Published online Feb 16, 2021. doi: 10.12998/wjcc.v9.i5.1139
Peer-review started: October 26, 2020
First decision: November 3, 2020
Revised: November 12, 2020
Accepted: November 21, 2020
Article in press: November 21, 2020
Published online: February 16, 2021
Processing time: 96 Days and 2.2 Hours
Thymic epithelial carcinomas are rare and have a poor prognosis. Treatment of thymic epithelial carcinoma is multimodal and includes surgery, post-operative radiation therapy, adjuvant and neoadjuvant chemotherapy, or exclusive chemotherapy based on disease resectability. However, there is currently no standard treatment regimen for metastatic and recurrent thymic carcinoma.
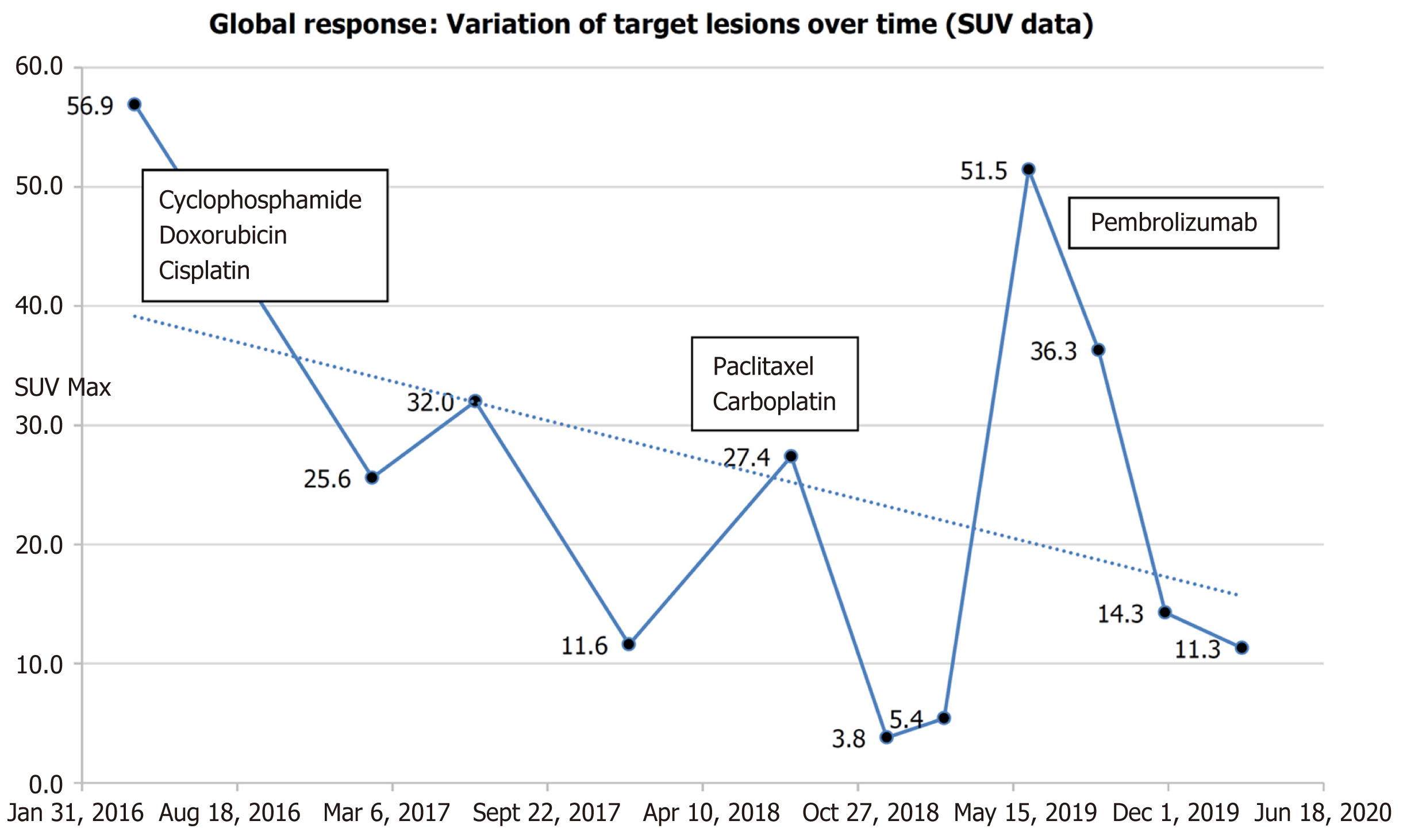
A 45-year-old Caucasian male, with no past medical history, presented with hepatalgia and a cervical mass. A computed tomography (CT) scan showed multiple suspect lesions in the lungs, liver, and anterior mediastinum associated with mediastinal and cervical adenopathy. CT-guided percutaneous biopsies of the liver lesions and anterior mediastinal mass were performed, confirming the histopathology of thymic epithelial carcinoma. Management consisted of several chemotherapy regimens and radiation therapy, administered between April 2016 and December 2018. The patient achieved complete metabolic response. Fluorodeoxyglucose positron emission tomography/CT performed in June 2019 showed disease relapse, with reappearance of a large hypermetabolic hepatic mass and involvement of mediastinal and axillary lymph nodes. Intravenous pembrolizumab (200 mg, every 3 wk) was administered after two prior systemic therapies. The patient’s response to treatment was last documented on March 5, 2020.
Pembrolizumab was successful in treatment of a patient with programmed death-ligand 1-negative metastatic thymic carcinoma, pretreated with chemotherapy.
Core Tip: Thymic epithelial carcinomas are rare and have poor prognosis. The overall 5-year survival rate for patients with thymic carcinoma is about 30%-50%. We present the case of a 45-year-old Caucasian male who presented with hepatalgia and a cervical mass, and was diagnosed with programmed death-ligand 1-negative metastatic thymic carcinoma. The patient underwent pretreatment with platinum-based chemotherapy, after which pembrolizumab was administered as salvage therapy and complete metabolic response was achieved.
- Citation: Wong-Chong J, Bernadach M, Ginzac A, Veyssière H, Durando X. Pembrolizumab as a novel therapeutic option for patients with refractory thymic epithelial tumor: A case report. World J Clin Cases 2021; 9(5): 1139-1147
- URL: https://www.wjgnet.com/2307-8960/full/v9/i5/1139.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i5.1139
Thymic epithelial carcinomas are rare and have a poor prognosis. The worldwide incidence is currently estimated to be 1.3 to 3.2 cases per million[1,2] and only 0.35 per 100000 persons-years in France[3]. Unfortunately, the overall 5-year survival rate is low as well, reportedly 30%-50%[4], and the risk factors are unknown. Epidemiologic studies have indicated that thymic carcinomas mainly occur between 40 and 60 years of age but show no sexual disparity[4,5]. The known symptoms are thoracic in nature and include chest pain, dyspnea, coughing, phrenic nerve palsy, and superior vena cava syndrome. Patients can also be asymptomatic, diagnosed incidentally on routine imaging. Paraneoplastic disorders, such as myasthenia gravis and autoimmune diseases, can be present but to an extent of association much less than with thymoma[6].
The initial assessment and staging of thymic carcinomas is carried out by computed tomography (CT) and/or magnetic resonance imaging and/or positron emission tomography (PET) scan. While these imaging modalities can differentiate thymic carcinomas from thymomas, confirmation of the entity requires histopathology. For the latter, it is recommended that both the Masaoka-Koga classification and Tumor-Node-Metastasis classification (8th edition) be used for proper staging[7]. Treatment is multimodal and includes surgery, postoperative radiation therapy, adjuvant and neoadjuvant chemotherapies, or exclusive chemotherapy based on disease resectability[7,8].
There is currently no standard treatment regimen for metastatic and recurrent thymic carcinoma. Second-line treatment options, including etoposide, ifosfamide, pemetrexed, fluorouracil, gemcitabine, and paclitaxel[9], as well as anti-vascular endothelial growth factor agents, such as sunitinib[10], and immune checkpoint inhibitors [anti-programmed cell death protein 1/programmed death-ligand 1 (PD-L1) antibodies] have provided promising results but further data are required to confirm their efficacy and safety profiles[11-13]. We report herein the successful use of the humanized antibody pembrolizumab as salvage therapy in a patient with a PD-L1-negative metastatic thymic carcinoma, following pretreatment with platinum-based chemotherapy.
A 45-year-old Caucasian male presented with hepatalgia and a cervical mass. CT scan at admission showed multiple suspect lesions in the lungs, liver, and anterior mediastinum associated with mediastinal and cervical adenopathy.
The patient had three episodes of severe right hypochondrium pain.
The patient’s medical history was unremarkable.
The patient’s family history was unremarkable.
Cardiovascular, abdominal, urinary, hematological and neurological examinations were performed. The cardiovascular, neurological and urinary examinations were normal. The patient presented sensitivity to hepatic palpation and various cervical adenopathies were found.
Our histopathology sample was negative for nuclear protein in testis (NUT) protein staining, and no NUT gene rearrangement was found by fluorescence in situ hybridization. Thus, the patient was not diagnosed with NUT midline carcinoma[14]. PD-L1 assessment was not performed on the histological sample, but it was performed on biopsies at diagnosis (see below) and the PD-L1 status was negative (PD-L1 = 0%). Tumor mutational burden (TMB) was not assessed.
CT-guided percutaneous biopsies were obtained from the liver lesions and anterior mediastinal mass detected in the initial CT scan. Subsequent histopathological analysis demonstrated epidermal differentiation, suggestive of thymus carcinoma, and thus confirmed the biopsied tissues to be thymic epithelial carcinoma. Follow-up was performed by fluorodeoxyglucose (FDG)-PET scans.
Thymic epithelial carcinoma.
The patient was administered combination chemotherapy with intravenous cyclophosphamide (500 mg/m2), doxorubicin (50 mg/m2), and cisplatin (50 mg/m2) every 3 wk[15] for six cycles (April to September 2016). CT scan and FDG-PET scan showed a partial morpho-metabolic response after the six cycles, with complete regression of the pulmonary and hepatic hypermetabolic foci but persistent uptake in mediastinal and cervical lymph nodes.
Disease recurrence occurred after 9 mo of surveillance (September 2016 to June 2017), with the appearance of a new mediastinal lymphadenopathy and a single liver metastasis. Second-line platinum-based chemotherapy regimen, consisting of paclitaxel (225 mg/m²) and carboplatin (area under the curve of 6 mg/mL), was administered every 3 wk[16] for six cycles (August to November 2017).
FDG-PET/CT performed in November 2017 assessed dissociated responses and showed complete metabolic response of the cervical and mediastinal lymph-adenopathy and the liver metastasis but appearance of a previously undetected right upper latero-cervical hypermetabolic lymphadenopathy. Thus, radiation therapy of the hypermetabolic lymph node with subsequent platinum-based chemotherapy by paclitaxel was planned.
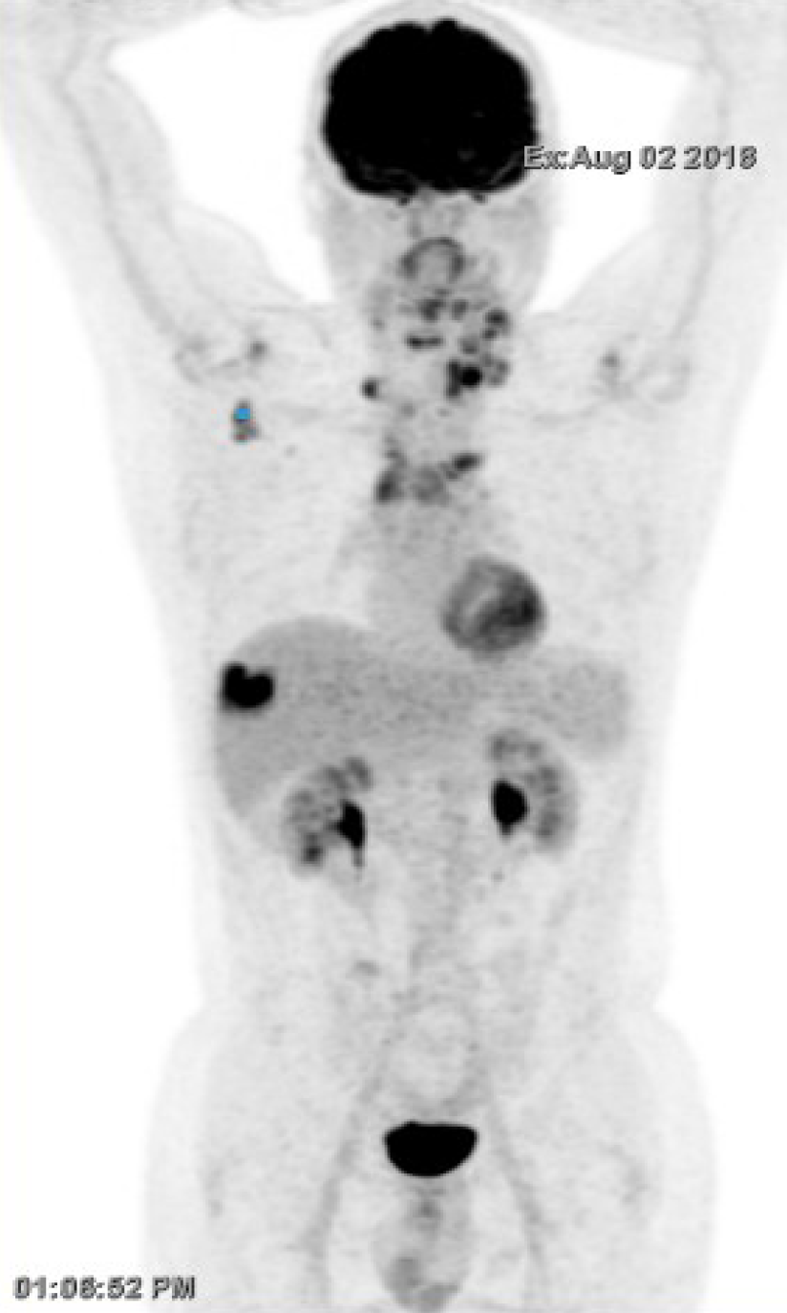
The patient discontinued treatment and presented to the medical oncology unit after 6 mo in July 2018, with complaint of dyspnea due to enlargement of the left cervical lymph nodes. Re-evaluation by FDG-PET/CT confirmed disease recurrence on the anterior mediastinal mass, superior and inferior diaphragmatic lymph nodes, and single hepatic mass (located between the sixth and seventh hepatic segment). The lungs were clear of any lesion (Figure 1). Radiation therapy (30 Gy in 10 fractions of 3 Gy per fraction) was performed urgently on the left cervical lymph nodes with acceptable toxicity in August 2018. Weekly administration of paclitaxel-carboplatin chemotherapy was resumed on August 29, 2018 (1 wk after radiotherapy).
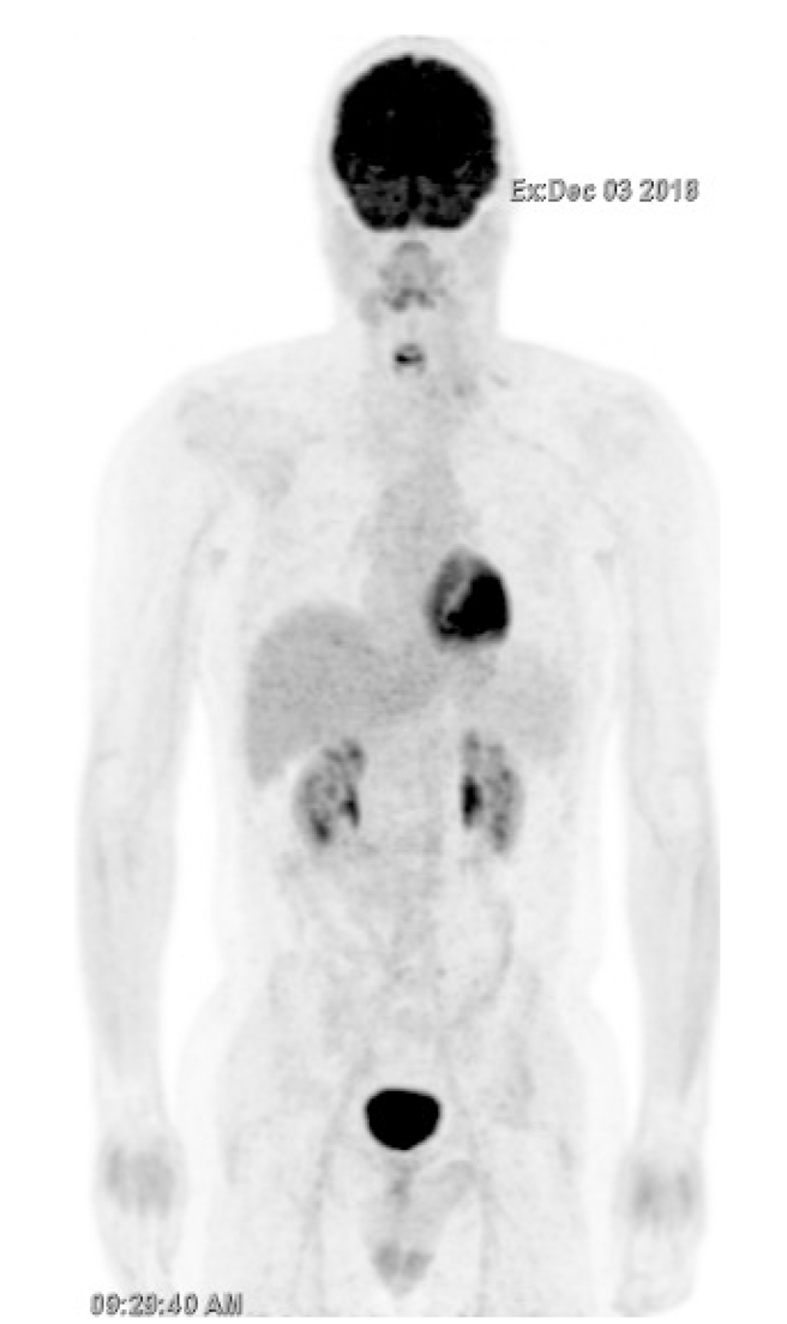
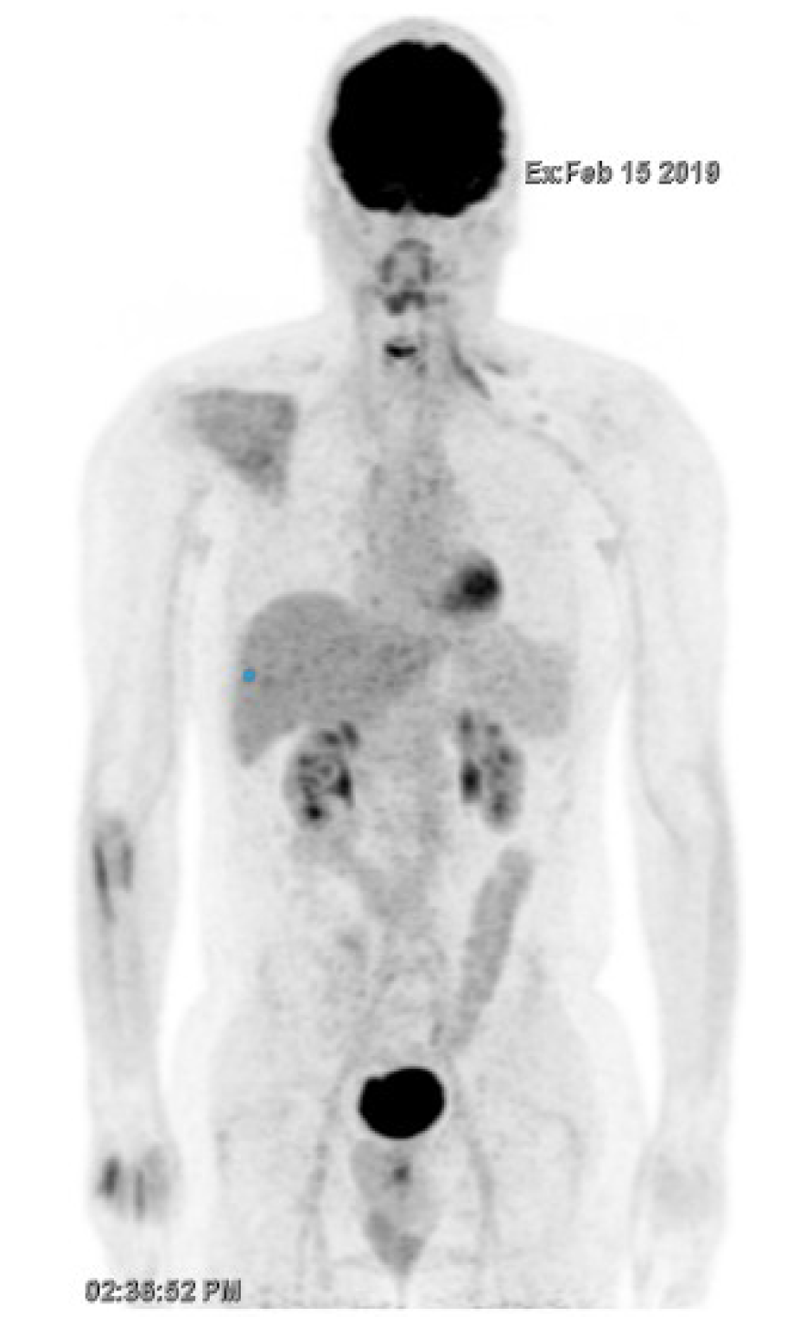
FDG-PET/CT performed in December 2018 showed a durable complete metabolic response after three cycles (Figure 2). Severe grade III peripheral neuropathy led to the discontinuation of both paclitaxel and carboplatin. FDG-PET/CT performed in February 2019 confirmed a complete metabolic response (Figure 3).
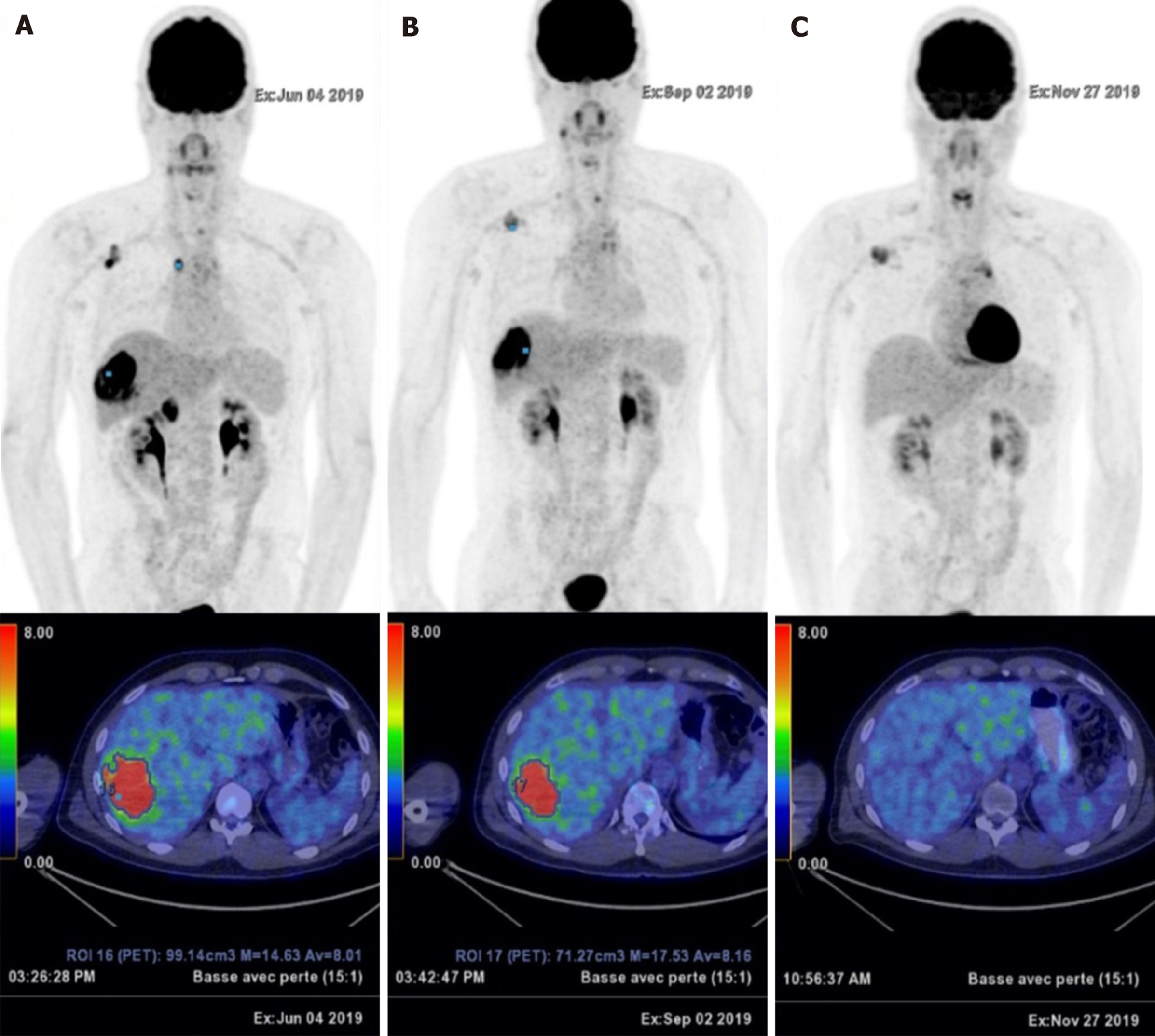
FDG PET/CT performed in June 2019 showed disease relapse with the reappearance of several hypermetabolic lesions (Figure 4A). The patient then received intravenous pembrolizumab (200 mg, every 3 wk)[11] after two prior systemic therapies. A dissociated response was obtained after four cycles of pembrolizumab, with a significant increase in FDG uptake of the single liver metastasis. Complete metabolic responses were achieved on some cervical lymph nodes, while other responses were hypermetabolic (Figure 4B). After four additional cycles, a complete metabolic response was observed on the hepatic mass and cervical lymphadenopathy, with stability of metabolism of the superior clavicular and right axillary lymph nodes (Figure 4C) (Figure 5).
The response to treatment was last documented on March 5, 2020. The patient experienced symptoms of fatigue, sleepiness, and residual chemotherapy-induced sensory neuropathy. No immune-related adverse events (irAEs), and notably no thyroiditis, hepatitis, or paraneoplastic syndrome occurred.
High expression of PD-L1 (> 50%) is known to provide a better response to immune checkpoint inhibitors than low or no PD-L1 expression in thymic carcinoma[5]. The successful use of pembrolizumab, a checkpoint inhibitor targeting PD-L1, was described in two open-label single-arm phase II trials[11,12] and case reports[13,17]. Nivolumab, another checkpoint inhibitor that blocks PD-L1, is routinely used as salvage therapy to treat refractory thymic epithelial carcinoma[18-20]. In a post-hoc analysis on patients treated by pembrolizumab, Giaccone et al[11] showed that progression-free survival was longer in patients with high PD-L1 expression (PD-L1 > 50%) than in those with low (PD-L1 1%-49%) or no expression (median survival: 24 mo vs 2.9 mo). Overall survival was also longer in patients with high expression than in those with low or no expression [median survival (50% of patients still alive at end of study): Not reached vs 15.5 mo].
Regarding irAEs, Cho et al[12] discouraged the use of immune checkpoint inhibitors in patients with thymoma and/or history of autoimmunity. Fatal AEs have been reported in that population. Most patients with thymoma (71.4%, 7 of 11 patients) presented with grade 3 or 4 irAEs such as myocarditis, hepatitis, myasthenia gravis, thyroiditis, colitis, conjunctivitis, and nephritis. By contrast, only 15.4% (4 of 26 patients) with thymic carcinoma report grade 3 or 4 irAEs including hepatitis, myasthenia gravis, and subacute myoclonus. Most patients who discontinue treatment due to irAEs recover. Some patients receive a combination of high-dose corticosteroids and immunosuppressive agents[15]. Reported cases of polymyositis and myocarditis are managed by high-dose corticosteroids but also require the placement of pacemakers[5]. Thus, the management of grade 3 or 4 irAES is challenging and should involve multidisciplinary teams.
The patient in our case report had a very good response to immunotherapy despite the absence of PD-L1 expression. PD-L1 is highly expressed in normal thymic epithelial cells. PD-L1 immunostaining is typically performed on liver biopsies to avoid confounders of that predictive biomarker, but this is not the standard practice for histopathological analysis of thymus biopsies[20]. Thus, the biopsies were performed at diagnosis, prior to the initiation of chemotherapy. As mentioned above, the PD-L1 status was negative (PD-L1 = 0%). However, PD-L1 immunostaining is an imperfect predictor of response, and it should not be used as a definitive biomarker for the selection of immunotherapy[21]. First, false negatives may arise when biopsy material is insufficient or archived, as the expressed protein can degrade over time. Second, PD-L1 expression may be focally heterogeneous at the target sites and can be missed if the biopsy is too small[22]. It can also vary according to anatomical site in an individual[23]. Third, prior platinum-based chemotherapy may exert immunomodulatory effects on the tumor microenvironment, leading to the up-regulation of PD-L1 expression in tumor cells[24], which would justify repeating biopsy collection and analysis before each new line of therapy is initiated. It is important to note that in our case, no repeat biopsy was performed to confirm those hypotheses. Finally, the scoring method for evaluating PD-L1 immunostaining is subjective and lacks standardization, with the use of multiple antibodies that may not be validated[25] and the use of variable thresholds for positivity[26]. However, a durable objective response has been reported in epithelial carcinoma and other solid tumors, regardless of PD-L1 status[26,27].
Other biomarkers, such as the combined positive score (CPS), are currently being evaluated[28]. CPS takes into account tumor-infiltrating immune cells, which represent a positive prognostic feature[21] and may be more relevant clinically and more reproducible. Immune pathways are complex and still need to be understood, including the clinical relevance of PD-L2- in PD-L1-negative status of patients[21,29].
We report herein the successful use of pembrolizumab in a patient with PD-L1-negative metastatic thymic carcinoma, pretreated with platin-based chemotherapy. Additional clinical trials are required to evaluate the role of immunotherapy in a first-line setting when considering the promising results and excellent tolerance of immunotherapy as second-line treatment. Research is also needed to develop more reliable predictive biomarkers and better understand immune pathways and their implications for cancer immunotherapy.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tovoli F S-Editor: Fan JR L-Editor: A P-Editor: Zhang YL
| 1. | de Jong WK, Blaauwgeers JL, Schaapveld M, Timens W, Klinkenberg TJ, Groen HJ. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer. 2008;44:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 209] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 2. | Siesling S, van der Zwan JM, Izarzugaza I, Jaal J, Treasure T, Foschi R, Ricardi U, Groen H, Tavilla A, Ardanaz E; RARECARE Working Group. Rare thoracic cancers, including peritoneum mesothelioma. Eur J Cancer. 2012;48:949-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 3. | Bluthgen M, Dansin E, Kerjouan M, Mazieres J, Pichon E, Thillays F, Massard G, Quantin X, Oulkhouir Y, Westeel V, Thiberville L, Clément-Duchêne C, Thomas PA, Girard N, Besse B. P2.04-006 Updated Incidence of Thymic Epithelial Tumors (TET) in France and Clinical Presentation at Diagnosis: Topic: Thymic Malignancies Clinical and Translational. J Thorac Oncol. 2017;12:S1000. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Rieker RJ, Muley T, Klein C, Schnabel PA, Herpel E, Meister M, Schirmacher P, Dienemann H, Pfannschmidt J. An institutional study on thymomas and thymic carcinomas: experience in 77 patients. Thorac Cardiovasc Surg. 2008;56:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Rieker RJ, Hoegel J, Morresi-Hauf A, Hofmann WJ, Blaeker H, Penzel R, Otto HF. Histologic classification of thymic epithelial tumors: comparison of established classification schemes. Int J Cancer. 2002;98:900-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | RESERVES IU--TD. Orphanet: Carcinome thymique [Internet]. [cited 2020 Feb 11]; Available from: https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=FR&Expert=99868. |
| 7. | Referentiel RYTHMIC [Internet]. [cited 2020 Feb 11]; Available from: https://www.rythmic.org/images/RYTHMIC/Doc/referentiel_RYTHMIC_2020.pdf. |
| 8. | Berghmans T, Durieux V, Holbrechts S, Jungels C, Lafitte JJ, Meert AP, Moretti L, Ocak S, Roelandts M, Girard N. Systemic treatments for thymoma and thymic carcinoma: A systematic review. Lung Cancer. 2018;126:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 9. | Tateishi K, Ko R, Shukuya T, Okuma Y, Watanabe S, Kuyama S, Murase K, Tsukita Y, Ashinuma H, Nakagawa T, Uematsu K, Nakao M, Mori Y, Kaira K, Mouri A, Miyabayashi T, Sakashita H, Matsumoto Y, Tanigawa T, Koizumi T, Morita S, Kobayashi K, Nukiwa T, Takahashi K; North East Japan Study Group. Clinical Outcomes of Second-Line Chemotherapy in Patients with Previously Treated Advanced Thymic Carcinoma: A Retrospective Analysis of 191 Patients from the NEJ023 Study. Oncologist. 2020;25:e668-e674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Thomas A, Rajan A, Berman A, Tomita Y, Brzezniak C, Lee MJ, Lee S, Ling A, Spittler AJ, Carter CA, Guha U, Wang Y, Szabo E, Meltzer P, Steinberg SM, Trepel JB, Loehrer PJ, Giaccone G. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol. 2015;16:177-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 222] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 11. | Giaccone G, Kim C, Thompson J, McGuire C, Kallakury B, Chahine JJ, Manning M, Mogg R, Blumenschein WM, Tan MT, Subramaniam DS, Liu SV, Kaplan IM, McCutcheon JN. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol. 2018;19:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 285] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 12. | Cho J, Kim HS, Ku BM, Choi YL, Cristescu R, Han J, Sun JM, Lee SH, Ahn JS, Park K, Ahn MJ. Pembrolizumab for Patients With Refractory or Relapsed Thymic Epithelial Tumor: An Open-Label Phase II Trial. J Clin Oncol. 2019;37:2162-2170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 207] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 13. | Isshiki T, Isobe K, Tochigi N, Sunakawa M, Nakamura Y, Shibuya K, Sakamoto S, Takai Y, Homma S. Successful Use of Pembrolizumab to Treat Refractory Thymic Carcinoma with High PD-L1 Expression. Case Rep Oncol. 2018;11:688-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | French CA. NUT Carcinoma: Clinicopathologic features, pathogenesis, and treatment. Pathol Int. 2018;68:583-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 15. | Loehrer PJ Sr, Kim K, Aisner SC, Livingston R, Einhorn LH, Johnson D, Blum R. Cisplatin plus doxorubicin plus cyclophosphamide in metastatic or recurrent thymoma: final results of an intergroup trial. The Eastern Cooperative Oncology Group, Southwest Oncology Group, and Southeastern Cancer Study Group. J Clin Oncol. 1994;12:1164-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 197] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 16. | Lemma GL, Lee JW, Aisner SC, Langer CJ, Tester WJ, Johnson DH, Loehrer PJ Sr. Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol. 2011;29:2060-2065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 17. | Zander T, Aebi S, Rast AC, Zander A, Winterhalder R, Brand C, Diebold J, Gautschi O. Response to Pembrolizumab in a Patient with Relapsing Thymoma. J Thorac Oncol. 2016;11:e147-e149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Yang PC, Guo JC, Hsieh MS, Lin CC, Hsu CH. Response to Nivolumab as Salvage Therapy in a Patient with Thymic Carcinoma. J Thorac Oncol. 2018;13:e36-e39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Konstantina T, Konstantinos R, Anastasios K, Anastasia M, Eleni L, Ioannis S, Sofia A, Dimitris M. Fatal adverse events in two thymoma patients treated with anti-PD-1 immune check point inhibitor and literature review. Lung Cancer. 2019;135:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Girard N. Thymic malignancies: Twisting between autoimmunity and immunotherapy. Lung Cancer. 2017;110:68-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1659] [Cited by in RCA: 2093] [Article Influence: 232.6] [Reference Citation Analysis (0)] |
| 22. | Padda SK, Riess JW, Schwartz EJ, Tian L, Kohrt HE, Neal JW, West RB, Wakelee HA. Diffuse high intensity PD-L1 staining in thymic epithelial tumors. J Thorac Oncol. 2015;10:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 23. | Jiménez-Sánchez A, Memon D, Pourpe S, Veeraraghavan H, Li Y, Vargas HA, Gill MB, Park KJ, Zivanovic O, Konner J, Ricca J, Zamarin D, Walther T, Aghajanian C, Wolchok JD, Sala E, Merghoub T, Snyder A, Miller ML. Heterogeneous Tumor-Immune Microenvironments among Differentially Growing Metastases in an Ovarian Cancer Patient. Cell 2017; 170: 927-938. e20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 369] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 24. | Katsuya Y, Horinouchi H, Asao T, Kitahara S, Goto Y, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Watanabe S, Tsuta K, Ohe Y. Expression of programmed death 1 (PD-1) and its ligand (PD-L1) in thymic epithelial tumors: Impact on treatment efficacy and alteration in expression after chemotherapy. Lung Cancer. 2016;99:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Rimm D, Schalper K, Pusztai L. Unvalidated antibodies and misleading results. Breast Cancer Res Treat. 2014;147:457-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Sunshine J, Taube JM. PD-1/PD-L1 inhibitors. Curr Opin Pharmacol. 2015;23:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 443] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 27. | Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington KJ, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Docampo LCI, Haddad R, Rordorf T, Kiyota N, Tahara M, Lynch M, Jayaprakash V, Li L, Gillison ML. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 574] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 28. | Kulangara K, Hanks DA, Waldroup S, Peltz L, Shah S, Roach C, Juco JW, Emancipator K, Stanforth D. Development of the combined positive score (CPS) for the evaluation of PD-L1 in solid tumors with the immunohistochemistry assay PD-L1 IHC 22C3 pharmDx. J Clin Oncol. 2017;35:e14589-e14589. [RCA] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Yearley JH, Gibson C, Yu N, Moon C, Murphy E, Juco J, Lunceford J, Cheng J, Chow LQM, Seiwert TY, Handa M, Tomassini JE, McClanahan T. PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer. Clin Cancer Res. 2017;23:3158-3167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 447] [Article Influence: 63.9] [Reference Citation Analysis (0)] |