Published online Feb 16, 2021. doi: 10.12998/wjcc.v9.i5.1087
Peer-review started: June 9, 2020
First decision: November 30, 2020
Revised: December 22, 2020
Accepted: December 28, 2020
Article in press: December 28, 2020
Published online: February 16, 2021
Processing time: 235 Days and 8.1 Hours
The drug interaction between warfarin and rifampicin is widely known, but there are still some difficulties in managing the combination of the two drugs.
A patient with brucellosis received strict monitoring from a Chinese pharmacist team during combination of warfarin and rifampicin. The dose of warfarin was increased to 350% in 3 mo before reaching the lower international normalized ratio treatment window. No obvious adverse reaction occurred during the drug-adjustment period. This is the first case report of long-term combined use of rifampicin and warfarin in patients with brucellosis and valve replacement in China based on the Chinese lower warfarin dose and international normalized ratio range.
Anticoagulation for valve replacement in Chinese patients differs from that in other races. Establishment of a pharmacist clinic provides vital assistance in warfarin dose adjustment.
Core Tip: Brucellosis is a zoonotic disease with increasing incidence. In order to improve the adherence and cure rate of outpatients, doxorubicin and rifampicin is the preferred treatment. We described a patient with brucellosis and mechanical valve replacement caused by infective endocarditis who required the combination of rifampicin and warfarin. The case highlighted the racial differences in the therapeutic range of the international normalized ratio and the adjustment range of warfarin dose. Individualized medication is required. Pharmacists can help reduce the burden on doctors and the risk of adverse reactions.
- Citation: Hu YN, Zhou BT, Yang HR, Peng QL, Gu XR, Sun SS. Effect of rifampicin on anticoagulation of warfarin: A case report. World J Clin Cases 2021; 9(5): 1087-1095
- URL: https://www.wjgnet.com/2307-8960/full/v9/i5/1087.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i5.1087
Warfarin is a commonly used oral anticoagulant that inhibits the synthesis of coagulation factors II, VII, IX and X in vitamin K cycloreductase complex subunit 1 in liver cells[1]. Its anticoagulant efficacy is affected by drugs and food[2,3], resulting in the risk of bleeding or embolism. Frequent follow-up and dose adjustment to ensure that a patient’s international normalized ratio (INR) value is within the therapeutic range is necessary, which takes time and effort. Due to the influence of Asian ethnicity and living environment, the dose of warfarin for Chinese patients is significantly lower than that required for western patients. Combined with the improvement of valve quality, the INR therapeutic range of warfarin in Chinese patients with valve replacement is 1.5-2.5[4,5]. This is lower than the therapeutic INR in the western population (2.0-3.0). Warfarin is cheaper than other nondimensional K antagonists and is widely used in the treatment of thromboembolic diseases and myocardial infarction, especially in patients with atrial fibrillation with artificial mechanical valve replacement[6]. Rifampicin is a liver enzyme inducer[7] that has a significant impact on the rational use of warfarin[8,9]. Rifampicin induces aromatic hydroxylated cytochrome P-450 isoenzyme CYP2C9 to accelerate the clearance of warfarin used in combination, thereby affecting the anticoagulant effect of warfarin[8].
Brucellosis is an important zoonotic disease, prevalent in most countries around the world[10] with an incidence of 500000 cases per year[11]. Occupations with frequent contact with livestock, such as travelers and herders, are susceptible. With economic development in recent years, the incidence of the disease has increased in China. It is common for the bacterium to cause endocarditis, resulting in heart valve replacement. Rifampicin is one of the first-line treatments for brucellosis.
Some previous cases of tuberculosis non-Asian patients treated with combined warfarin and rifampicin have been reported. Considering the significant difference between warfarin dose and the therapeutic INR range of Asian populations vs non-Asian populations, the specificity of brucellosis and the significant effect that rifampicin has on the anticoagulant effect of warfarin, cases of long-term combination of the two drugs in Asia and a clinical rationale for their use have not yet been reported.
In this report, we describe a 10-mo follow-up of a patient treated with warfarin after mechanic valve replacement and rifampicin for brucellosis. Following discontinuation of rifampicin, warfarin dose was adjusted to reach the INR therapeutic range. We also reviewed similar case reports with different warfarin doses and therapeutic INR ranges.
A 45-year-old man presented to the outpatient department of our hospital complaining of repeated fever 1 year ago. He had fever again 10 d ago with no obvious cause.
Blood culture at the local hospital was positive for Brucella many times, but the specific treatment is unknown.
The patient had no special medical history. He denied any history of other diseases or exposure to endemic or infectious diseases or endemic living areas.
The patient had a history of smoking for 15 years but had quit smoking for 2 years. He had a history of drinking but had quit drinking for 1 year. He denied any family history.
The patient’s temperature was 37.6 °C, heart rate was 100 bpm, respiratory rate was 20 breaths/min, blood pressure was 120/41 mmHg, and weight was 53 kg. Systolic murmur could be heard in the aortic area. Other measures were normal.
Routine blood tests revealed a low albumin level of 29.4 g/L, red blood cell count of 4.15 × 1012/L, lymphocyte count of 1.0 × 109/L and platelet specific volume of 0.14%. Blood culture was positive for Brucella. Urinalysis was normal.
Computed tomography showed falling lung inflammation and a small amount of pericardial effusion. Echocardiography demonstrated aortic valve lesions that appeared as faster aortic valve jet velocity and aortic valve prolapse and perforation.
The patient was admitted to the hospital with a diagnosis of infective endocarditis, lung infection and sepsis.
Doxycycline 100 mg twice daily and rifampicin 600 mg/d were prescribed on admission. On day 3 of admission, the patient underwent prosthetic valve replacement and began taking warfarin 2.5 mg daily for anticoagulation with a target INR range of 1.5-2.5. Pantoprazole, beclomethasone, terbutaline, acetylcysteine, digoxin, hydrochlorothiazide, potassium chloride and metoprolol were used during the 9 d of hospitalization. Before discharge, the patient’s INR (0.97) had not yet reached the target INR range (1.5-2.5). The drugs prescribed at discharge were doxycycline 100 mg twice daily, rifampicin 600 mg/d, metoprolol 23.75 mg/d, warfarin 3.8 mg/d, hydrochlorothiazide 25 mg/d and potassium chloride 500 mg twice daily.
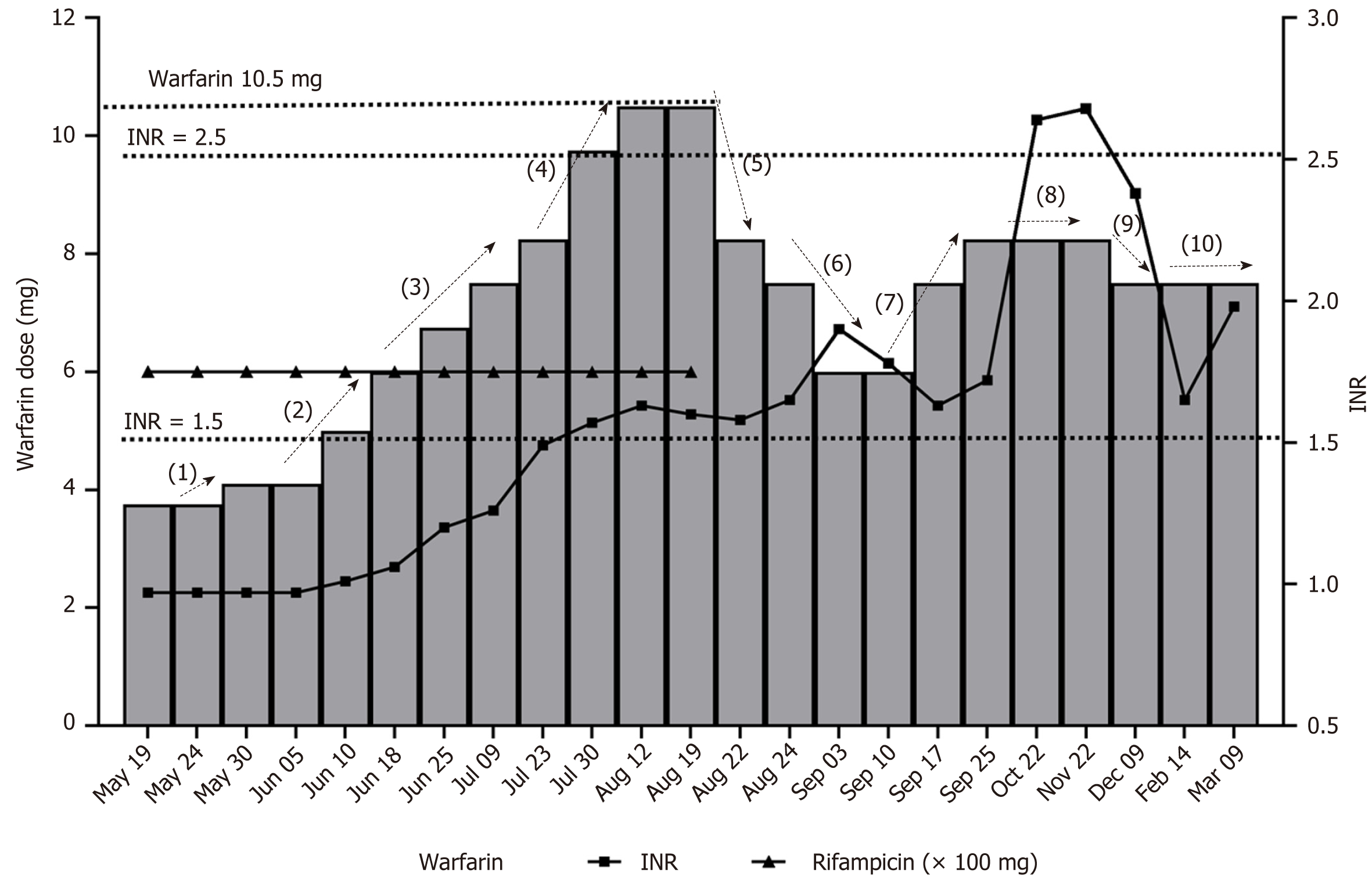
The dose of warfarin was increased to 5 mg/d in the third week after discharge in order to reach the therapeutic INR range. Then it was conservatively increased by 0.25-0.5 mg/wk in the following 4 wk with virtually no response in INR. Dose was increased weekly at 0.75-1.5 mg/wk increments to 10.5 mg/d. INR reached the lower limit of the therapeutic value of 1.56 while the patient was receiving warfarin 10.5 mg/d. After that, the does was not adjusted until termination of anti-brucellosis treatment (discontinuation of rifampicin) because he had completed a 3 mo course of therapy. During this period, the patient did not experience obvious adverse reactions such as embolism or bleeding. For the purpose of maintaining therapeutic INR, the pharmacist gradually reduced the warfarin dose within 2 wk. Finally, the patient reached the treatment INR at a dose of 8.25/d. However, gum bleeding occasionally occurred at a value of 2.2, so the dose was reduced to 7.5 mg/d and the INR dropped below 2.0. Figure 1 tracks the anticoagulant and anti-infective treatment of this patient over 10 mo after discharge, and Table 1 presents this information in more detail.
| Date | Warfarin dosage in mg/d | INR, | Pharmacist intervention node | Pharmacist medication intervention | Warfarin dosage changes |
| 1.5-2.5 | |||||
| 2019-05-19 | 3 | 0.97 | Taking rifampicin, doxycycline and other drugs at the same time | None | |
| 2019-05-24 | 3 | 0.97 | Taking rifampicin, doxycycline and other drugs at the same time | None | |
| 2019-05-30 | 3.75 | 0.97 | (1) | Taking rifampicin, doxycycline and other drugs at the same time | Increased to 3.75 mg/d (+ 25%) |
| 2019-06-10 | 3.75 | 0.97 | Taking rifampicin, doxycycline and other drugs at the same time | None | |
| 2019-06-10 | 5 | 1.01 | (2) | Taking rifampicin, doxycycline and other drugs at the same time. Considering there was no significant change in INR value when warfarin dosage was 3.75 mg/d, warfarin dosage increased and changed to imported warfarin | Increased to 5.00 mg/d (+ 33%) |
| 2019-06-18 | 6 | 1.06 | (2) | Taking rifampicin, doxycycline and other drugs at the same time. Considering there was no significant change in INR value when warfarin dosage was 5.00 mg/d, warfarin dosage increased and changed to imported warfarin | Increase to 6.00 mg/d (+ 20%) |
| 2019-06-20 | 6.75 | 1.2 | (3) | Taking rifampicin, doxycycline and other drugs at the same time. The INR value increased, and the warfarin dose continued to increase slightly | Increased to 6.75 mg/d (+ 12%) |
| 2019-07-09 | 7.5 | 1.26 | (4) | Taking rifampicin, doxycycline and other drugs at the same time. The INR value increased, and the warfarin dose continued to increase slightly | Increased to 7.50 mg/d (+ 11%) |
| 2019-07-23 | 8.25 | 1.49 | (4) | Taking rifampicin, doxycycline and other drugs at the same time. The INR value increased, and the warfarin dose continued to increase slightly | Increased to 8.25 mg/d (+ 10%) |
| 2019-07-30 | 9.75 | 1.57 | Taking rifampicin and doxycycline at the same time. The lower limit of the INR value guideline was reached, but the increase was limited, so continued to increase warfarin dosage significantly | Increased to 9.75 mg/d (+ 18%) | |
| 2019-08-12 | 10.5 | 1.63 | Taking rifampicin and doxycycline at the same time. Reaching the INR value therapeutic range. Continued to increase slightly | Increased to 10.5 mg/d (+ 8%) | |
| 2019-08-19 | 10.5 | 1.6 | (5) | Taking rifampicin and doxycycline at the same time | None |
| 2019-08-22 | 8.25 | 1.58 | (5) | Stopped taking rifampicin and doxycycline. There is no more rifampicin interaction. Taking into account that 10.5 mg/d was the highest dose we used and stopping rifampicin could have a great impact, we greatly reduced the warfarin dose | Reduced to 8.25 mg/d (-21%) |
| 2019-08-24 | 7.5 | 1.65 | (6) | Considering the long half-life of warfarin, we continued to reduce the warfarin dose | Reduced to 7.50 mg/d (-9%) |
| 2019-09-03 | 6 | 1.9 | (6) | 7.50 mg/d is already a large dose for Asians. INR value was increased after the 9% dosage reduction. Continued to reduce the dosage | Reduced to 6.00 mg/d (-20%) |
| 2019-09-10 | 6 | 1.78 | (7) | In the therapeutic range. No change | None |
| 2019-09-17 | 7.5 | 1.63 | (7) | INR value decreased, so increased warfarin dose | Increased to 7.50 mg/d (+ 25%) |
| 2019-09-25 | 8.25 | 1.72 | (7) | Limited rise in INR, so increased warfarin dose | Increased to 8.25 mg/d (+ 10%) |
| 2019-10-22 | 8.25 | 2.64 | (8) | Occasionally higher INR value observed | None |
| 2019-11-22 | 8.25 | 2.68 | (8) | INR was still high. Occasional bleeding gums | None |
| 2019-12-09 | 7.5 | 2.38 | (9) | INR was in the range of 1.5-2.5 | Reduced to 7.50 mg/d (-9%) |
| 2020-02-14 | 7.5 | 1.78 | (10) | INR was in the range of 1.5-2.5 | None |
| 2019-03-09 | 7.5 | 1.98 | INR was in the range of 1.5-2.5 | None |
Brucellosis is a class B infectious disease in China that appeared in the 1950s and 1960s, gradually declined, and then saw a rise again after the 1990s. Brucella can cause fever and even endocarditis and encephalitis. The main source of infection is animal food, and it can also be spread by close contact with animals. Although our patient did not visit any infectious disease areas, he did keep goats in his house, which was presumably the cause of his brucellosis.
To treat brucellosis, the World Health Organization recommends doxycycline 100 mg twice daily combined with rifampicin 600-900 mg/d continuously for 6 wk (DOX-RIF) or streptomycin 1 g/d for 2-3 wk (DOX-STR). DOX-RIF for 6 wk is the most common treatment for pulmonary infections[12,13], whereas a treatment period of 6 wk to 6 mo is common for endocarditis. Although the efficacy and pharmacokinetic data of DOX-STR are more advantageous, clinicians and patients prefer DOX-RIF because it is a full-oral treatment that can help improve patient compliance and overall success rate and can be better implemented clinically in areas with developing medical infrastructure. DOX-RIF also eliminates the need for parenteral administration of streptomycin as part of the DOX-STR regimen. Additionally, the shortage of streptomycin in many parts of the world may hinder the implementation of DOX-STR[14]. In the present case, due to aortic valve prolapse caused by infective endocarditis, warfarin was the first choice after artificial heart valve replacement, giving the two drugs the opportunity to interact.
Warfarin is a racemic mixture. S-warfarin, as a major anticoagulant, is mainly metabolized by CYP2C9, while R-warfarin is mainly metabolized by CYP3A4, but both are important members of hepatic cytochrome enzyme P-450[15]. With its 35-h elimination half-life, warfarin is almost completely absorbed in the body. Due to the 17 h elimination half-life of prothrombin, it takes 3 d for warfarin to reach a steady blood concentration[16]. Rifampicin is an aromatic hydroxylated cytochrome P-450 isoenzyme drug that reduces the half-life of warfarin and accelerates its clearance rate (clearance rate of R-warfarin triples while that of S-warfarin doubles), thereby reducing the anticoagulant effect of warfarin and leading to increased risk of embolism[17]. The sudden withdrawal of rifampin in the combination of the two drugs also increases the risk of bleeding.
In the present case, the interaction between warfarin and rifampicin was confirmed. At first, the increasing dose of warfarin and unchanged does of rifampicin caused the patient to reach the lower limit of therapeutic INR (1.5-2.5). Although the dose of warfarin decreased to 8.5 mg/d, discontinuation of rifampicin resulted in increasing INR, leading to bleeding gums. Lowering the dose of warfarin could help that.
Unlike the INR of mechanical heart valve replacement (2.5-3.5) established by the American College of Chest Physicians and the American Heart Association and American College of Cardiology, the anticoagulation strength of the Chinese population (1.5-2.5) is significantly lower than in the non-Chinese population because the prognosis of bleeding events is more serious than embolic events in Chinese people[5], who tend to eat more vitamin-K-rich vegetables.
Table 2 summarizes case reports on the interaction between rifampicin and warfarin. The INR therapeutic range and warfarin dose listed in Table 2 from other cases are markedly higher than those used for Chinese patients, meaning that Chinese people may not require high-intensity anticoagulation to achieve their therapeutic range and have fewer adverse reactions. Additionally, most of the other cases had tuberculosis, while the present case is the first report of the combined use of rifampicin and warfarin in patients with brucellosis and can thus provide a reference for similar patients.
| Ref. | Country | Race | Age in yr | Sex | Therapeutic INR range | Disease | Warfarin dose range in mg/d | Warfarin dose adjustment |
| Romankiewicz et al[8], 1975 | United States | Black | 48 | Male | Prothrombin time | Tuberculosis, pulmonary embolism | 7.5-20 | Starting dose of 7.5 mg/d, increased to 20 mg/d within 8 d, and warfarin dose decreased by 50% 7 d after rifampicin was discontinued |
| Self et al[25], 1975 | United States | Unknown | 72 | Male | Prothrombin time | Pulmonary tuberculosis, pulmonary embolism | 5-20 | Initially 5 mg/d, increased to 20 mg/d, decreased to 15 mg/d after 8 d of rifampicin discontinuation, and gradually decreased to 10 mg/d |
| Almog et al[26], 1988 | Israel | White | 30 | Female | Prothrombin time | Chronic obstructive lung disease, pulmonary tuberculosis, deep vein thrombosis, pulmonary embolism | 8.7-15.3 | Average 15.3 mg/d to attain therapeutic INR and decreased by 50% to average 8.7 mg/d after rifampicin discontinued |
| Casner et al[27], 1996 | United States | Black | 36 | Male | 2.0-3.0 | Staphylococcus aureus infection, pulmonary embolism | 5-20 | 10 mg/d, increased to 20 mg/d in 20 d, decreased to 5 mg/d immediately after rifampin is stopped |
| Lee et al[22], 2001 | United States | Unknown | 58 | Female | 2.0-3.0 | Pulmonary tuberculosis, left ventricular dysfunction | 5-25 | Starting at 5 mg/d, increased by 233% over 4 mo and unable to attain therapeutic INR during rifampicin combination. A gradual 70% reduction in warfarin dose over 4-5 wk after rifampicin discontinued |
| Baciewicz et al[9], 2013 | United States | Unknown | 79 | Male | 2.0-3.0 | Deep vein thrombosis, pulmonary embolism, osteomyelitis | 5-25 | Taking long-term warfarin at 5 mg/d, the dose increased by 10%-25% weekly to 25 mg/d after combined use. The dose was reduced by 70% within 4-5 wk to 5 mg/d after rifampicin discontinued |
| Unal et al[28], 2007 | Turkey | Unknown | 16 | Female | 2.0-3.0 | Drug abuse | — | Acute event, not comparable |
| Krajewski et al[18], 2010 | United States | White | 71 | Male | 2.0-3.0 | Deep vein thrombosis, prosthetic knee infection due to methicillin-resistant Staphylococcus aureus | 5-25 | Starting at 5 mg/d, increased to 25 mg/d in 2 mo, and the 5-fold increase did not reach the therapeutic INR. After stopping rifampicin, it was reduced by 30% to 17.5 mg/d, and after 3 d it was reduced by 20% to 12.5 mg/d, reduced to 10 mg/d after 1 wk, then gradually reduced to 5-5.5 mg/d in the next 2-3 mo |
| Martins et al[19], 2013 | Brazil | Non-white | 59 | Female | 2.0-3.0 | Atrial fibrillation, pleural TB | 5.3-11 | Long-term use of 7.5 mg/d, increased to 11 mg/d within 3 mo after combined use, hematuria occurred after rifampicin was discontinued, the drug was stopped twice, the dose decreased by 33% to 7.5 mg/d, and then adjusted to 5.3 mg/d |
| Fahmi et al[20], 2016 | Sri Lanka | White | 34 | Female | 2.5-3.5 | Mitral valve replacement, infective endocarditis | 7.5-30 | 7.5 mg/d, conservatively increased to 15 mg/d in 1 mo, 22 mg/d in 2 wk, and then increased to 30 mg/d. Continuous monitoring of INR value after antibiotics discontinued without dose adjustment. At 11 d of discontinuation, the INR value increased to 10.2, then dose reduced to 7.5 mg/d |
| Raru et al[29], 2019 | United States | White | 67 | Male | 2.0-3.0 | Pulmonary vein thrombosis, tuberculosis | - | 10 mg/d |
The Chinese population is also significantly different from other populations in terms of dose adjustment of warfarin. From Table 1, it can be concluded that the dose of warfarin in the non-Chinese population to control the INR value in the therapeutic range is large, with a maximum of 30 mg/d. Our patient reached the therapeutic range at 10.5 mg/d, which further reflects the differences in the population, suggesting that ethnicity may play a role in warfarin therapy. It is necessary to conduct special research on the Chinese population.
In the present case, the pharmacist reduced the dose by 21% immediately after discontinuing rifampicin. Because of the half-life of warfarin, it decreased by another 9% within 2 d and by 42% within 3 d, which is like other case reports. A 71-year-old Caucasian woman had the dose of warfarin reduced by 30% after discontinuing rifampicin and subsequently by 20% within 3 d[18]. In most cases, warfarin dose was directly reduced by 25%-75% within 1 wk after stopping rifampicin, while in a few cases warfarin was stopped for 1-2 d or unchanged[19,20].
Knowledge of the interaction of warfarin with other drugs is changing rapidly, and the active participation of pharmacists in the adjustment of clinical medications will help to reduce the burden on doctors[21]. Rapid and effective adjustment of warfarin dose is still a problem[22]. Chinese pharmacists are gradually developing anti-coagulation management for patients[23]. In this case, the dose of warfarin was adjusted for up to 6 mo, which increased the patient’s financial burden and inconvenience. Although doctors and nurses provide anticoagulation management services, the active participation of pharmacists plays a vital role. This case is the embodiment of the advantages of the pharmacist clinic. In other countries, pharmacist outpatient management has proved to be effective. Outpatient anticoagulation services based on pharmacist management have a lower incidence of adverse reactions and a higher control rate than conventional anticoagulation care[24].
The present study had some limitations. The patient’s initial dose based on genetic testing results was not applied to this case nor after rifampicin was discontinued. Second, this case referred to instructions for a 20% weekly increase in warfarin without considering the large effect of rifampicin on warfarin metabolism. We should have increased titration dose and reduced titration times. In the future, effective experience will be drawn to benefit more patients.
The combination of rifampicin makes warfarin dose control difficult. The anticoagulation of valve replacement in the Chinese population is different from that in other races. The establishment of a pharmacist clinic provides vital assistance in warfarin dose adjustment.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Schoenhagen P, Turner AM S-Editor: Fan JR L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Mosnier LO. Warfarin, a juggler's demise. Blood. 2018;131:2742-2743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Wells PS, Holbrook AM, Crowther NR, Hirsh J. Interactions of warfarin with drugs and food. Ann Intern Med. 1994;121:676-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 358] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 3. | Greenblatt DJ, von Moltke LL. Interaction of warfarin with drugs, natural substances, and foods. J Clin Pharmacol. 2005;45:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Haibo Z, Jinzhong L, Yan L, Xu M. Low-intensity international normalized ratio (INR) oral anticoagulant therapy in Chinese patients with mechanical heart valve prostheses. Cell Biochem Biophys. 2012;62:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Zhang H, Dong Y, Ao X, Zhu D, Dong L. Optimal oral anticoagulant therapy in Chinese patients with mechanical heart valves. Eur J Pharm Sci. 2020;144:105202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, Blatchford J, Devenny K, Friedman J, Guiver K, Harper R, Khder Y, Lobmeyer MT, Maas H, Voigt JU, Simoons ML, Van de Werf F; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 978] [Cited by in RCA: 1027] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 7. | Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivistö KT. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet. 2003;42:819-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 544] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 8. | Romankiewicz JA, Ehrman M. Rifampin and warfarin: a drug interaction. Ann Intern Med. 1975;82:224-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Baciewicz AM, Chrisman CR, Finch CK, Self TH. Update on rifampin, rifabutin, and rifapentine drug interactions. Curr Med Res Opin. 2013;29:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Olsen SC, Palmer MV. Advancement of knowledge of Brucella over the past 50 years. Vet Pathol. 2014;51:1076-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 11. | de Figueiredo P, Ficht TA, Rice-Ficht A, Rossetti CA, Adams LG. Pathogenesis and immunobiology of brucellosis: review of Brucella-host interactions. Am J Pathol. 2015;185:1505-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 319] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 12. | Solera J, Solís García Del Pozo J. Treatment of pulmonary brucellosis: a systematic review. Expert Rev Anti Infect Ther. 2017;15:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7:775-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 716] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 14. | Ariza J, Bosilkovski M, Cascio A, Colmenero JD, Corbel MJ, Falagas ME, Memish ZA, Roushan MR, Rubinstein E, Sipsas NV, Solera J, Young EJ, Pappas G; International Society of Chemotherapy; Institute of Continuing Medical Education of Ioannina. Perspectives for the treatment of brucellosis in the 21st century: the Ioannina recommendations. PLoS Med. 2007;4:e317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 265] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 15. | Kaminsky LS, Zhang ZY. Human P450 metabolism of warfarin. Pharmacol Ther. 1997;73:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 579] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 16. | Holford NH. Clinical pharmacokinetics and pharmacodynamics of warfarin. Understanding the dose-effect relationship. Clin Pharmacokinet. 1986;11:483-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 192] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Heimark LD, Gibaldi M, Trager WF, O'Reilly RA, Goulart DA. The mechanism of the warfarin-rifampin drug interaction in humans. Clin Pharmacol Ther. 1987;42:388-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Krajewski KC. Inability to achieve a therapeutic INR value while on concurrent warfarin and rifampin. J Clin Pharmacol. 2010;50:710-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Martins MA, Reis AM, Sales MF, Nobre V, Ribeiro DD, Rocha MO, Ribeiro AL. Rifampicin-warfarin interaction leading to macroscopic hematuria: a case report and review of the literature. BMC Pharmacol Toxicol. 2013;14:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Fahmi AM, Abdelsamad O, Elewa H. Rifampin-warfarin interaction in a mitral valve replacement patient receiving rifampin for infective endocarditis: a case report. Springerplus. 2016;5:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Vazquez SR. Drug-drug interactions in an era of multiple anticoagulants: a focus on clinically relevant drug interactions. Hematology Am Soc Hematol Educ Program. 2018;2018:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Lee CR, Thrasher KA. Difficulties in anticoagulation management during coadministration of warfarin and rifampin. Pharmacotherapy. 2001;21:1240-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Zhang J, Liu M, Chen Q, Wu J, Cao H. Outcomes of an online pharmacist-managed anticoagulation clinic for individuals on warfarin therapy living in rural communities. Thromb Res. 2017;157:136-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Manzoor BS, Cheng WH, Lee JC, Uppuluri EM, Nutescu EA. Quality of Pharmacist-Managed Anticoagulation Therapy in Long-Term Ambulatory Settings: A Systematic Review. Ann Pharmacother. 2017;51:1122-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 25. | Self TH, Mann RB. Interaction of rifampin and warfarin. Chest. 1975;67:490-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Almog S, Martinowitz U, Halkin H, Bank HZ, Farfel Z. Complex interaction of rifampin and warfarin. South Med J. 1988;81:1304-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Casner PR. Inability to attain oral anticoagulation: warfarin-rifampin interaction revisited. South Med J. 1996;89:1200-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Unal S, Bayrakci B, Yasar U, Karagoz T. Successful treatment of propafenone, digoxin and warfarin overdosage with plasma exchange therapy and rifampicin. Clin Drug Investig. 2007;27:505-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Raru Y, Abouzid M, Zeid F, Teka S. Pulmonary vein thrombosis secondary to tuberculosis in a non-HIV infected patient. Respir Med Case Rep. 2019;26:91-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |