS | 15 yr |
| Esophagus | Squamous cell carcinoma | 22 mo | ||||
| Tympanic membrane | 18 mo | |||||
| Egashira et al[33], 2013 | 63/F | Esophagus | Esophageal cancer | Surgery | DFS | 10 mo |
| Colon | Ascending colon cancer | |||||
| Jejunum | Jejunal cancer | |||||
| Takalkar et al[34], 2013 | 64/F | Small intestine | Moderately differentiated adenocarcinoma | Surgery CT | NM | NM |
| Breast | Well differentiated infiltrated duct carcinoma | |||||
| Ovary | Papillary adenocarcinoma | |||||
| Arikan-Sengul et al[35], 2009 | 70/M | Bladder | Transitional cell carcinoma | NM | NM | NM |
| Prostate | ||||||
| Renal | Renal cell carcinoma | |||||
| Yamanaka et al[36], 2008 | 24/F | Humerus | Steosarcoma | Surgery CT | NM | NM |
| Breast | The light breast cancer the left breast cancer | |||||
| Oztop et al[37], 2008 | 76/M | Rectum | Metastatic rectal cancer | Surgery CT RT ET | DOD | 1 yr |
| Prostate | Prostate cancer | |||||
| Lymphocyte | Philadelphia (+) chronic myeloid leukemia | |||||
| Baba et al[38], 2002 | 64/M | Lung | Small cell lung cancer | Surgery | DFS | 20 mo |
| Prostate | Moderately or poorly differentiated adenocarcinoma | |||||
| Breast | Scirrhous carcinoma | |||||
| Iioka et al[39], 2000 | 60/M | Colon | Sigmoid colon cancer | Surgery RT | DOD | 13 mo |
| Stomach | Early gastric cancer | |||||
| Esophagus | The middle thoracic esophagus cancer | |||||
| Saitoh et al[40], 1995 | 64/M | Lung | Moderately differentiated squamous cell carcinoma | Surgery RT | NM | NM |
| Colon | The rectum carcinoma | |||||
| Trachea | Moderately differentiated squamous cell carcinoma with mediastinal lymph node metastasis |
In this study, the patient was 6 years past the first onset of primary gastric cancer, 2 more years past the second primary nasopharyngeal cancer, and 7 mo past the third primary rectal cancer. All the treatment of this patient followed the guidelines for each primary cancer. Currently, this patient is in good survival condition without any evidence of recurrence or metastasis. Generally, patients with MPMN have a better prognosis than other cancer patients with no MPMN. As expected, the misdiagnosis of a primary lesion as the metastasis of the prior cancer can lead to incorrect treatment and a poor prognosis.
The occurrence of triple heterochronic primary malignancies associated with EBV infection and genetic mutations is an extremely rare event. To the best of our knowledge, this is the first case of a 39-year-old male patient with triple heterochronic primary tumors including EBV-associated stomach cancer and nasopharyngeal cancer and TP53 mutant rectal carcinoma in the literature, and appropriate therapy for each primary cancer led to his good clinical outcome. This case further confirms the value of precision medicine; only a precise diagnosis can lead to appropriate treatment and good prognosis. Further research is needed to elucidate the role of EBV infection and the TP53 gene in the carcinogenesis of MPMN.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Frascio M S-Editor: Fan JR L-Editor: Filipodia P-Editor: Xing YX
| 1. | Demandante CG, Troyer DA, Miles TP. Multiple primary malignant neoplasms: case report and a comprehensive review of the literature. Am J Clin Oncol. 2003;26:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Lv M, Zhang X, Shen Y, Wang F, Yang J, Wang B, Chen Z, Li P, Zhang X, Li S, Yang J. Clinical analysis and prognosis of synchronous and metachronous multiple primary malignant tumors. Medicine (Baltimore). 2017;96:e6799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 3. | Tsao SW, Tsang CM, Lo KW. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos Trans R Soc Lond B Biol Sci. 2017;372:20160270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 417] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 4. | Naseem M, Barzi A, Brezden-Masley C, Puccini A, Berger MD, Tokunaga R, Battaglin F, Soni S, McSkane M, Zhang W, Lenz HJ. Outlooks on Epstein-Barr virus associated gastric cancer. Cancer Treat Rev. 2018;66:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 5. | Ghosh SK, Perrine SP, Faller DV. Advances in Virus-Directed Therapeutics against Epstein-Barr Virus-Associated Malignancies. Adv Virol. 2012;2012:509296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Tao Q, Young LS, Woodman CB, Murray PG. Epstein-Barr virus (EBV) and its associated human cancers--genetics, epigenetics, pathobiology and novel therapeutics. Front Biosci. 2006;11:2672-2713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Otsuka K, Ishioka C. [TP53 mutations and molecular epidemiology]. Gan To Kagaku Ryoho. 2007;34:683-689. [PubMed] |
| 8. | Li XL, Zhou J, Chen ZR, Chng WJ. P53 mutations in colorectal cancer - molecular pathogenesis and pharmacological reactivation. World J Gastroenterol. 2015;21:84-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 261] [Cited by in RCA: 247] [Article Influence: 24.7] [Reference Citation Analysis (1)] |
| 9. | Warren S. Multiple primary malignant tumors: A survey of the literature and a statistical study. Am J Cancer. 1932;93:1358-1414. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | MOERTEL CG, DOCKERTY MB, BAGGENSTOSS AH. Multiple primary malignant neoplasms. II. Tumors of different tissues or organs. Cancer. 1961;14:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Hildesheim A. Invited commentary: Epstein-Barr virus-based screening for the early detection of nasopharyngeal carcinoma: a new frontier. Am J Epidemiol. 2013;177:251-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Oeffinger KC, Baxi SS, Novetsky Friedman D, Moskowitz CS. Solid tumor second primary neoplasms: who is at risk, what can we do? Semin Oncol. 2013;40:676-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Jiao F, Yao LJ, Zhou J, Hu H, Wang LW. Clinical features of multiple primary malignancies: a retrospective analysis of 72 Chinese patients. Asian Pac J Cancer Prev. 2014;15:331-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Wang L, Wang H, Wang T, Liu J, Chen W, Wang Y, Chen C, Zhu H, Dai P. Analysis of polymorphisms in genes associated with the FA/BRCA pathway in three patients with multiple primary malignant neoplasms. Artif Cells Nanomed Biotechnol. 2019;47:1101-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Yin H, Qu J, Peng Q, Gan R. Molecular mechanisms of EBV-driven cell cycle progression and oncogenesis. Med Microbiol Immunol. 2019;208:573-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 16. | Kanda T, Yajima M, Ikuta K. Epstein-Barr virus strain variation and cancer. Cancer Sci. 2019;110:1132-1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 17. | Gruhne B, Sompallae R, Masucci MG. Three Epstein-Barr virus latency proteins independently promote genomic instability by inducing DNA damage, inhibiting DNA repair and inactivating cell cycle checkpoints. Oncogene. 2009;28:3997-4008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Iwakiri D, Takada K. Role of EBERs in the pathogenesis of EBV infection. Adv Cancer Res. 2010;107:119-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Banerjee AS, Pal AD, Banerjee S. Epstein-Barr virus-encoded small non-coding RNAs induce cancer cell chemoresistance and migration. Virology. 2013;443:294-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Samanta M, Takada K. Modulation of innate immunity system by Epstein-Barr virus-encoded non-coding RNA and oncogenesis. Cancer Sci. 2010;101:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Kato T, Suzuki K, Muto Y, Sasaki J, Tsujinaka S, Kawamura YJ, Noda H, Horie H, Konishi F, Rikiyama T. Multiple primary malignancies involving primary sporadic colorectal cancer in Japan: incidence of gastric cancer with colorectal cancer patients may be higher than previously recognized. World J Surg Oncol. 2015;13:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Li G, Yao J, Wu T, Chen Y, Wang Z, Wang Y, Wang F, Zhong R, Yang S. Triple metachronous primary cancer of uterus, colon, and breast cancer: A case report and review of the literature. Medicine (Baltimore). 2020;99:e21764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Feng Y, Zhong M, Zeng S, Xiao D, Liu Y. Metachronous triple primary neoplasms with primary prostate cancer, lung cancer, and colon cancer: A case report. Medicine (Baltimore). 2018;97:e11332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Gheonea IA, Popp CG, Ivan ET, Gheonea DI. Unusual triple combination of prostate, lung and skin cancer. Rom J Morphol Embryol. 2017;58:567-574. [PubMed] |
| 25. | Kim JH, Park SY, Park SJ, Chung MJ, Lee HB. Different histological types of triple metachronous primary lung carcinomas in 1 patient: Case report. Medicine (Baltimore). 2017;96:e8923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Yoshikawa M, Ikemoto T, Morine Y, Imura S, Saito Y, Yamada S, Teraoku H, Takata A, Yoshimoto T, Shimada M. Aggressive resection of metachronous triple biliary cancer. J Med Invest. 2017;64:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Pastore AL, Palleschi G, Leto A, Silvestri L, Porta N, Petrozza V, Carbone A. A novel combination of triple metachronous malignancies of the kidney, oropharynx and prostate: A case report. Oncol Lett. 2015;10:917-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Nishikawa K, Kawada J, Fujitani K, Hirao M, Yamamoto K, Fukui A, Takagi M, Fushimi H, Iwase K, Endo S, Harada Y, Fukuda Y, Haraguchi N, Miyake M, Asaoka T, Miyamoto A, Miyazaki M, Ikeda M, Nakamori S, Sekimoto M. [A case of metachronous triple cancer treated with a multimodal approach including surgical resection]. Gan To Kagaku Ryoho. 2014;41:2033-2035. [PubMed] |
| 29. | Okumura A, Tsuritani S, Takagawa K, Fuse H. [Case of heterochronous triple urogenital cancer (renal cell carcinoma, bladder cancer, prostatic cancer)]. Nihon Hinyokika Gakkai Zasshi. 2013;104:702-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Zargar-Shoshtari MA, Saffari H, Moslemi MK. Metachronous occurrence of triple malignancies of kidneys, prostate, and breast. A case report and review of the literature. Case Rep Urol. 2013;2013:194620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Freeman HJ. Triple metachronous colon cancer. World J Gastroenterol. 2013;19:4443-4444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Chang HY, Jiang H, Zhou F. A rare case of metachronous triple cancers involving the tympanic membrane. Pak J Med Sci. 2013;29:218-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Egashira A, Taguchi K, Toh Y, Yamamoto M, Okamura T, Saeki H, Oki E, Morita M, Ikeda T, Mimori K, Watanabe M, Maehara Y. Successful treatment of primary jejunal cancer after esophageal and colon cancer resection. Fukuoka Igaku Zasshi. 2013;104:435-441. [PubMed] |
| 34. | Takalkar U, Asegaonkar BN, Kodlikeri P, Asegaonkar S, Sharma B, Advani SH. An elderly woman with triple primary metachronous malignancy: A case report and review of literature. Int J Surg Case Rep. 2013;4:593-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Arikan-Sengul C, Pehlivan Y, Sevinc A, Karakok M, Kalender ME, Camci C. A case of metachronous triple primary urogenital cancer: urinary bladder, prostate, and renal cancer. Onkologie. 2009;32:122-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Yamanaka S, Arai H, Rino Y, Masuda M. [Pulmonary metastasis of metachronous triple cancer occurring in a young patient]. Kyobu Geka. 2008;61:541-544. [PubMed] |
| 37. | Oztop I, Yaren A, Demirpence M, Alacacioglu I, Tuna B, Piskin O, Yilmaz U. The development of metachronous prostate cancer and chronic myeloid leukemia in a patient with metastatic rectal cancer. J BUON. 2008;13:267-270. [PubMed] |
| 38. | Baba M, Higaki N, Ishida M, Kawasaki H, Kasugai T, Wada A. A male patient with metachronous triple cancers of small cell lung, prostate and breast. Breast Cancer. 2002;9:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Iioka Y, Tsuchida A, Okubo K, Ogiso M, Ichimiya H, Saito K, Osaka Y, Sato S, Aoki T, Koyanagi Y. Metachronous triple cancers of the sigmoid colon, stomach, and esophagus: report of a case. Surg Today. 2000;30:368-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Saitoh Y, Umemoto M, Imamura H, Yamanaka E, Hioki K, Okamura A. [A case of metachronous primary cancer of the lung, rectum and trachea]. Nihon Kyobu Geka Gakkai Zasshi. 1995;43:78-81. [PubMed] |