Published online Feb 16, 2021. doi: 10.12998/wjcc.v9.i5.1111
First decision: November 3, 2020
Revised: November 14, 2020
Accepted: December 6, 2020
Article in press: December 6, 2020
Published online: February 16, 2021
Processing time: 154 Days and 8.4 Hours
Fibrous dysplasia (FD) is a common benign intramedullary fibro-osseous lesion. Involvement of the spine is rare, with the literature including only case reports, and cases of monostotic FD (MFD) in the sacrum are extremely rare. A correct preoperative diagnosis of spinal MFD is important for clinicians to select proper treatment.
We retrospectively assessed a case report of MFD in the sacrum. This patient was examined by computed tomography (CT) and magnetic resonance imaging (MRI), and the diagnosis was confirmed by pathology. A review of the literature was performed to analyze the imaging characteristics and differential diagnoses of spinal MFD. For our patient, the CT scan showed the lesion to be expansile, with ground glass opacity and a sclerotic rim. On MRI, the lesion showed iso-low signal intensity on T1WI and iso-high signal intensity on T2WI. A low signal rim was found on T1WI and T2WI. Our patient was treated by posterior focal excision, decompression, bone grafting, fusion and pedicle screw fixation. A satisfactory result was achieved, with pain disappearance. No complications had occurred at the 1-year follow up.
MFD is an expansile osteolytic change. Ground glass opacity and a sclerotic margin are obvious characteristics. The lesion often involves the vertebral body and posterior element. Knowledge of these imaging characteristics of spinal FD could be helpful for diagnosis and prevent unnecessary procedures.
Core Tip: This report presents a rare case of monostotic fibrous dysplasia (MFD) involving the sacrum. The imaging manifestations of MFD include expansile lesions, ground glass opacity, and sclerotic rims. Most lesions show iso-low signal intensity on T1WI and iso-high signal intensity on T2WI. These features can provide a suggestive diagnosis to distinguish MFD from giant cell tumour, aneurysmal bone cyst, and vertebral haemangioma. Accurate diagnosis of MFD in the spine is of great value for clinicians to choose an appropriate treatment.
- Citation: Liu XX, Xin X, Yan YH, Ma XW. Imaging characteristics of a rare case of monostotic fibrous dysplasia of the sacrum: A case report. World J Clin Cases 2021; 9(5): 1111-1118
- URL: https://www.wjgnet.com/2307-8960/full/v9/i5/1111.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i5.1111
Fibrous dysplasia (FD) is a benign intramedullary fibro-osseous lesion originally described by Lichtenstein in 1938[1]. FD is most commonly found in the long bones[2], and it is rare in the spine and extremely rare in the sacrum[3]. In the spine, FD is found either in the monostotic or polyostotic form, but monostotic FD (MFD) is rare[4]. According to our literature search, this is the seventh case reported in the sacrum. Kinnunen et al[5] reviewed the literature and collected 136 cases of FD confirmed by pathology, including all parts of the body, in which the incidence in a vertebral body was 24/136 (18%). The proportions of vertebral body FD were 17% in the cervical region, 29% in the thoracic region, 29% in the lumbar region, and only 8% in the sacral region (2/24). Firat et al[6] reviewed a single case of MFD in the sacrum, and Schoenfeld et al[7] described three patients with monostotic disease of the sacrum.
Treatment of spinal MFD depends on the clinical symptoms. Asymptomatic patients with stable lesions can undergo clinical observation. Patients with severe pain can undergo surgery. A correct diagnosis of spinal MFD is important for the clinician to select an appropriate treatment. Therefore, in this rare case report of MFD in the sacrum, we reviewed the literature to analyze the clinical, radiographic, and pathologic features of spinal MFD.
A 60-year-old woman suffered from low back pain for more than 2 years and aggravating radiating pain and numbness in both lower limbs for more than 2 months. The study was approved by the institutional review board of the hospital, and the patient signed an informed consent form.
Two years prior, the patient had low back pain and discomfort with no obvious cause that could be relieved after rest. The patient paid no attention to the pain, and no further examination or treatment was performed. Then, the symptoms recurred, which were sometimes mild and sometimes severe. In the previous two months, the patient had experienced radiating pain of both lower limbs and numbness on the posterolateral side of the leg, especially on the right side. After rest, the symptoms were partially relieved, and then the pain gradually aggravated and numbness occurred.
The patient denied any history of hepatitis, tuberculosis, malaria, hypertension, heart disease, diabetes, cerebrovascular disease, mental illness, surgery, trauma, blood transfusion, or food or drug allergy.
The patient had no history of contact with an epidemic area, situation or contaminated water; no residential history in a pastoral, mining, high fluoride or low iodine area; no contact history of chemical, radioactive or toxic substances; and no history of drug abuse, smoking, drinking, or recreational tourism.
Tenderness and percussion pain were observed in the L4-S1 spinal space, and the pain radiated to the right lower limb, buttocks, posterolateral areas of the upper and lower legs, and right foot including the toes. The lumbar range of motion was limited. The straight leg raising test was positive at 40° in the right lower limb and negative in the left lower limb. The temperature and tactile sensation of lateral pain were decreased in the right leg. No skin lesion or soft-tissue mass was noted.
Laboratory tests included examinations of liver and kidney function, electrolytes, tumour markers and coagulation and routine blood tests. Among them, the glutamic oxaloacetic transaminase level was 11.6 U/L (reference range 13-35 U/L), the total protein level was 57.5 g/L (reference range 65-85 g/L), the albumin level was 36.4 g/L (reference range 40-55 g/L), the urea level was 7.7 mmol/L (reference range 2.6-7.5 mmol/L), and the chlorine level was 108.4 mmol/L (reference range 96-108 mmol/L). The tumour markers were all in the normal range. Routine blood tests were normal.
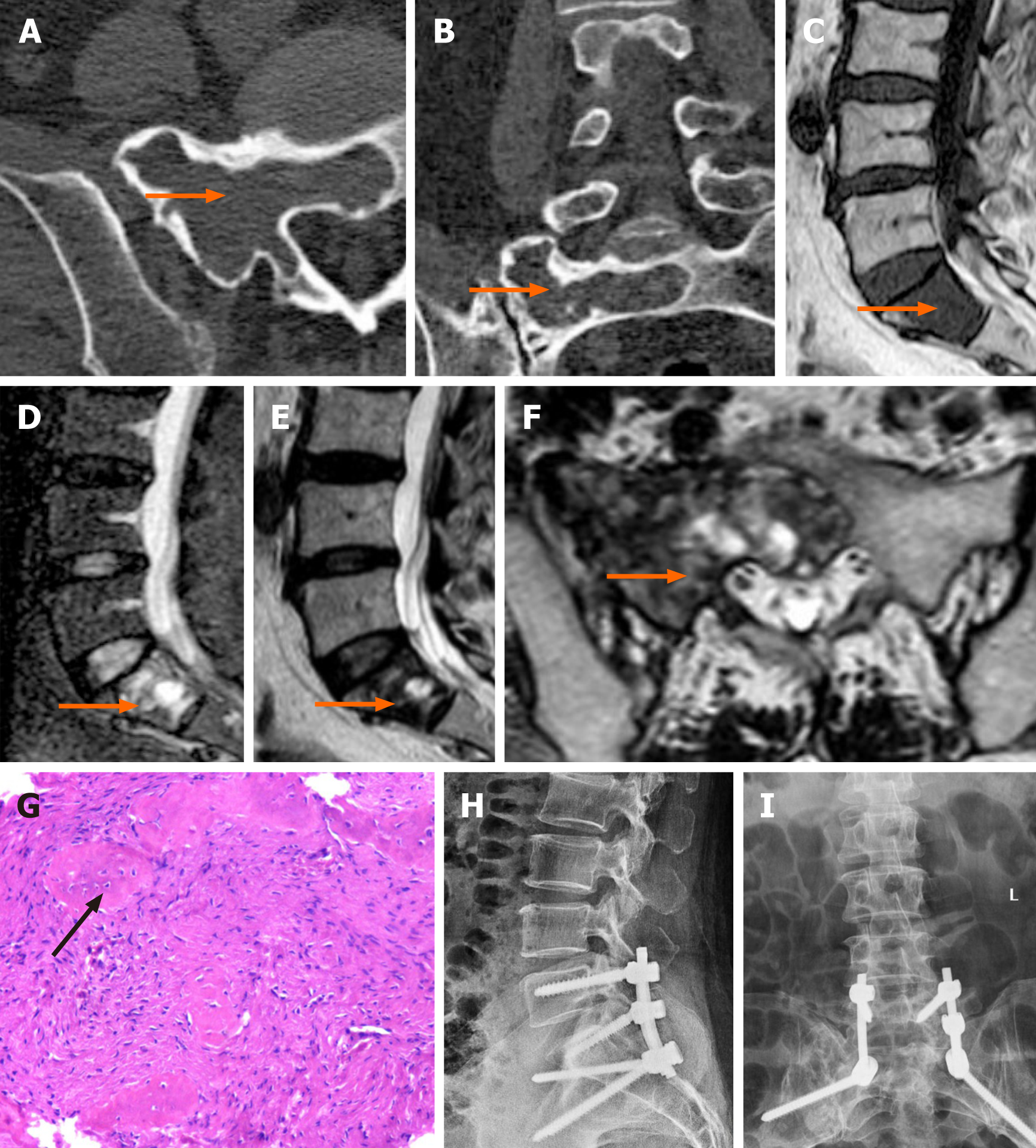
The patient did not undergo X-ray examination before the operation. Imaging studies included computed tomography (CT) and magnetic resonance imaging (MRI), which demonstrated an expansile lesion involving both the anterior and the posterior elements of the vertebral body and no involvement of the surrounding structures. The lesion exhibited ground glass opacity with a high-density sclerotic rim on CT and presented a mixed signal intensity on MRI (Figure 1A-F).
The patient underwent surgery, and the lesion in the sacral vertebra was fully removed along the S1 pedicle channel and sent for pathological examination. Pathological haematoxylin and eosin (HE) staining results supported the diagnosis of FD (Figure 1G).
Posterior debridement, iliac bone grafting and internal fixation were adopted. The patient underwent an X-ray examination at three days after the operation (Figure 1H and I).
Currently, neither clinical symptoms nor signs of tumour recurrence have been detected during a follow-up period of more than 18 months.
According the World Health Organization's (WHO) 2020 version of the bone tumour classification, FD is classified as a benign tumour in the category of other mesenchymal tumours of bone. It is a common non-malignant fibro-osseous lesion, accounting for 7% of benign bone tumours[2]. MFD occurs more frequently (70%-80%) than polyostotic FD (20%-30%) or McCune-Albright syndrome, which is a variable condition with endocrine and cutaneous abnormalities (3%)[8]. The occurrence of FD in the spine is rare, but among these cases, occurrence in the cervical and thoracic regions is relatively more common. Reports that describe FD in the sacrum are rare. Based on a literature review[9-11], the demographic details of spinal MFD patients are summarized in Table 1.
| Cases | Percentage (%) | |
| Sex | ||
| Male | 24 | 60 |
| Female | 16 | 40 |
| Age (y) | ||
| Range (mean ± SD) | 11-77 (37.85 ± 15.84) | |
| Presentation | ||
| Posttraumatic | 3 | 7.5 |
| Pain | 19 | 47.5 |
| Pain and radiculopathy | 7 | 17.5 |
| Incidental finding | 5 | 12.5 |
| Other | 6 | 15 |
At present, the exact aetiology of MFD is unknown. A sporadic activating mutation of Gs alpha on chromosome 20q13.2-13.3 has been reported to be associated with the occurrence of FD[12], which leads to increased utilization of cyclic adenosine monophosphate[13].
The peak incidence of spinal MFD is in the third to fifth decade of life, and there is no significant gender difference[7,14]. MFD develops during skeletal development. However, clinical manifestations depend on the site of the lesion[13,15]. Pain is usually proportional to the degree of vertebral involvement[16].
MFD is characterized by the replacement of bone marrow with poorly organized spicules of immature bone[10,15]. Insufficient bone mineralization results in loss of mechanical strength, which may result in skeletal deformity and vertebral collapse.
A typical CT characteristic is “ground glass opacity”[16]. The ground glass opacity appearance is due to the presence of many irregular spicules of bone within the fibrous stroma[17]. Furthermore, expansile and lytic changes are common features. A sclerotic rim is observed around the lesion, and the cortical bone is thin with no disruption.
MRI is useful for evaluating the entire extent of the lesion and the soft-tissue components. The MRI characteristics of FD are variable; typically, imaging results demonstrate hypo-intensity with several iso-intense regions on T1WI and iso-intensity with several hyperintense regions on T2WI. The heterogeneous signal may be related to the trabecular bone, collagen fibres, cystic changes, and haemorrhage in the lesion. MFD may be surrounded by a thick, sclerotic rim, called a rind[18]. The rind can be seen as hypo-intense on T1WI and T2WI. No obvious soft-tissue involvement is observed.
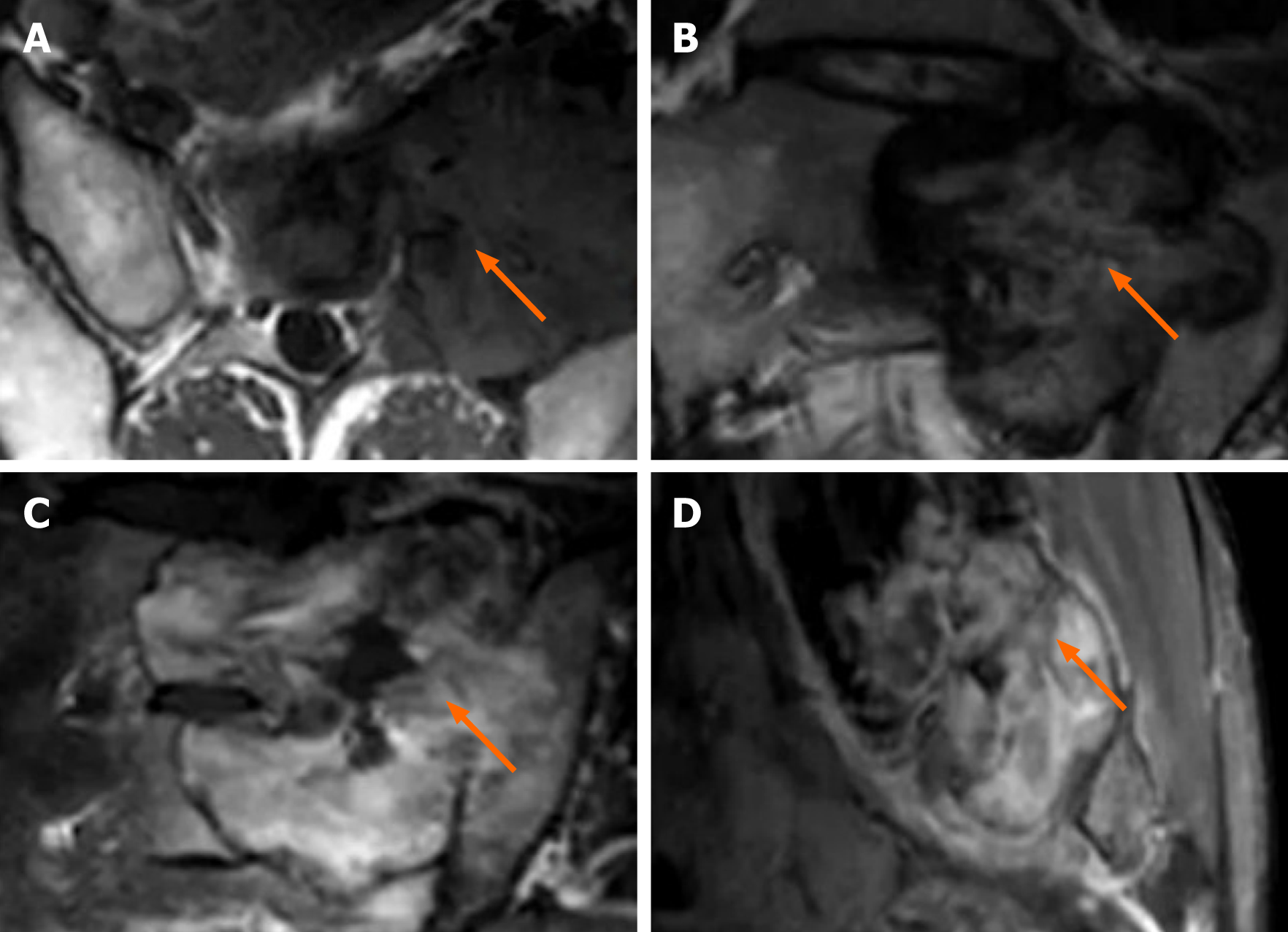
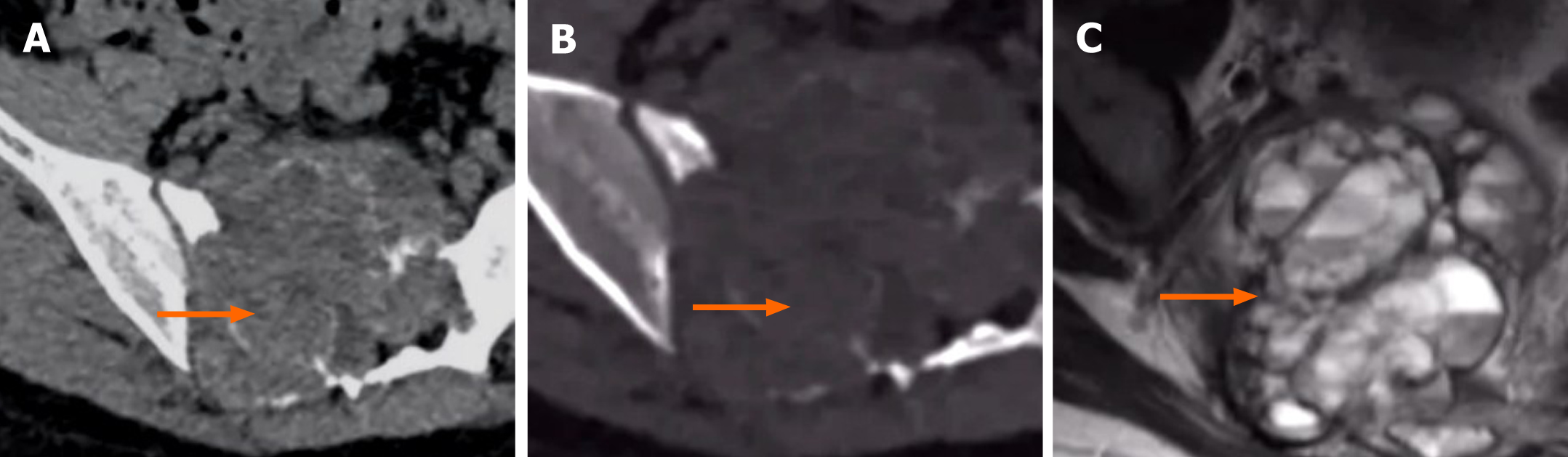
The differential diagnosis of spinal MFD should include benign and intermediate (locally aggressive) tumours, such as giant cell tumour (GCT), vertebral haemangioma (VH), and aneurysmal bone cyst (ABC)[4,8,17]. GCTs commonly occur in the first and second sacral vertebrae in patients 20-40 years of age. A GCT is an invasive intermediate tumour with swelling and irregular changes. Multiple vacuoles are visible, the surrounding sclerotic rim is not obvious, and the cortical bone is partially interrupted (Figure 2)[19,20]. An ABC is classified as a tumour with an intermediate, undefined, neoplastic nature[8]. It is common in patients younger than 20 years old. It involves expansive bone destruction, thinning of the cortical bone, and partial cortical interruption. The presence of a “fluid-fluid” level on MRI is characteristic[21], and the hardening margin is visible (Figure 3). A typical sign of haemangioma is the “corduroy cloth” manifestation[7]. Large trabecular bone can be seen on CT and MRI, which is usually non-expandable and has a circular-like shape. It exhibits high or low signal intensity on T1WI and high signal intensity on T2WI.
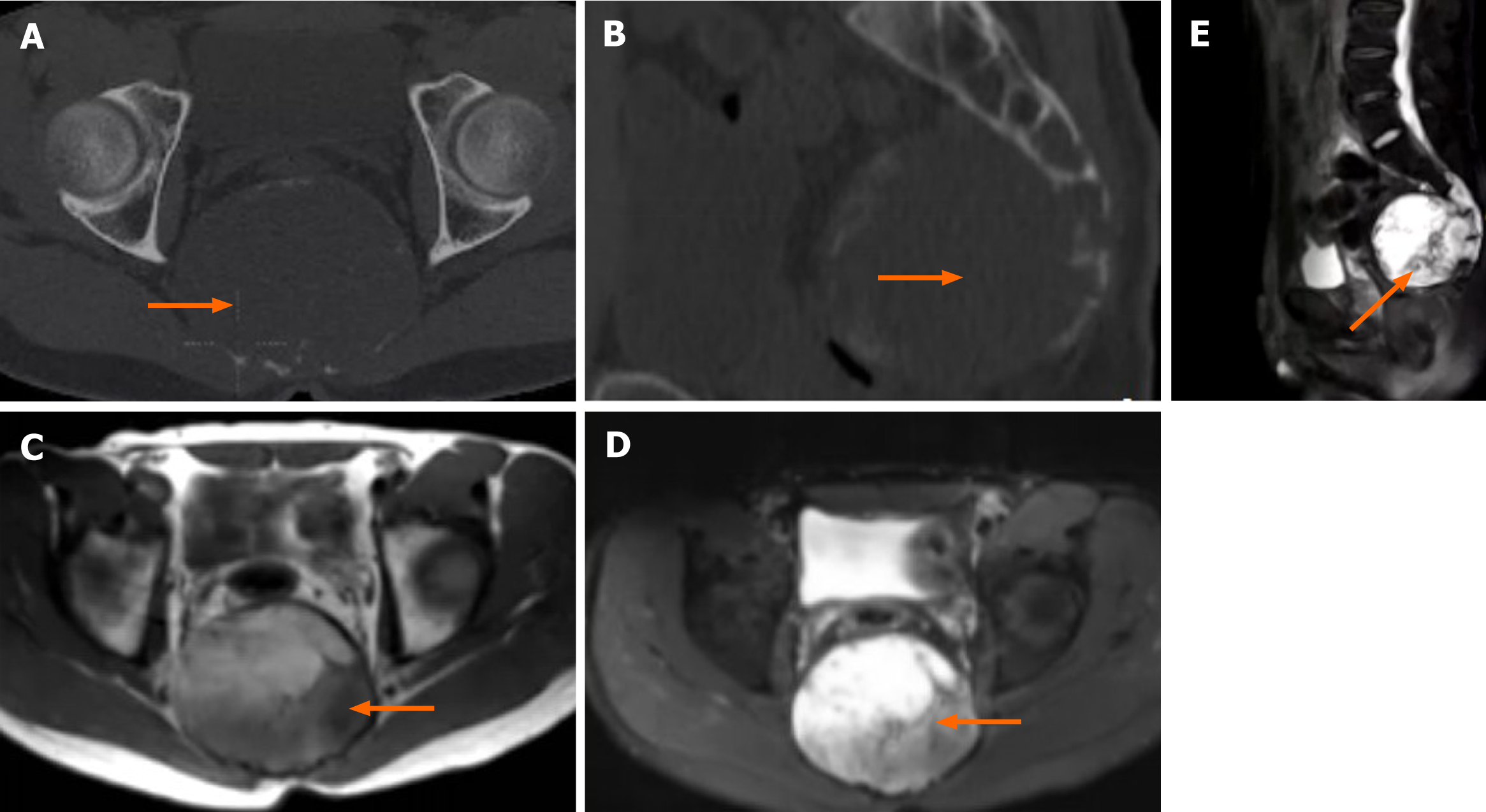
In addition, chordoma should also be excluded because it occurs in the sacrum, often in the midline of the third to fifth sacral vertebrae. It is a malignant tumour with osteolytic bone destruction and a large soft tissue mass. Chordoma is relatively easy to differentiate from FD. It exhibits low and high signal intensity (related to haemorrhage) on T1WI, and it exerts high signal intensity (related to the intratumoural gel and mucinous tissue) on T2WI (Figure 4).
The treatment of MFD focuses on symptom relief and preventing lesion pro-gression[22]. Bisphosphonates are first-line drugs for pain relief[7,10]. When conservative management fails, surgery should be considered to restore spinal stability.
This report presents a rare case of MFD involving the sacrum. The imaging manifestations of MFD include expansile lesions, ground glass opacity, and sclerotic rims. Most lesions show iso-low signal intensity on T1WI and iso-high signal intensity on T2WI. These features can provide a suggestive diagnosis to distinguish MFD from GCT, ABC, and VH. Accurate diagnosis of spinal MFD is of great value for the clinician to choose an appropriate treatment.
Conflict of interest statement: The authors declare no conflicts of interest.
Manuscript source: Unsolicited manuscript
Peer review started: August 29, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Corvino A S-Editor: Gao CC L-Editor: Filipodia P-Editor: Zhang YL
| 2. | DiCaprio MR, Enneking WF. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint Surg Am. 2005;87:1848-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 212] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Ropper AE, Cahill KS, Hanna JW, McCarthy EF, Gokaslan ZL, Chi JH. Primary vertebral tumors: a review of epidemiologic, histological, and imaging findings, Part I: benign tumors. Neurosurgery. 2011;69:1171-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Ehara S, Kattapuram SV, Rosenberg AE. Fibrous dysplasia of the spine. Spine (Phila Pa 1976). 1992;17:977-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Kinnunen AR, Sironen R, Sipola P. Magnetic resonance imaging characteristics in patients with histopathologically proven fibrous dysplasia-a systematic review. Skeletal Radiol. 2020;49:837-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Firat D, Stutzman L. Fibrous dysplasia of the bone. Review of twenty-four cases. Am J Med. 1968;44:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 57] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Schoenfeld AJ, Koplin SA, Garcia R, Hornicek FJ, Mankin HJ, Raskin KA, Springfield D, Rosenberg AE, Schwab JH. Monostotic fibrous dysplasia of the spine: a report of seven cases. J Bone Joint Surg Am. 2010;92:984-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Sharifudin MA, Zakaria Z, Awang MS, Mohamed Amin MA, Abd Aziz A. A Rare Case of Monostotic Spinal Fibrous Dysplasia Mimicking Solitary Metastatic Lesion of Thyroid Carcinoma. Malays J Med Sci. 2016;23:82-86. [PubMed] |
| 9. | Park SK, Lee IS, Choi JY, Cho KH, Suh KJ, Lee JW, Song JW. CT and MRI of fibrous dysplasia of the spine. Br J Radiol. 2012;85:996-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Ren K, Lan T, Yu Z, Chen Y, Tian CQ, Gu HS, Yang XJ. Monostotic fibrous dysplasia of the thoracic spine: A case report. J Back Musculoskelet Rehabil. 2016;29:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Teixeira JC, Simão DC, Pimentel J, Livraghi S. A Rare Case of Radiculopathy: Monostotic Fibrous Dysplasia of the Sacrum. Acta Med Port. 2019;32:466-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Rosenblum B, Overby C, Levine M, Handler M, Sprecher S. Monostotic fibrous dysplasia of the thoracic spine. Spine (Phila Pa 1976). 1987;12:939-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Lee SH, Han IH, Kang DW, Choi BK. Cervical fibrous dysplasia presenting as a pathologic fracture in an older patient. J Korean Neurosurg Soc. 2011;50:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325:1688-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1002] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 15. | Wu FL, Jiang L, Liu C, Yang SM, Wei F, Dang L, Liu XG, Liu ZJ. Fibrous dysplasia of the mobile spine: report of 8 cases and review of the literature. Spine (Phila Pa 1976). 2013;38:2016-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Meredith DS, Healey JH. Twenty-year follow-up of monostotic fibrous dysplasia of the second cervical vertebra: a case report and review of the literature. J Bone Joint Surg Am. 2011;93:e74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Wright JF, Stoker DJ. Fibrous dysplasia of the spine. Clin Radiol. 1988;39:523-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Jee WH, Choi KH, Choe BY, Park JM, Shinn KS. Fibrous dysplasia: MR imaging characteristics with radiopathologic correlation. AJR Am J Roentgenol. 1996;167:1523-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 99] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Si MJ, Wang CG, Wang CS, Du LJ, Ding XY, Zhang WB, Lu Y, Zu JY. Giant cell tumours of the mobile spine: characteristic imaging features and differential diagnosis. Radiol Med. 2014;119:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Sigwalt L, Bourgeois E, Eid A, Durand C, Griffet J, Courvoisier A. A thoracic spinal bone giant cell tumor in a skeletally immature girl. A case report and literature review. Childs Nerv Syst. 2016;32:873-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Cugati G, Pande A, Jain PK, Symss NP, Ramamurthi R, Vasudevan CM. Aneurysmal bone cyst of the lumbar spine. Asian J Neurosurg. 2015;10:216-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Gaudino S, Martucci M, Colantonio R, Lozupone E, Visconti E, Leone A, Colosimo C. A systematic approach to vertebral hemangioma. Skeletal Radiol. 2015;44:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |