Published online Feb 16, 2021. doi: 10.12998/wjcc.v9.i5.1096
Peer-review started: July 25, 2020
First decision: November 26, 2020
Revised: December 10, 2020
Accepted: December 23, 2020
Article in press: December 23, 2020
Published online: February 16, 2021
Processing time: 188 Days and 20.2 Hours
Guillain-Barré syndrome (GBS) is a rare disorder that typically presents with ascending weakness, pain, paraesthesias, and numbness, which mimic the findings in lumbar spinal stenosis. Here, we report a case of severe lumbar spinal stenosis combined with GBS.
A 70-year-old man with a history of lumbar spinal stenosis presented to our emergency department with severe lower back pain and lower extremity numbness. Magnetic resonance imaging confirmed the diagnosis of severe lumbar spinal stenosis. However, his symptoms did not improve postoperatively and he developed dysphagia and upper extremity numbness. An electromyogram was performed. Based on his symptoms, physical examination, and electromyogram, he was diagnosed with GBS. After 5 d of intravenous immunoglobulin (0.4 g/kg/d for 5 d) therapy, he gained 4/5 of strength in his upper and lower extremities and denied paraesthesias. He had regained 5/5 of strength in his extremities when he was discharged and had no symptoms during follow-up.
GBS should be considered in the differential diagnosis of spinal disorder, even though magnetic resonance imaging shows severe lumbar spinal stenosis. This case highlights the importance of a careful diagnosis when a patient has a history of a disease and comes to the hospital with the same or similar symptoms.
Core Tip: A 70-year-old man with a history of lumbar spinal stenosis presented to our emergency department because of severe lower back pain and lower extremity numbness. On the physical examination, he had 4/5 of strength in both legs and decreased sensation below the knees. Magnetic resonance imaging demonstrated lumbar spinal stenosis (L4/5). Based on these findings, he was diagnosed with lumbar spinal stenosis. After conservative treatment failed, he underwent transforaminal lumbar interbody fusion. However, his symptoms worsened postoperatively and dysphagia appeared. An electromyogram was performed. Finally, he was diagnosed with Guillain-Barré syndrome. After 5 d of intravenous immunoglobulin therapy, he gained 4/5 of strength in his upper and lower extremities and denied paraesthesias. This case demonstrates that Guillain-Barré syndrome should be considered in the differential diagnosis of spinal disorder and highlights the importance of a careful diagnosis when a patient has a history of a disease and comes to the hospital with the same or similar symptoms.
- Citation: Xu DF, Wu B, Wang JX, Yu J, Xie JX. Severe lumbar spinal stenosis combined with Guillain-Barré syndrome: A case report. World J Clin Cases 2021; 9(5): 1096-1102
- URL: https://www.wjgnet.com/2307-8960/full/v9/i5/1096.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i5.1096
Lumbar spinal stenosis is common in older people. The spinal canal is constricted by osteophytes, disc degeneration, and the ligamentum flavum, compressing the dural sac, spinal cord, or nerve roots, which leads to low back and leg pain, numbness, lower limb weakness, claudication, etc[1]. Decompression of the spinal canal is an effective treatment for severe lumbar spinal stenosis and the pain, numbness, and walking ability improve postoperatively[1-3].
Guillain-Barré syndrome (GBS) is an autoimmune peripheral neuropathy characterized by demyelination of the peripheral nerves and nerve roots and infiltration of inflammatory cells in small blood vessels[4-6]. It is rare, with an incidence is 1 to 2 per 100000 per year worldwide[7]. The typical symptoms of GBS are ascending weakness, pain, paraesthesia, and numbness, which are also those of lumbar spinal stenosis. Since GBS is rare, patients might be misdiagnosed and subsequently receive the wrong treatment, especially patients with a history of lumbar spinal stenosis.
Here, we present a 70-year-old man who had severe lower back pain and lower extremity numbness. Magnetic resonance imaging (MRI) confirmed the diagnosis of severe lumbar spinal stenosis. However, his symptoms did not improve postoper-atively. An electromyogram was performed. Based on his symptoms, physical examination, and electromyogram, he was diagnosed with GBS and subsequently treated correctly. This case highlights that GBS should be considered in the differential diagnosis of spinal diseases.
Lower back pain and lower extremity numbness for 10 h.
A 70-year-old man with a history of lumbar spinal stenosis, hypertension, and gout presented to our emergency department because of severe lower back pain and lower extremity numbness. He has been receiving intermittent physical therapy and medical treatment for lower back pain and lower extremity numbness. On the physical examination, he had tenderness in the lower back, and no obvious decrease in skin sensation around the anus. He had 4/5 of strength in both legs and decreased sensation below the knees. Lasègue signs were negative. The bilateral knee and Achilles tendon reflexes were normal. He had joint deformity and gout nodules between the fingers and toes. Babinski’s sign and other pathological reflex signs were negative. Laboratory testing was largely unremarkable, aside from a uric acid level of 462 μmol/L. X-rays showed degenerative changes of the lumbar spine and the L4 vertebral body had slipped forward slightly. He was diagnosed with spinal stenosis by an orthopedic surgeon and admitted to the Department of Spinal Surgery for further workup.
After admission, he was treated with steroid injections, analgesia, and nervous system nutrients for symptom relief. MRI demonstrated lumbar spinal stenosis (L4/5). Based on these findings, he was diagnosed with lumbar spinal stenosis. After conservative treatment failed, he underwent transforaminal lumbar interbody fusion. Postoperatively, his paraesthesias and muscle weakness did not improve markedly and he reported numbness and weakness in both upper extremities. On postoperative day 2, he had 2/5 of strength in his upper extremities and 1/5 of strength in his lower extremities. Deep tendon reflexes (for example triceps reflex, biceps reflex, and knee and Achilles tendon reflexes) disappeared. He also reported dysphagia and numbness in the upper extremities. We requested a neurology consultation. Careful review of the patient’s history, as provided by his family and community doctor, revealed that he got the flu vaccination 10 d ago.
An electromyogram was obtained, but no lumbar puncture was performed because of his recent surgery. The electrophysiological study showed reduced motor and sensory responses in his extremities. Given his symptoms, physical examination, and laboratory tests, he was diagnosed with GBS (the form is acute inflammatory demyelinating polyneuropathy). Intravenous immunoglobulin (IVIG) was started (0.4 g/kg/day for 5 d)[6,8]. He reported some improvement during therapy. He gained 4/5 of strength in his upper and lower extremities and denied paraesthesias after 5 d of therapy. Then, he was given ultrasound therapy, electroacupuncture, and electronic biofeedback therapy and postoperative rehabilitation. He regained 5/5 of strength in his extremities and could ambulate without aid after about 4 wk, when he was discharged. At the follow-up, he was asymptomatic.
The patient had a history of hypertension for nearly 20 years, but the blood pressure is normal after taking valsartan capsule (80 mg per day). He also had a history of gout for 15 years, and was receiving the oral administration of febuxostat (40 mg per day).
The patient worked as a farmer. He neither smokes nor drinks. He got the flu vaccina-tion 10 d ago.
He had tenderness in the lower back, and no obvious decrease in skin sensation around the anus. He had 4/5 of strength in both legs and decreased sensation below the knees. Lasègue signs were negative. The bilateral knee and Achilles tendon reflexes were normal. He had joint deformity and gout nodules between the fingers and toes. Babinski’s sign and other pathological reflex signs were negative.
The uric acid level was 462 μmol/L.
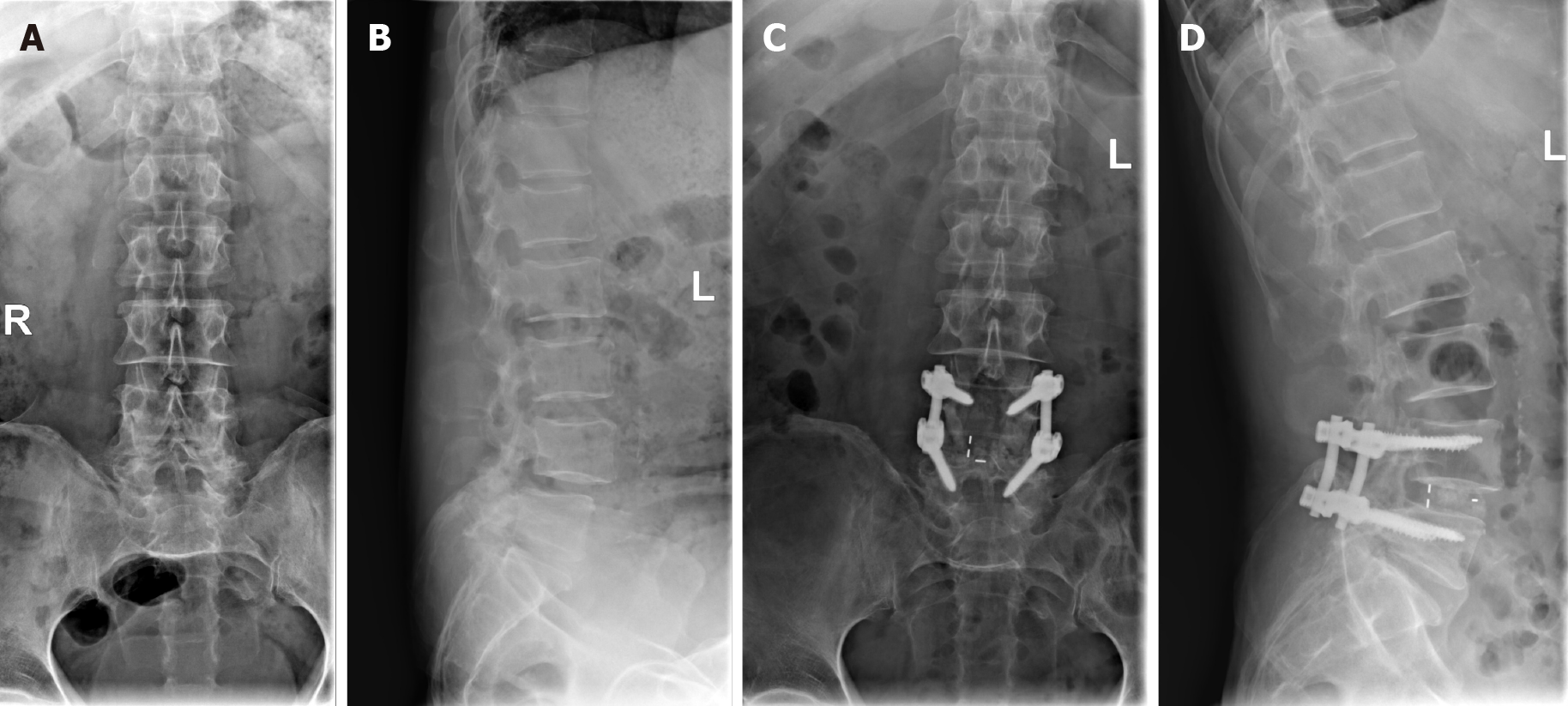
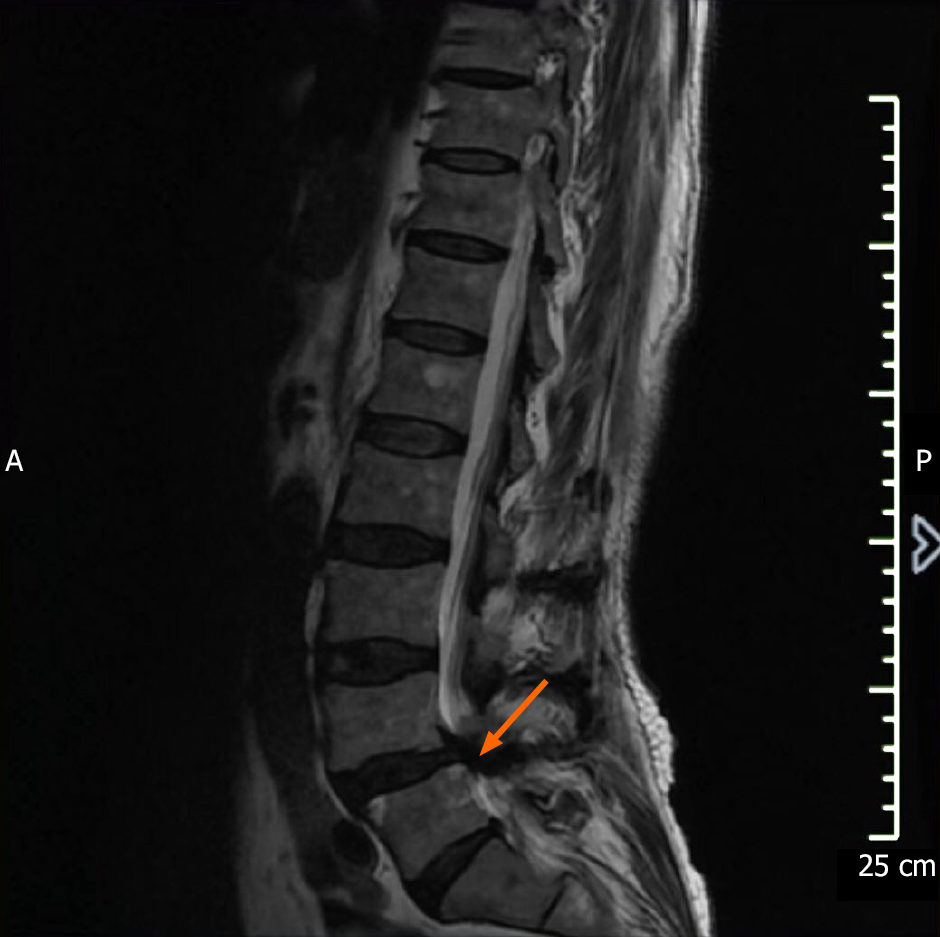
X-rays (Figure 1) showed degenerative changes of the lumbar spine and the L4 vertebral body had slipped forward slightly. MRI (Figure 2) demonstrated lumbar spinal stenosis (L4/5). The results of electromyogram are listed in Table 1.
| Nerve | Conduction | NCV (m/s) | Stimulating points | Record points | Incubation period (ms) | CMAP (mv) or SNAP (μv) | |||
| Left | Right | Left | Right | Left | Right | ||||
| Median nerve (S) | 38.8 | Middle finger | Wrist | No | 3.3 | 1.2 | |||
| Ulnar nerve (S) | 33.7 | Little finger | Wrist | No | 3.0 | 1.0 | |||
| Median nerve (M) | Wrist to elbow | 25.2 | Abductor pollicis brevis | No | 5.8 | 0.6 | |||
| Ulnar nerve (M) | Wrist to elbow | 32.9 | 33.7 | Abductor digiti minimi | 3.2 | 3.6 | 0.7 | 0.8 | |
| Common peroneal nerve (M) | Ankle to capitula fibula | 26.7 | 17.8 | Extensor digitorum brevis | 5.1 | 4.4 | 0.5 | 0.3 | |
| Superficial peroneal nerve (S) | Middle fibula | Ankle | No | No | |||||
| Tibial nerve (M) | Malleolus medialis to popliteal space | 27.6 | 28.5 | Flexor hallucis brevis | 5.2 | 5.9 | 0.3 | 0.4 | |
| Sural nerve (S) | Lateral malleolus | No | No | ||||||
The patient was diagnosed with Guillain-Barré syndrome, and the form is acute inflammatory demyelinating polyneuropathy.
The patient was started on intravenous immunoglobulin (0.4 g/kg/day for 5 d). Then, he was given ultrasound therapy, electroacupuncture, and electronic biofeedback therapy and postoperative rehabilitation.
He gained 4/5 of strength in his upper and lower extremities and denied paraesthesias after 5 d of intravenous immunoglobulin therapy. He regained 5/5 of strength in his extremities and could ambulate without aid after about 4 wk, when he was dis-charged. At the follow-up, he was asymptomatic.
We present a patient with a history of lumbar spinal stenosis who suffered severe pain in the lower back and lower extremity numbness. Based on his medical history, symptoms, physical examination, and MRI results, he was diagnosed with lumbar spinal stenosis and underwent surgical treatment. However, his symptoms worsened postoperatively and dysphagia appeared. He was diagnosed with GBS by a neurologist after electromyography. After 5 d of IVIG therapy, his symptoms improved.
GBS is a rare disorder and the typical symptoms are ascending weakness, pain, paraesthesias, and numbness, which are similar to those of a spinal disorder. This makes it difficult for most spinal surgeons to diagnose. Our patient had a history of lumbar spinal stenosis, with lower back pain and lower extremity numbness, which improved after medication. This time he was admitted because his symptoms were worsening. After admission, MRI showed severe lumbar spinal stenosis (L4/5), consistent with the clinical findings. We unanimously believed that he was showing progression of his original disease, which caused the misdiagnosis. Careful review of the patient’s history and physical examination showed that his muscle strength in the lower extremities was symmetrical. After several days of conservative treatment including steroid injections, his symptoms did not improve markedly. This is atypical of lumbar spinal stenosis. Patients with lumbar spinal stenosis have numbness and weakness in the lower extremities, but they are not always symmetrical and their symptoms will improve after conservative treatment, especially steroid injections[1,3,9]. By contrast, steroid injections are not beneficial in the management of GBS[6,10]. When we consider only the initial findings, the diagnosis is challenging. This case demonstrates that GBS should be considered in the differential diagnosis of spinal disorder.
The diagnosis of GBS is usually confirmed by electromyography and lumbar puncture[5,6,8]. Because our patient had had lumbar spine surgery, lumbar puncture was inappropriate. We obtained an electromyogram and, with the help of a neurologist, obtained the correct diagnosis, enabling prompt treatment.
There have been several recent reports of GBS after spinal surgery. Rashid et al[11] reported a 62-year-old woman who underwent lumbar spine surgery revision and developed leg weakness and respiratory failure approximately 2 wk postoperatively. After an electromyogram, she was diagnosed with GBS and placed on IVIG. Abode-Iyamah et al[12] present a case of GBS after lumbar spine surgery. Postoperatively, their patient’s symptoms of paraesthesia, pain, and weakness relieved markedly. However, on postoperative day 5, she reported weakness that worsened progressively. Finally, she was diagnosed with GBS after MRI and lumbar puncture. Chen et al[13] described a patient who showed characteristics of GBS on postoperative day 9. GBS was a postoperative spinal complication in these reports[14]. Our report differs from these cases in that our patient’s symptoms of numbness and lower extremity weakness worsened progressively postoperatively and he also developed dysphagia and upper extremity numbness. These are not typical postoperative spinal complications[1,15]. We made a mistake in the diagnosis, and the spinal surgery was inappropriate.
GBS is an immune-mediated disorder. Infection and vaccination have been considered to be associated with its occurrence. The patient denied any respiratory infection or gastroenteritis recently, but he was found to get the flu vaccination by carefully reviewing his history. Cases of GBS have been reported after vaccination[16]. The vaccination might be the etiology of our patient. After vaccination, an autoimmune response is initiated, and antibodies that attack myelin protein are produced, leading to demyelination and axonal damage. The operation has been thought to alter the balance of the immune system and accelerate the progression of his original disease[17,18].
A patient with a history of lumbar spinal stenosis developed pain in the lower back and lower extremity numbness. Although MRI showed severe lumbar spinal stenosis, we must still consider other spinal or neurological diseases, including GBS. When a patient has a history of one disease that is not responding to conventional therapy, other illnesses with similar symptoms should be considered carefully and invest-igated.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cure E, Shimada S, Tarbox J S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Katz JN, Harris MB. Clinical practice. Lumbar spinal stenosis. N Engl J Med. 2008;358:818-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 375] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 2. | Zaina F, Tomkins-Lane C, Carragee E, Negrini S. Surgical versus non-surgical treatment for lumbar spinal stenosis. Cochrane Database Syst Rev. 2016: CD010264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 3. | Lurie J, Tomkins-Lane C. Management of lumbar spinal stenosis. BMJ. 2016;352:h6234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 344] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 4. | van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. Lancet Neurol. 2008;7:939-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 514] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 5. | Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016;388:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 902] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 6. | van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA. Guillain-Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2014;10:469-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 682] [Article Influence: 62.0] [Reference Citation Analysis (2)] |
| 7. | Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain-Barré syndrome: a systematic review and meta-analysis. Neuroepidemiology. 2011;36:123-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 726] [Cited by in RCA: 608] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 8. | Leonhard SE, Mandarakas MR, Gondim FAA, Bateman K, Ferreira MLB, Cornblath DR, van Doorn PA, Dourado ME, Hughes RAC, Islam B, Kusunoki S, Pardo CA, Reisin R, Sejvar JJ, Shahrizaila N, Soares C, Umapathi T, Wang Y, Yiu EM, Willison HJ, Jacobs BC. Diagnosis and management of Guillain-Barré syndrome in ten steps. Nat Rev Neurol. 2019;15:671-683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 446] [Cited by in RCA: 491] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 9. | Suri P, Rainville J, Kalichman L, Katz JN. Does this older adult with lower extremity pain have the clinical syndrome of lumbar spinal stenosis? JAMA. 2010;304:2628-2636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 10. | Hughes RA, Brassington R, Gunn AA, van Doorn PA. Corticosteroids for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2016;10:CD001446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Rashid A, Kurra S, Lavelle W. Guillain-Barré Syndrome After Revision Lumbar Surgery: A Case Report. Cureus. 2017;9:e1393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Abode-Iyamah K, Bohnen AM. Guillain-Barre Syndrome After Minimally Invasive Transforaminal Interbody Fusion: A Case Report. Cureus. 2019;11:e6222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Chen EY, Stratton C, Mercer B, Hohler A, Tannoury TY, Tannoury C. Guillain-Barré Syndrome After Elective Spinal Surgery. J Am Acad Orthop Surg. 2017;25:587-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Huang SL, Qi HG, Liu JJ, Huang YJ, Xiang L. A Rare Complication of Spine Surgery: Guillain-Barré Syndrome. World Neurosurg. 2015;84:697-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Willson MC, Ross JS. Postoperative spine complications. Neuroimaging Clin N Am. 2014;24:305-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Hartung HP, Keller-Stanislawski B, Hughes RA, Lehmann HC. [Guillain-Barré syndrome after exposure to influenza]. Nervenarzt. 2012;83:714-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Hogan BV, Peter MB, Shenoy HG, Horgan K, Hughes TA. Surgery induced immunosuppression. Surgeon. 2011;9:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Miyazaki M. Immunosuppression following surgical and traumatic injury. Surg Today. 2010;40:793-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |