Published online Feb 16, 2021. doi: 10.12998/wjcc.v9.i5.1148
Peer-review started: October 18, 2020
First decision: December 13, 2020
Revised: December 19, 2020
Accepted: January 5, 2021
Article in press: January 5, 2021
Published online: February 16, 2021
Processing time: 104 Days and 1.9 Hours
In the development of coronary stent technology, bioresorbable scaffolds are promising milestones in improving the clinical treatment of coronary artery disease. The “leave nothing behind” motto is the premise of the fourth revolution in percutaneous coronary intervention (PCI). Studies proving the safety and efficacy of the magnesium-based resorbable scaffolds (MgBRSs) include the BIOSOLVE-I and BIOSOLVE-II trials and the latest BIOSOLVE-IV registry. However, spontaneous retrograde dissection of a partially absorbed MgBRS may still occur, albeit rarely.
We describe an unusual case of coronary artery disease in a patient who had undergone a successful PCI 8 mo earlier, where an MgBRS was implanted into the left anterior descending artery (LAD) and left circumflex artery with drug-coated balloons for a ramus intermedius branch stenosis to achieve the “leave nothing behind” therapeutic intention and was currently presenting with a gradual worsening of chest tightness. The distal edge vascular response, during subsequent attempts with balloon angioplasty was performed smoothly. However, spontaneous retrograde dissection of a partially absorbed MgBRS in the LAD ensued. Successful bailout stenting was performed with revascularization of the entry and exit sites created by spontaneous dissection and complete sealing of the intramural hematoma. The patient recovered well and was discharged after 2 d of intervention. When followed up in August 2020 (7 mo later), the patient showed uneventful recovery.
Spontaneous retrograde dissection of a partially absorbed MgBRS was successfully treated using bailout sirolimus-eluting coronary stent strategy.
Core Tip: Coronary revascularization with percutaneous coronary intervention primarily involves using balloon angioplasty and intracoronary stenting with either drug-eluting stents or bare metal stents. Other methods of improving coronary blood flow include atherectomy and radiation. Resorbable metallic scaffolds have been developed to reduce adverse events after permanent metallic stent placement, such as restenosis or stent thrombosis. These adverse events have been attributed to persistent inflammation, impaired vasomotion, ongoing tissue growth within the stent frame, and neoatherosclerosis. Spontaneous coronary artery dissection is rare. Reverse dissection of post-magnesium-based resorbable scaffold stenting is even rarer.
- Citation: Liao ZY, Liou JY, Lin SC, Hung HF, Chang CM, Chen LC, Chua SK, Lo HM, Hung CF. Successful bailout stenting strategy against rare spontaneous retrograde dissection of partially absorbed magnesium-based resorbable scaffold: A case report. World J Clin Cases 2021; 9(5): 1148-1155
- URL: https://www.wjgnet.com/2307-8960/full/v9/i5/1148.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i5.1148
Currently, perpetual metallic stents are the most commonly used stents for percu-taneous myocardial re-vascularization[1] because they meet a broad range of technical and clinical demands, including exceptional deliverability, sufficient radial force on the vessel wall, uniform and adequate scaffolding, restriction of neointimal hyperplasia, decreased restenosis rate, and reduced occurrence of major cardiac adverse events[2]. Although the current generation of metallic stents, especially drug-eluting stents (DES), perform excellently, concerns about their long-term efficacy continue to pose a dilemma for interventional cardiologists[3]. Although magnesium-based resorbable scaffolds (MgBRSs) overcome many problems and obstacles, including permanent caging of the vessels and its sequelae, late stent thrombosis, eventful restenosis, and neoatherosclerosis may still occur. Many of these issues are due to the drug, polymer, or metallic platform left behind in the arterial wall[4].
A 45-year-old man was admitted to the cardiovascular department with complains of ongoing chest tightness, dyspnea, and left shoulder pain that had worsened over the preceding weeks.
The chest tightness progressed and worsened. The patient described it as a dull, non-spreading tightness over the retrosternal area. However, angina occurred more commonly in the morning. Each episode lasted a few minutes and usually subsided after taking a short rest. He experienced slight limitations in his daily activities.
The patient had a history of hypertension, dyslipidemia, coronary artery disease, and he had undergone percutaneous coronary intervention (PCI) eight months ago using a new MgBRSs over the left anterior descending artery (LAD) and left circumflex artery. He was on several medications for many years, including angiotensin-receptor blockers, calcium channel blockers, and HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A) reductase inhibitors. He had no known history of allergies.
He had a family history of coronary artery disease. He was also a chain smoker (20 cigarettes per day), with more than 20 years of smoking history before he quit smoking in 2018. He denied any illicit drug or alcohol use.
The patient’s body-mass index was approximately 29.7 (kg/m2), indicating obesity. His blood pressure was 120/79 mmHg with a regular heart rate of 72 beats per minute. Cardiac examination, including auscultation for carotid bruits, jugular venous pulse, heart sounds, and murmurs was normal.
On admission, laboratory data, such as blood cell count, electrolytes, and biochemistry blood analysis, were within normal limits. A 12-lead electrocardiogram showed normal sinus rhythm with low voltage and borderline right axis deviation. Myocardial perfusion scans were previously performed to determine the extent and location of myocardial ischemia and revealed partial reversibility with notable ischemia at the apical segment.
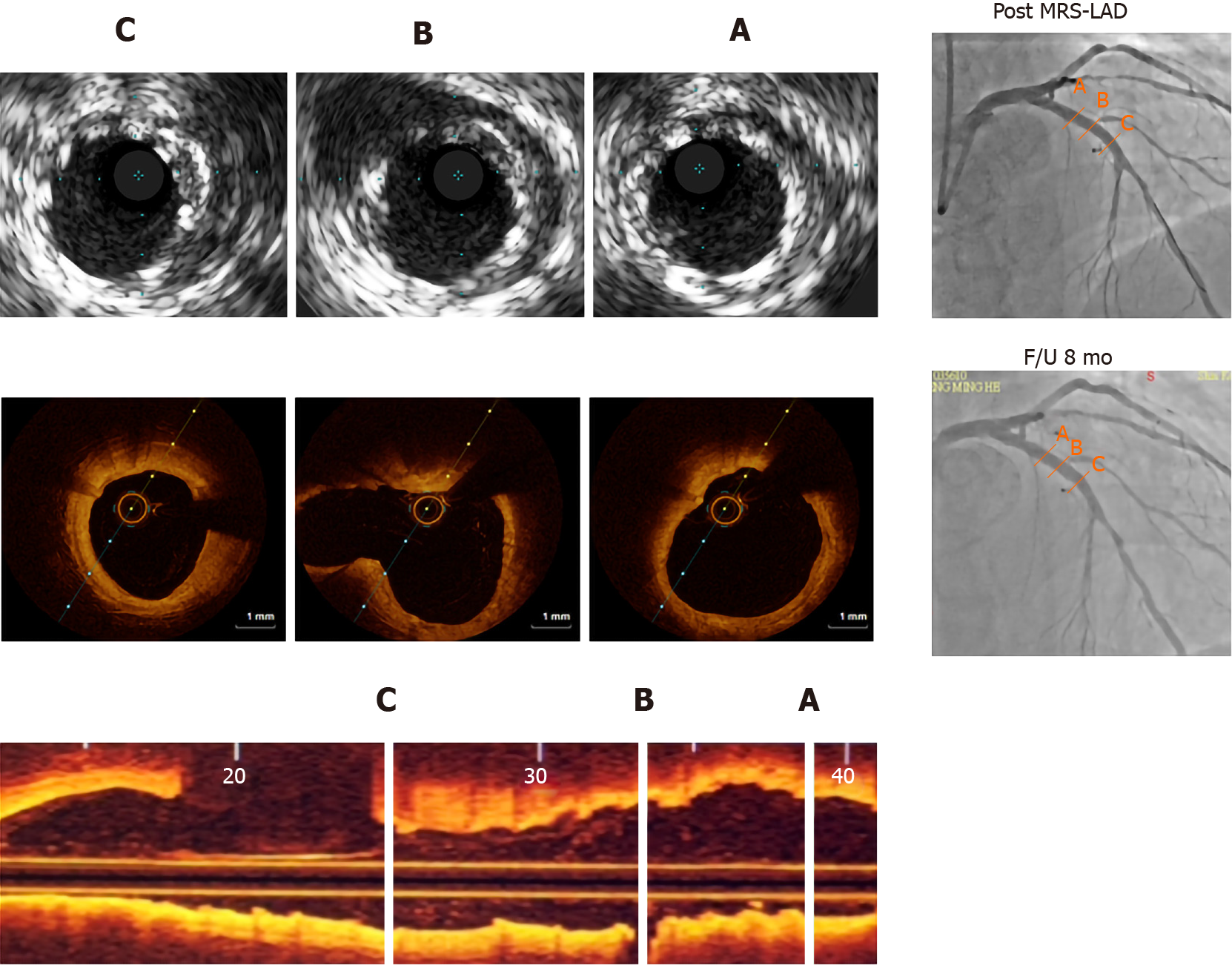
Chest radiography showed normal cardiac size and configuration. Patients underwent post-implantation intravascular ultrasound (IVUS) and optical coherence tomography (OCT) evaluation during admission. The main OCT imaging findings were near total resorption of the MgBRS struts eight months after implantation without substantial vessel recoil, which could have been caused by early radial strength deficiency (Figure 1). A distal edge vascular response (EVR) was apparent (Figure 2A).
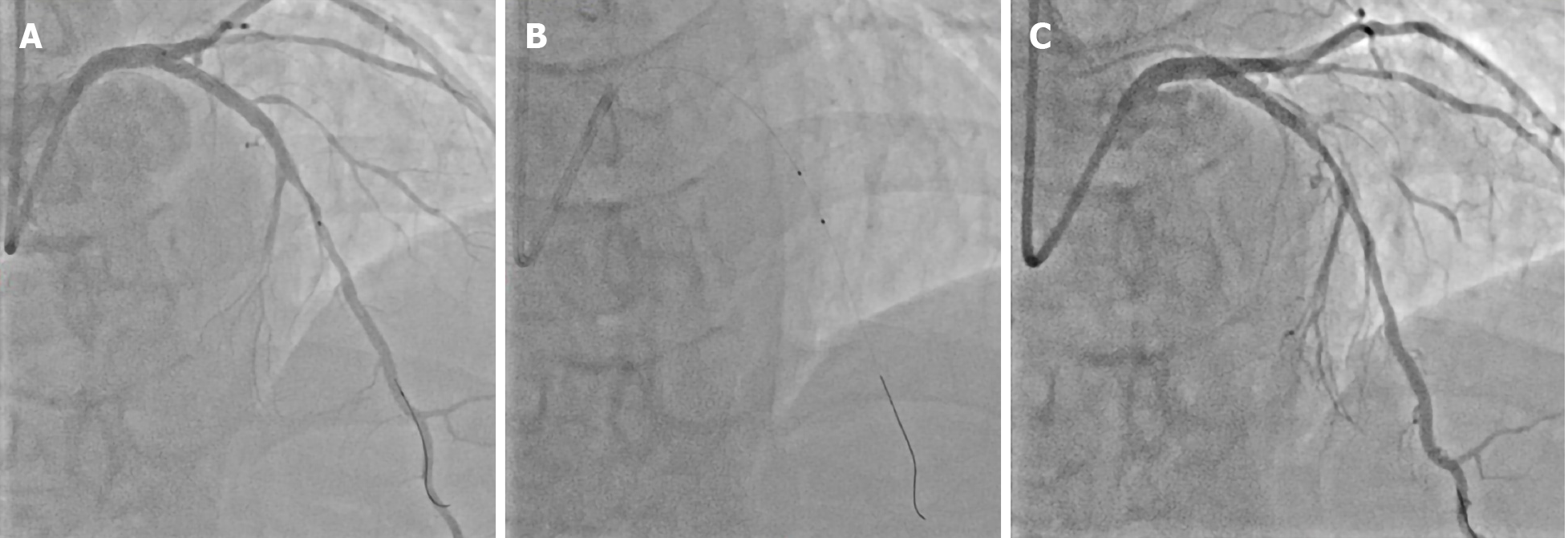
For distal EVR (Figure 2A), the patient underwent coronary angiography following balloon angioplasty (Figure 2B), which led to type B dissections in the partially absorbed MgBRS of the LAD. Coronary angiography revealed localized parallel strips and a double lumen separated by a radiolucent area during dye injection with obvious residual enhancement after imaging contrast clearance (Figure 2C).
The final diagnosis was spontaneous retrograde dissection of a partially absorbed MgBRS.
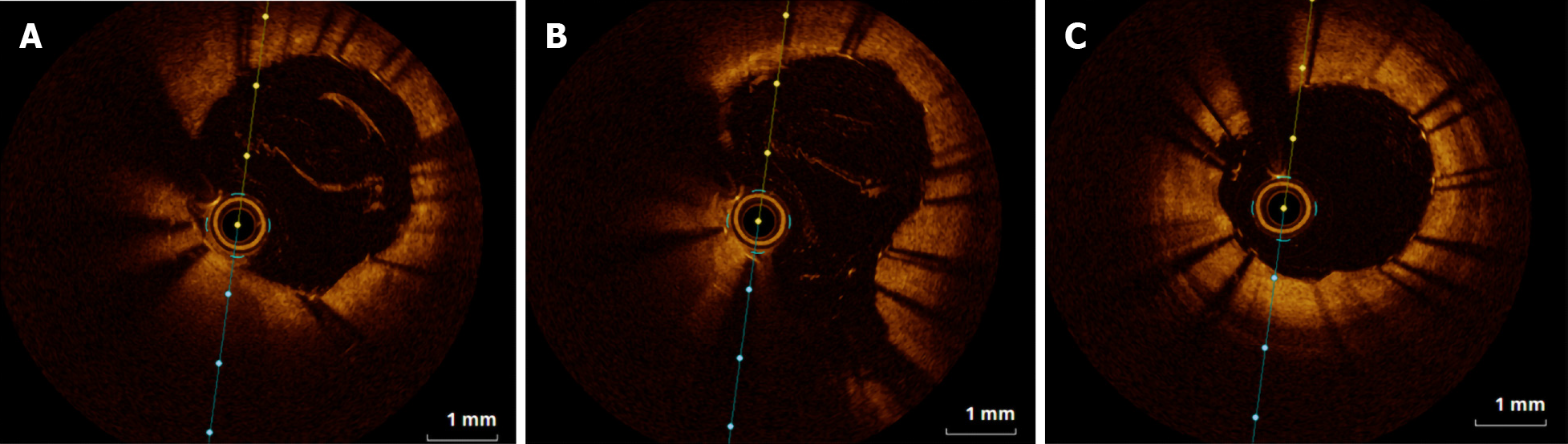
A 6F EBU SH guiding catheter (Cordis Corporation, Miami Lakes, FL, United States) was uneventfully inserted into the left coronary artery via the patient’s right radial artery. Workhorse guidewires are usually used during this procedure, including the RUNTHROUGH NS Extra Floppy (Terumo Medical Corporation, Somerset, NJ, United States). Subsequent bailout stenting was accomplished with revascularization of entry and exit sites created by spontaneous dissection and by complete sealing of the intramural hematoma (Figure 3).
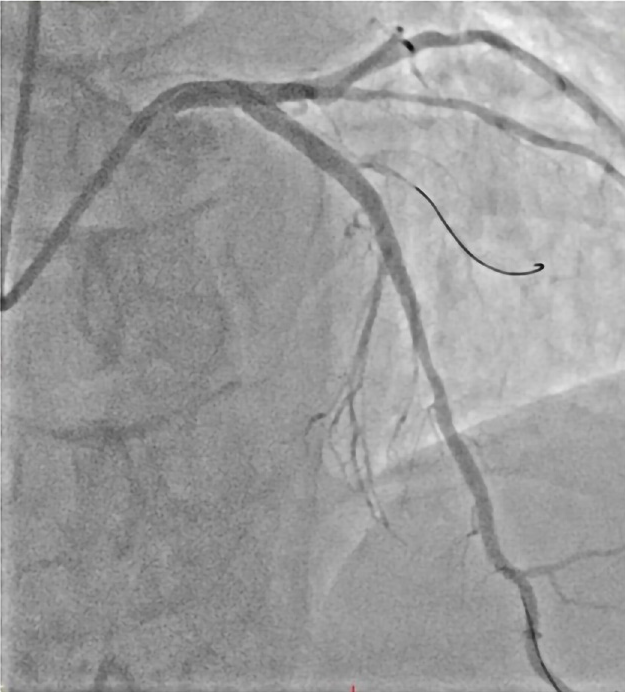
Following validation of possible complications associated with spontaneous dissection and final LAD coronary flow status, all rescue strategies and treatments were successful (Figure 4).
After the intervention treatment, oppressive retrosternal chest discomfort during procedure subsided. Mild myocardial damage was observed (Max Troponin I: 10.5093 ng/mL) without ST segment elevation in electrocardiogram interpretation, and myocardial contractility was well preserved (left ventricular ejection fraction: 74%). The patient was closely followed-up for 10 mo in the cardiovascular outpatient department with cardiac rehabilitation, and follow-up period was uneventful.
This is the first case report of a rare spontaneous dissection of a partially absorbed MgBRS, successfully treated with bailout drug-eluting intracoronary stenting. PCI is a safe and effective procedure used to improve blood flow through the coronary circulation. Procedures that improve coronary revascularization, including atherectomy and radiation, involve the use of balloon angioplasty and intracoronary stenting with DES, bare metal stents, or resorbable scaffolds (BRS)[5]. Patients with long life expectancies and simple coronary artery disease benefit more from bioresorbable scaffold technology that provides short duration vessel support (12 mo) and a drug-delivery effect, compared to DES from permanent metallic stents that pose long-term limitations[6].
Our real-world experience includes 21 consecutive subjects who underwent PCI with MgBRS at the Cardiology Department of Shin Kong Wu Ho-Su Memorial Hospital between May 2019 and August 2020. Data were collected from clinical and PCI records, which included demographic characteristics, PCI details, medical history, and in-hospital complications (Table 1).
| Baseline characteristics | n (%) |
| Age (mean ± SD) | 58.14 ± 8.60 |
| Male | 19 (90.5) |
| Hypertension | 17 (81.0) |
| Hyperlipidemia | 17 (81.0) |
| Smoking | 4 (19.0) |
| Diabetes mellitus | 7 (33.3) |
| Insulin dependent | 0 (0) |
| Non-insulin dependent | 7 (33.3) |
| History of MI | 0 (0) |
| Previous percutaneous intervention | 4 (19.0) |
| NSTEMI | 0 (0) |
Most of our patients were men, with ages ranging from 43 to 70 years. The rates of hypertension, dyslipidemia, and diabetes mellitus were 81%, 81%, and 33.3%, respectively. None of the patients had anemia or chronic kidney disease. We adhered to the implantation guidelines, including selection of de novo lesions, quantitative coronary angiography, IVUS, and/or OCT for quantitative lesion evaluation, oriented lesion preparation using cutting balloon or scoring technologies, and image-guided implantation with non-compliant balloon post-dilatation[7]. Furthermore, we also avoided MgBRS implantation with special coronary artery geometries, including left main lesions, ostial lesions, and lesions with heavy calcification, tortuosity or angulation, and diffuse long coronary arteries[8].
OCT provides a significantly superior resolution that allows precise evaluation of apposition struts, resorption, and relevant vessel wall pathology. Patients underwent post-procedural OCT or IVUS evaluation, a planned angiographic and OCT follow-up at 6 and 12 mo, if available, and scheduled clinical assessments. Double anti-platelet therapy was mandatory for at least 12 mo.
In the first-generation BRS era, the ABSORB Cohort B study used serial OCT imaging to examine the EVR and its relationship with in-scaffold vascular response (SVR) after Absorb Bioresorbable Vascular Scaffold (Abbott Vascular) implantation with a 3-year follow-up period[9]. In a previous study, Zhang et al[10] assumed that the geometric modification at the edges of the Absorb Bioresorbable Vascular Scaffold (Abbott Vascular) was not a separate pathological occurrence, but just an extension of the changes in luminal dimension revealed at the margins of the in-scaffold[10-12]. The Magmaris EVR invasive imaging analysis in the Biosolve-II trial (123 patients) sub-study was a single-arm, prospective, multi-center study that included 20 patients. In the proximal and distal EVR assessments, segment- and frame-level analysis of the 5 mm segments proximal and distal to the actual MgBRS revealed that there were no meaningful changes in intracoronary imaging, including OCT, grayscale IVUS, and virtual histology IVUS[13].
The types of coronary artery dissection, according to the NHLBI classification system, include type A and B, which are clinically benign, whereas types C-F may lead to catastrophic clinical events unless they are promptly and safely treated. Laceration of the coronary endothelium and rupture of the vasa vasorum are possible pathological mechanisms that may explain the spontaneous separation of the layers of the vascular wall[14]. Side effects of the degradation products from MgBRSs are not expected since magnesium plays a key role in many biological systems. However, no data are available regarding the possible consequences of a rare spontaneous retrograde dissection of a partially absorbed MgBRS.
In the absence of evidence-based randomized trials to analyze the outcomes of different strategies, the optimal treatment for spontaneous coronary artery dissection remains unknown. Several recent strategies have refined the outcome of spontaneous coronary artery dissection, including traditional DESs, BRSs, and drug eluting balloons[15]. Similarly, due to the lack of conventional data, expert consensus has recommended the use of biodegradable polymer sirolimus-eluting stents (Orsiro; Biotronik, Bulach, Switzerland) because its coating does not interfere with the residual magnesium alloy.
To achieve the therapeutic intention of “leave nothing behind” BRSs have been materialized to conquer the limitations of metallic drug-eluting stents. We reported a rare case, the first in Taiwan, which demonstrated successful treatment of rare spontaneous retrograde dissection of a partially absorbed MgBRS with bailout stenting of the SES. This rare case report provides new information on MgBRS gathered from precious clinical experience, review of current data, anticipation, advice, and recommendations for interventional cardiologist. It also hints the possible future perspectives on MgBRS.
We appreciate the expert comments of the reviewers and editors and the contacts they shared. We also thank for the Shin Kong Wu Ho-Su Memorial Hospital (108-SKH-FJU-02) in Taiwan.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abdel Razek AAK, Lei YC S-Editor: Gao CC L-Editor: A P-Editor: Ma YJ
| 1. | Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic' PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO. [2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and European Association for Cardio-Thoracic Surgery (EACTS)]. G Ital Cardiol (Rome). 2019;20:1S-61S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 2. | Iqbal J, Serruys PW, Silber S, Kelbaek H, Richardt G, Morel MA, Negoita M, Buszman PE, Windecker S. Comparison of zotarolimus- and everolimus-eluting coronary stents: final 5-year report of the RESOLUTE all-comers trial. Circ Cardiovasc Interv. 2015;8:e002230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 3. | Schmidt T, Abbott JD. Coronary Stents: History, Design, and Construction. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Forrestal B, Case BC, Yerasi C, Musallam A, Chezar-Azerrad C, Waksman R. Bioresorbable Scaffolds: Current Technology and Future Perspectives. Rambam Maimonides Med J. 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Rapetto C, Leoncini M. Magmaris: a new generation metallic sirolimus-eluting fully bioresorbable scaffold: present status and future perspectives. J Thorac Dis. 2017;9:S903-S913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Regazzoli D, Leone PP, Colombo A, Latib A. New generation bioresorbable scaffold technologies: an update on novel devices and clinical results. J Thorac Dis. 2017;9:S979-S985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Wlodarczak A, Garcia LAI, Karjalainen PP, Komócsi A, Pisano F, Richter S, Lanocha M, Rumoroso JR, Leung KF. Magnesium 2000 postmarket evaluation: Guideline adherence and intraprocedural performance of a sirolimus-eluting resorbable magnesium scaffold. Cardiovasc Revasc Med. 2019;20:1140-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Fajadet J, Haude M, Joner M, Koolen J, Lee M, Tölg R, Waksman R. Magmaris preliminary recommendation upon commercial launch: a consensus from the expert panel on 14 April 2016. EuroIntervention. 2016;12:828-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Serruys PW, Onuma Y, Dudek D, Smits PC, Koolen J, Chevalier B, de Bruyne B, Thuesen L, McClean D, van Geuns RJ, Windecker S, Whitbourn R, Meredith I, Dorange C, Veldhof S, Hebert KM, Sudhir K, Garcia-Garcia HM, Ormiston JA. Evaluation of the second generation of a bioresorbable everolimus-eluting vascular scaffold for the treatment of de novo coronary artery stenosis: 12-month clinical and imaging outcomes. J Am Coll Cardiol. 2011;58:1578-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 297] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 10. | Zhang YJ, Iqbal J, Nakatani S, Bourantas CV, Campos CM, Ishibashi Y, Cho YK, Veldhof S, Wang J, Onuma Y, Garcia-Garcia HM, Dudek D, van Geuns RJ, Serruys PW; ABSORB Cohort B Study Investigators. Scaffold and edge vascular response following implantation of everolimus-eluting bioresorbable vascular scaffold: a 3-year serial optical coherence tomography study. JACC Cardiovasc Interv. 2014;7:1361-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Gogas BD, Bourantas CV, Garcia-Garcia HM, Onuma Y, Muramatsu T, Farooq V, Diletti R, van Geuns RJ, De Bruyne B, Chevalier B, Thuesen L, Smits PC, Dudek D, Koolen J, Windecker S, Whitbourn R, McClean D, Dorange C, Miquel-Hebert K, Veldhof S, Rapoza R, Ormiston JA, Serruys PW. The edge vascular response following implantation of the Absorb everolimus-eluting bioresorbable vascular scaffold and the XIENCE V metallic everolimus-eluting stent. First serial follow-up assessment at six months and two years: insights from the first-in-man ABSORB Cohort B and SPIRIT II trials. EuroIntervention. 2013;9:709-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Tateishi H, Suwannasom P, Sotomi Y, Nakatani S, Ishibashi Y, Tenekecioglu E, Abdelghani M, Cavalcante R, Zeng Y, Grundeken MJ, Albuquerque FN, Veldhof S, Onuma Y, Serruys PW; investigators of the ABSORB Cohort B study. Edge Vascular Response After Resorption of the Everolimus-Eluting Bioresorbable Vascular Scaffold - A 5-Year Serial Optical Coherence Tomography Study. Circ J. 2016;80:1131-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Hideo-Kajita A, Garcia-Garcia HM, Haude M, Joner M, Koolen J, Ince H, Abizaid A, Toelg R, Lemos PA, von Birgelen C, Christiansen EH, Wijns W, Neumann FJ, Kaiser C, Eeckhout E, Teik LS, Escaned J, Azizi V, Kuku KO, Ozaki Y, Dan K, Waksman R. First Report of Edge Vascular Response at 12 Months of Magmaris, A Second-Generation Drug-Eluting Resorbable Magnesium Scaffold, Assessed by Grayscale Intravascular Ultrasound, Virtual Histology, and Optical Coherence Tomography. A Biosolve-II Trial Sub-Study. Cardiovasc Revasc Med. 2019;20:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Rogers JH, Lasala JM. Coronary artery dissection and perforation complicating percutaneous coronary intervention. J Invasive Cardiol. 2004;16:493-499. [PubMed] |
| 15. | Yang C, Alfadhel M, Saw J. Spontaneous Coronary Artery Dissection: Latest Developments and New Frontiers. Curr Atheroscler Rep. 2020;22:49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |