Published online Feb 16, 2021. doi: 10.12998/wjcc.v9.i5.1079
Peer-review started: June 4, 2020
First decision: November 30, 2020
Revised: December 13, 2020
Accepted: December 23, 2020
Article in press: December 23, 2020
Published online: February 16, 2021
Processing time: 234 Days and 21.3 Hours
Chloracne is a rare skin condition that is caused by systemic exposure to halogenated aromatic compounds. The main characteristic of chloracne is blackhead, and in severe cases, it can be accompanied by systemic symptoms. Sodium 3,5,6-trichloropyridin-2-ol (STCP) is a necessary precursor compound for the production of chlorpyrifos and triclopyr, which are extensively used as a pesticide and herbicide, respectively. STCP is also a chlorophenol that has been associated with chloracne. STCP poisoning could induce mild myelin sheath damage. We herein report three cases with chloracne due to exposure to STCP.
Three young men, aged 29, 33, and 26 years, respectively, in the same workplace had polymorphic skin lesions, characterized mainly by comedones and cysts, and one of them also had acne like lesions in the genital area. These clinical manifestations appeared when they were exposed to STCP for 3 d, 1 wk, and 2 wk, respectively. Among them, polyneuropathy and liver damage occurred. We performed dermoscopy and clinical and laboratory tests on these patients. Additionally, histopathology was used for further diagnosis in the serious patient. These patients were diagnosed with chloracne and separated from STCP. The patients were prescribed oral viaminate capsules, topical adapalene gel, and regular hematologic follow-up for aspartate transaminase and lipids. They are still under follow-up. There was no new lesions and the laboratory tests returned to normal in two patients. Pigmentation and shallow scars remained in the original areas of papules. However, in the most serious patient, new papules still appeared intermittently. All these remind us that the treatment of chloracne caused by STCP is difficult, and we should attach great importance to this new compound related with the neuropathy and chloracne.
STCP is becoming a new chemical product to induce chloracne, which should attract the attention of all medical professionals, especially dermatologists. Due to the lack of knowledge on the new chemical, the diagnosis of chloracne cannot be made in time. Chloracne still deserves our attention.
Core Tip: Chloracne, an acneiform eruption resulting from poisoning by halogenated aromatic compounds, is a rare clinical disease with systemic effects in severe instances. In recent years, new chloracnegens are emerging with the increasing number of new chemical compounds. Chloracne is on the rise again, and it is often misdiagnosed and missed, and cannot be treated. We report three cases of chloracne patients who were delayed due to not getting a correct diagnosis in time, which reminds us to be alert to the emergence of chloracne induced by sodium 3,5,6-trichloropyridin-2-ol.
- Citation: Ma Y, Cao X, Zhang L, Zhang JY, Qiao ZS, Feng WL. Neuropathy and chloracne induced by 3,5,6-trichloropyridin-2-ol sodium: Report of three cases. World J Clin Cases 2021; 9(5): 1079-1086
- URL: https://www.wjgnet.com/2307-8960/full/v9/i5/1079.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i5.1079
Chloracne is caused by exposure to halogenated aromatic compounds. It is characterized by comedo-like lesions and yellowish cysts on the face and involves body areas that are not usually affected by acne. The skin becomes greyish, hyperproliferative at times, and is associated with folliculitis and systemic manifestations occasionally[1]. Chloracne commonly occurs in workers who were occupationally exposed to naphthalene and chlorinated biphenyls (e.g., chemical industry workers exposed to pesticides). A number of chloracnegenic chemicals have been identified, such as chlorinated phenols, chloronaphthalenes, polychlorinated biphenyls (PCBs), and other polychlorinated compounds, including polyhalogenated dibenzofurans, polychlorinated dibenzo-p-dioxins, and chlorinated azo- and azoxybenzenes[2]. Exposure to dioxins is regarded as the major cause of chloracne. They induce chloracne and transient hepatotoxicity. As a hallmark lesion, chloracne is specific and sensitive to halogenated aromatic hydrocarbon intoxication and can be observed during the epidemiological assessment for occupational exposure thresholds[3]. According to a report in 1974, the patients who were exposed to pentachlorophenol, trichlorobenzene (TCB), and 2,3,7,8-tetrachlorodibenzop-dioxin (TCDD) developed chloracne in different proportions of poisoning cases[2]. Synthetic resins have replaced these compounds in 1960s, and since then the incidence of chloracne has dramatically dropped[4]. Sodium 3,5,6-trichloropyridine-2-ol (STCP) is a precursor compound for the production of chlorpyrifos and triclopyr, which are extensively used as a pesticide and herbicide, respectively[5]. Little is known regarding STCP intoxication. With the increased production and use of STCP, the chance of STCP intoxication also rises. In this study, three workers were exposed to STCP when installing the roof for a factory producing STCP, and they developed symptoms just 3 d after the exposure. We hope that these three cases can provide some information for conducting future studies on STCP[6].
Three young man came to the clinic due to skin papule on the face and trunk for 5 mo.
Between May 5, 2019 and May 7, 2019, three male young constructors installed the roof for a factory producing STCP for many years without undertaking any standard preventive measures. Three days later, all the three had fatigue, and was accompanied by bilateral lower limb pain in patient 1 and patient 3. Patient 1 could not walk due to severe bilateral lower limb pain, while the other two had mild symptoms, with no dizziness, headache, or limb numbness. Patient 1 visited a local clinic, and took Chinese herbs, which provided no significant efficacy. Sequentially, he experienced increased eye secretions with itching, difficulty to keep the eye open, and nausea but with no abdominal pain or vomiting. On June 11, he was transferred to another hospital, where he was diagnosed as having “acrylonitrile poisoning”. He was given glucocorticoids and treated for liver function protection, myocardial nutrition, and neurotherapy. After that, the bilateral feet pain alleviated, but the papules remained unchanged. On June 14, he visited a local dermatology hospital where he was diagnosed with STCP intoxication, chemical liver injury, and peripheral nerve injury. After a week of STCP exposure, the patient 2 also developed papules on the head, face, and neck. Two weeks later, similar papules occurred in the patient 3. In all workers, tender papules occurred in multiple regions including the head, face, neck, torso, and four limbs. However, there was obvious acne like rash in the perineum in patient 1. On September 6, 2019, these three patients were transferred to our hospital due to minimal papule improvement and newly occurring papules.
None of the three young men had obvious diseases in the past.
The family histories were unremarkable.
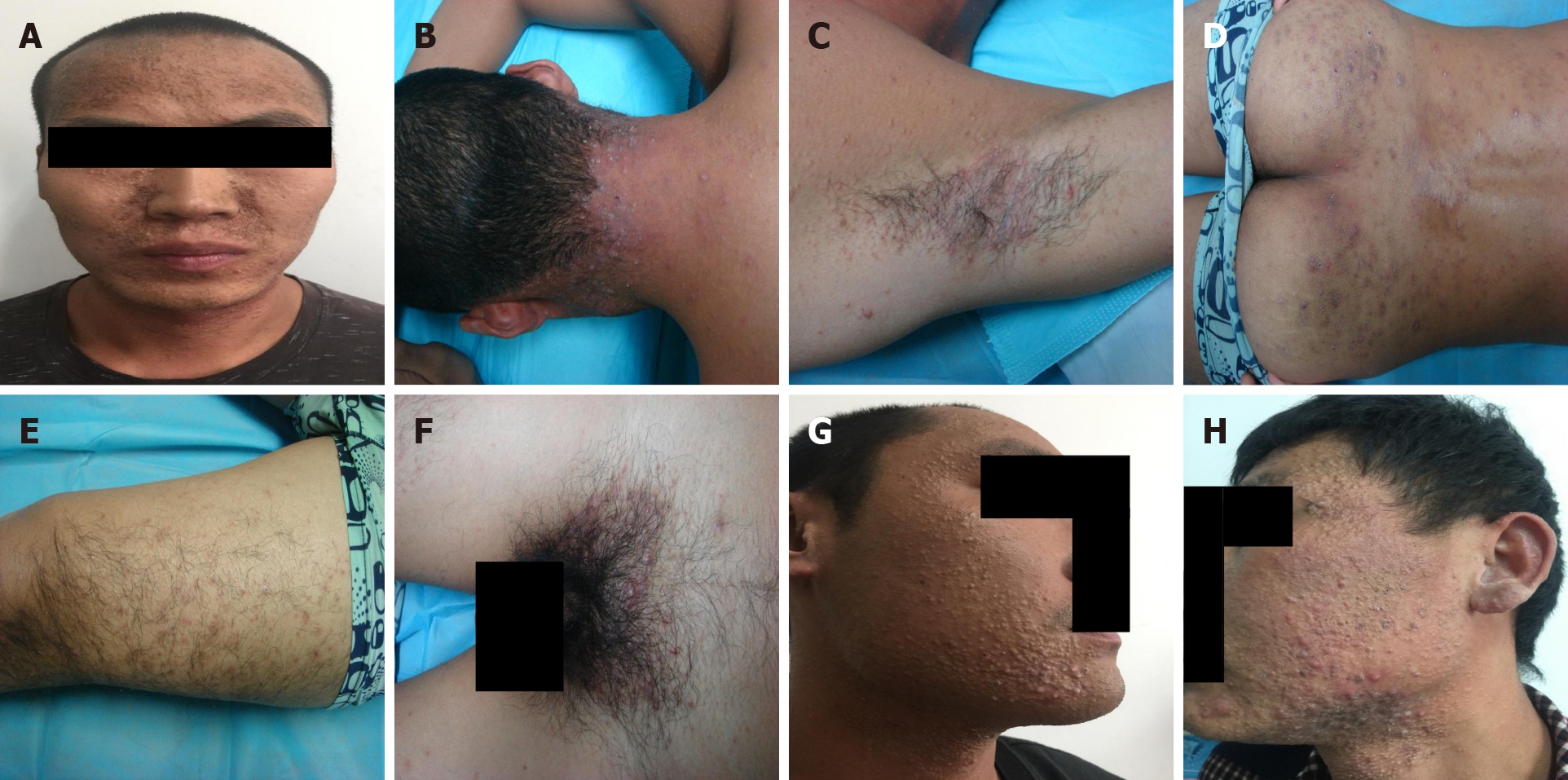
All the three patients were conscious and moderately nourished, and could speak clearly. No lymph node enlargement was found in any part of the body. The pupillary light reflex appeared normal and no obvious abnormality was found in cardiopulmonary auscultation. The abdomen was soft, with no tenderness or rebound tenderness. The motor and sensory functions of the limbs remained good, the physiological reflex existed, but no pathological reflex was elicited. Dermatological examination showed intensive distribution of yellow to brownish, miliary to mung bean-sized papules and nodules on the face, head, neck, retroauricular region, and torso of the three patients. Some nodules were covered with pus plugs and blackheads. Similar papules were discretely found on all the four limbs. In patient 1, the acne-like lesions were also seen in the perineal region (Figure 1).
Liver function tests indicated significant alanine aminotransferase and aspartate aminotransferase elevations in patients 1 and patient 3, reaching the highest level of up to 23000 U/L and patient 2 had normal aspartate aminotransferase (AST) levels. Laboratory examination indicated that there was a significant increase in white blood cell count and percentage of neutrophils in patients 1 and 3. No abnormality was found in electrocardiogram, chest X-ray, and abdominal color ultrasound (Table 1).
| Characteristics | Patient 1 | Patient 2 | Patient 3 |
| Sex | Male | Male | Male |
| Age | 29 | 33 | 26 |
| Manifestation of the skin eruptions | Grey-black changes on the face, intensive distribution of yellow to brownish, miliary to mung bean-sized papules and nodules were seen over the head, face, neck, torso, armpit, lower limb, hips, and perineum; some lesions were covered with pus plugs and blackheads; some lesions were fused | Intensive distribution of yellow to brownish, miliary to mung bean-sized papules and nodules were seen over the head and face; some lesions were covered with pus plugs and blackheads; similar lesions were distributed discretely over the other body parts | Intensive distribution of yellow to brownish, miliary to mung bean-sized papules and nodules over the face, neck, and chest were observed; some lesions were covered with pus plugs and blackheads; similar lesions were distributed discretely over the other body parts |
| Time to skin lesion | 3 d | 1 wk | 2 wk |
| Neurological damages | Bilateral calf pain | None | Bilateral calf pain |
| Ocular symptoms | Increased eye secretions with itching, and difficulty to keep eye open | None | None |
| Gastrointestinal symptoms | Nausea, but no abdominal pain, diarrhea, or vomiting | None | None |
| Liver function | ALT 20105.30 U/L, AST 8074.50 U/L (significantly increased AST) | ALT 45.30 U/L, AST 38.80 U/L (normal) | ALT 23453.40 U/L, AST 9735.23 U/L (significantly increased AST) |
| Routine blood test | WBC 23.20 × 109/L, NE% 93.80% | Normal | WBC 21.40 × 109/L, NE% 89.70% |
| Liver function (3 mo later) | ALT 22.70 U/L, AST 28.50 U/L | ALT 31.10 U/L, AST 20.80 U/L | ALT 35.20 U/L, AST 54.90 U/L |
| Routine blood test (3 mo later) | WBC 8.58 × 109/L, NE% 78.74% | WBC 7.22 × 109/L | WBC 9.43 × 109/L |
| Treatments | Valentate, mecobalamin, oral mediaction with diclofenac sodium and codeine phosphate tablet, and topical use of adapalene gel | Valentate, mecobalamin, oral mediaction with diclofenac sodium and codeine phosphate tablet, and topical use of adapalene gel | Valentate, mecobalamin, oral mediaction with diclofenac sodium and codeine phosphate tablet, and topical use of adapalene gel |
| Current status (1 yr later) | Newly occurring rashes are still seen intermittently on the neck | Skin eruptions on the face have subsided at present | Skin eruptions on the face have subsided at present |
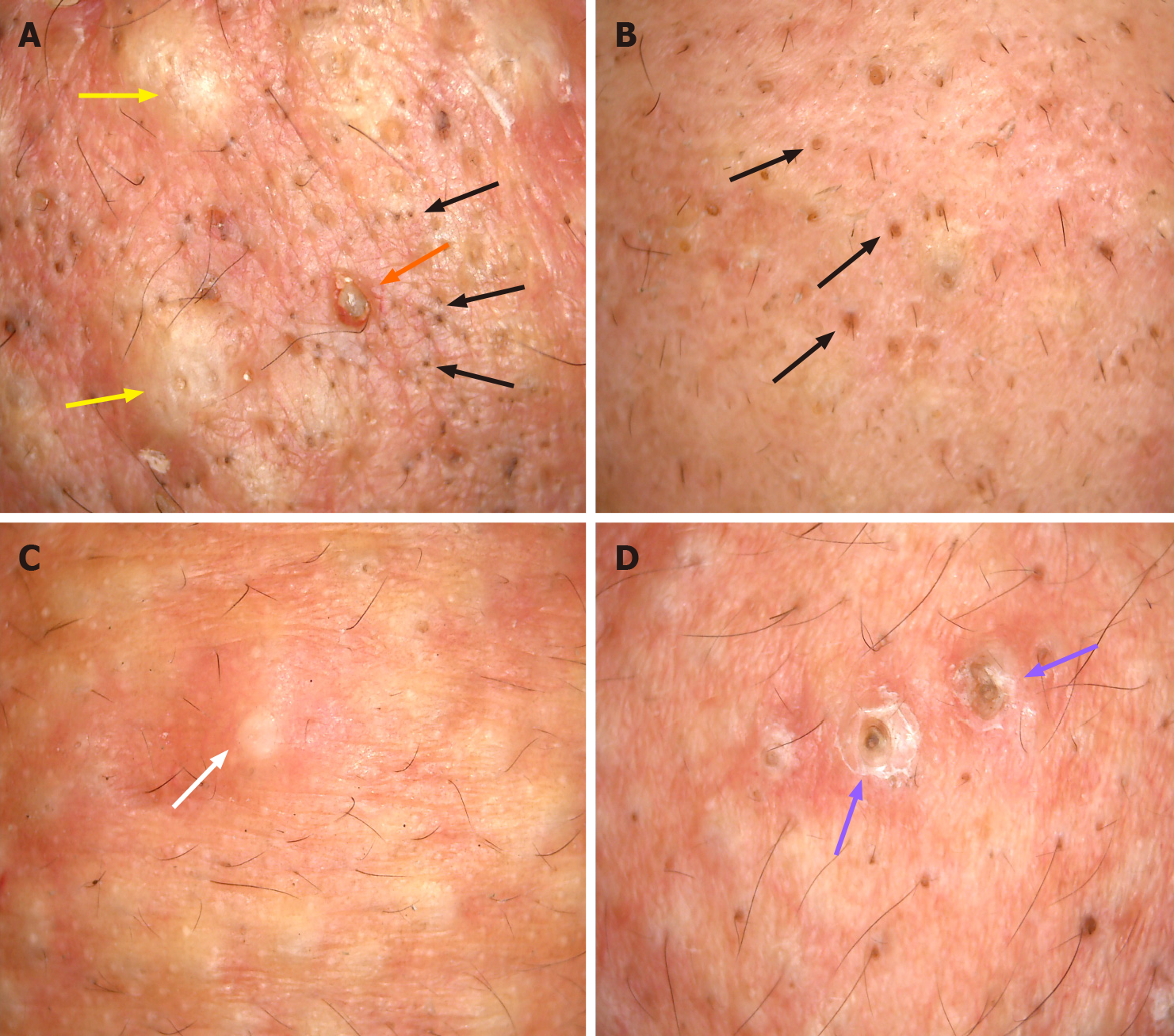
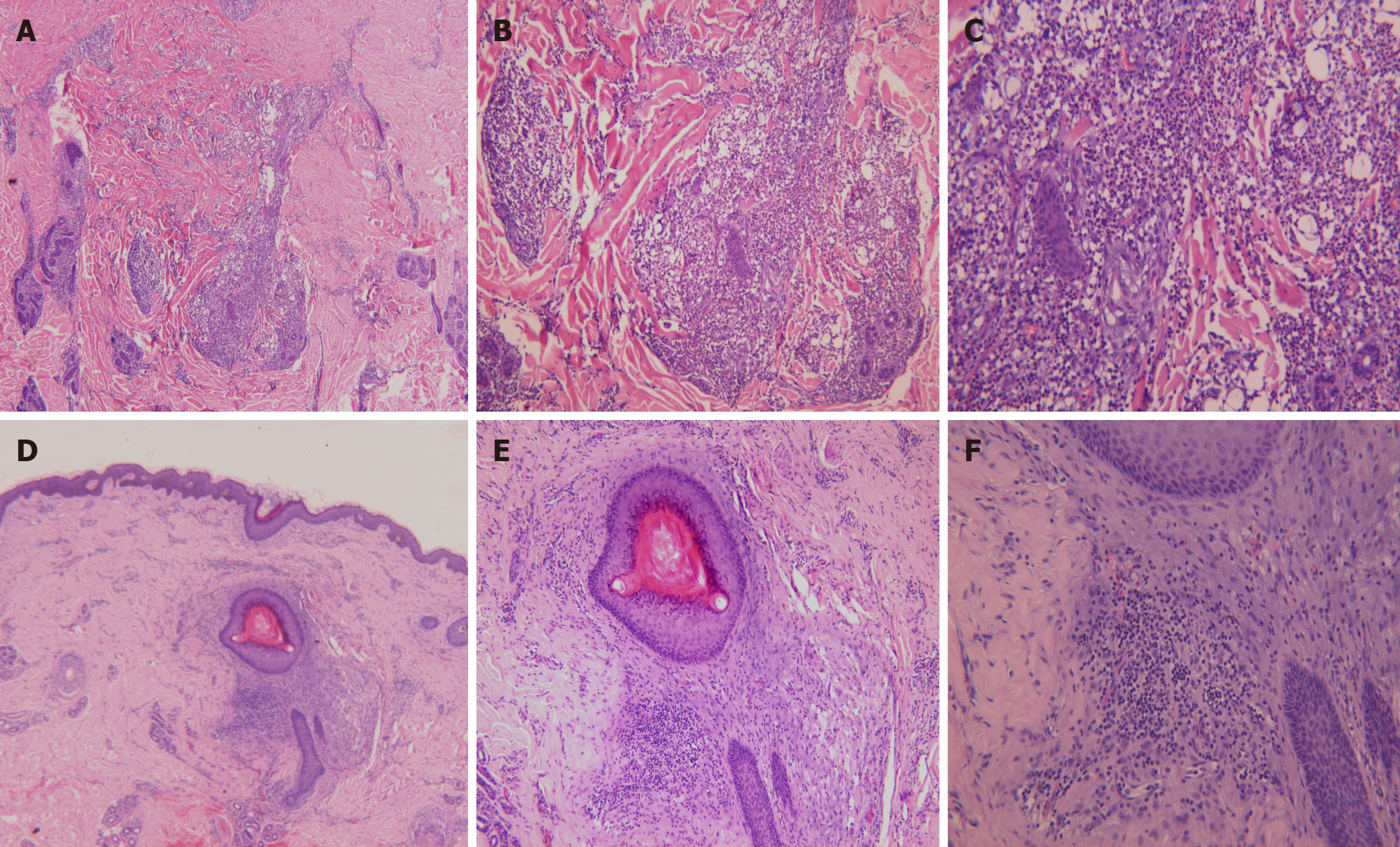
All the three patients underwent dermoscopy on a light red background, with multiple small blackheads, slightly larger dark brown solid plugs, yellow-white inflammatory papules and pustules, inflammatory erythema, and focally branched atypical blood vessels observed (Figure 2). Biopsies from the ear and back tissues were taken from patient 1 whose condition was more severe. Biopsy results of the back tissues revealed folliculitis and perifolliculitis as evidenced by hyperkeratinization, follicular keratotic plugs, focal epidermal hyperproliferation, vasodilatation and hyperemia in the dermis, destruction of hair follicles and sebaceous glands, and a large number of perifollicular neutrophil-dominant inflammatory infiltration. Biopsy results of the ear tissues showed fairly normal epidermis and perifollicular lymphocyte-dominant inflammatory infiltration, suggesting perifolliculitis (Figure 3).
Based on the exposure history, clinical manifestations, biopsy results, patient occupa-tion and clustering onset disease, a clear diagnosis of chloracne was made.
The local dermatology hospital prescribed viaminate capsules (Chongqing Huapont Pharm Co., Ltd.) 50 mg tid, po, mecobalamin (Eisai Co., Ltd.) 500 μg, tid, po, and glcyrrhizin injection, 60 mg, qd, iv (Minophagen Pharmaceutical Co., Ltd. Zama Factory). On September 6, 2019, the patients were transferred to our hospital due to minimal papule improvement and newly occurring papules. We recommended continuation of oral medication of viaminate capsules and adapalene gel (once each night, topical application), and regular check of AST as well as blood lipids monthly.
The last follow-up visit occurred in May 2020, which showed no newly occurring papules or pigmentation in patient 1 and patient 3; a shallow scar remained in the original areas of papules, and both patients had normal laboratory results. Unfortunately, intermittent papules were still occurring in patient 1 who was in severe condition at that time.
Chloracne, which is also known as occupational acne, is a unique acne-like lesion induced by exposure to halogenated aromatic compounds. It was named in 1899 by Herxheimer, and was described in a 22-year-old worker who developed skin eruptions including blackheads, pustules, and abscesses after exposing to chlorine compounds[7]. STCP is synthesized from trichloroacetyl chloride and acrylonitrile, and acts as a precursor compound for the production of chlorpyrifos. This is a highly efficient and low-toxic organophosphorus pesticide obtained after condensation reaction between STCP and ethyl chloride. Most of the chloracnegenic substances have a common characteristic of the presence of a halogen at the periphery of the molecule. In STCP molecule, a halogen is present at the periphery and therefore it is considered as chloracnegenic. In this report, all three patients have developed papules and neurological symptoms after a 3-d stay in an STCP factory. Similar cases have been reported in the past[8]. The very short time for the onset of the disease after exposure might be due to the lack of undertaking preventions and their physical conditions. In addition, studies have shown that older, taller, less educated workers with a history of alcohol adverse reactions have an increased risk of neurological deterioration with the increase of chlorpyrifos exposure time[9]. Repeated exposures could give rise to cumulative NTE inhibition, which could eventually reach the critical level for triggering neuropathy[10].
Based on the history of unprotected acute exposure to chloracnegenic substances, clinical manifestations, dermoscopic findings, biopsy results, and career identification results at our hospital, a diagnosis of chloracne due to STCP toxicity was made in these three patients. However, toxic levels of STCP are unable to detect in time due to the poor local medical level and the fact that the factory failed to provide the test report of occupational harmful factors. The typical skin manifestations of chloracne are regarded as acneiform lesions. Chloracne is different from acne vulgaris and can affect all age groups and all follicles of the areas exposed. Chloracne has a unique skin distribution, and the typical regions included cheek (para- and suborbital regions), retroauricular region, and genital areas (especially the scrotum). In severe cases, the skin eruptions can also spread to shoulder, torso, hip, and abdomen regions. Nasal and oral regions are generally not affected. The skin eruptions are usually ac-companied by pigmentation or skin hyperproliferation, and inflammatory pustules and large cysts appear in severe cases. Contrarily, acne vulgaris occurs on the face, neck, chest, back, shoulder and in 15-to-25-year-old individuals, and is closely related to androgen hormone. Dermoscopic features also differ between acne vulgaris and chloracne. Acne vulgaris under dermoscopy is manifested as: (1) Inflammatory acne characterized by a round structure with sharp white center, brown edge, and erythema; and (2) Whitehead acne is mainly manifested as yellow solid central plug with discrete erythema. Chloracne is mainly presented as numerous small blackheads, slightly larger dark brown solid plugs, yellow-white inflammatory papules and pustules, inflammatory erythema, and focally branched atypical blood vessels. Intoxication following STCP absorption led to other consequences such as neurological lesions, liver damage, gastrointestinal reaction, conjunctivitis, and loss of libido. Except skin eruptions, the remaining symptoms showed improvement in two of the three patients after undergoing symptomatic treatment.
At present, cases of dioxin chloracne induced by 2,3,7,8-tetrachlorodibenzop are more frequently reported[11]. Gawkrodger et al described an outbreak of chloracne among seven discovery chemists who synthesized novel polycyclic halogenated chemical compounds that were classified as triazoloquinoxalines, not known to be chloracnegenic[12]. However, STCP-induced chloracne is considered a rare skin disease. Between 2012 and 2019, there were only 27 cases of STCP intoxication in China, which included six cases from the previously published reports in the international journals[6,13]. In the previously reported cases, the time of onset after STCP exposure varied from 2 to 10 mo, but cases with such a short duration of onset after exposure has never been reported. The dermoscopic findings revealed features that are significantly different from the characteristics of acne vulgaris. These features, which cannot be detected by the naked eye, assisted in providing a novel detection method for diagnosing chloracne and therefore merit attention.
So far, the pathogenesis of STCP-induced chloracne is still unknown. Currently, the molecular mechanisms regarding the pathogenesis of chloracne included aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear transporter pathway[14], protein phosphorylation signaling transduction pathway[15], theory of hair follicle stem cell homeostasis[11], and homeostatic functions of AhR in stem/progenitor cells[16]. All these provided clues for the mechanisms of STCP-induced skin eruptions. The relationship between the severity of the disease and its toxicometric effect cannot be determined.
The key method to control chloracne is to avoid contact with chloracnegenic substances. All the three patients were separated from the work environment since the onset of this disease and received symptomatic treatments, but the acne-like rash on the face did not change significantly. It has been reported that in six patients with chloracne unresponsive to conventional therapy, the lesions were cleared by treatment with EMLA [eutectic mixture of lignocaine (lidocaine) 25 mg/g and prilocaine 25 mg/g] topical anesthesia and light cautery[17]. A recent study indicated that agents with AHR inhibiting and NRF2 activating activities can treat chloracne caused by dioxins. Cinnamaldehyde (a functional component of C. cassia) and perillaldehyde (a functional component of P. frutescens) are potent inhibitors of AHR–CYP1A1 signaling and activates the NRF2–antioxidative axis. The cinnamaldehyde is capable of improving chloracne that is induced by dioxin[18]. These natural phytochemicals are useful for managing chloracne. Surgery can achieve efficacious results for blackheads failing to respond to other therapies. Psychological treatment and caring methods including reasonable lower limb function training and skin cleansing assist in effectively improving peripheral nerve and skin symptoms in patients with STCP intoxication. Patients were advised to apply enhanced personal preventive measures during work, separate themselves from the working environment, and visit the doctor regularly. Follow-up 1 year after the onset showed persistent and reoccurring skin eruptions and mild hand and foot numbness in all the three patients.
Due to inadequate knowledge of STCP, the correct diagnosis or timely treatment was not provided to these three patients when presenting with initial symptoms. These three cases warn the dermatologists regarding the complexity in the causes of chloracne and the possibility of chloracne induced by emergent novel chemicals. Chloracne can be a difficult diagnosis to make when it occurs in a novel setting: Occupational physicians and dermatologists need to be vigilant when dealing with unusual eruptions. Chloracne is still a problem that is worthy of concern.
We wish to express our gratitude for the cooperation of the three patients during the examination and therapeutic process and their approval for sharing the cases.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bansal A, Langford M, Youness RA S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Passarini B, Infusino SD, Kasapi E. Chloracne: still cause for concern. Dermatology. 2010;221:63-70. [PubMed] [DOI] [Full Text] |
| 2. | Ju Q, Zouboulis CC, Xia L. Environmental pollution and acne: Chloracne. Dermatoendocrinol. 2009;1:125-128. [PubMed] [DOI] [Full Text] |
| 3. | Suskind RR. Chloracne, "the hallmark of dioxin intoxication". Scand J Work Environ Health. 1985;11:165-171. [PubMed] [DOI] [Full Text] |
| 4. | Pesatori AC, Consonni D, Rubagotti M, Grillo P, Bertazzi PA. Cancer incidence in the population exposed to dioxin after the "Seveso accident": twenty years of follow-up. Environ Health. 2009;8:39. [PubMed] [DOI] [Full Text] |
| 5. | Liu N, Cui HY, Yao D. Decomposition and oxidation of sodium 3,5,6-trichloropyridin-2-ol in sub- and supercritical water. Process Saf Environ Prot. 2009;87:387-394. [DOI] [Full Text] |
| 6. | Niu YM, Hao FT, Xia YJ. Sodium 3,5,6-trichloropyridin-2-ol poisoning: report of four cases. Toxicol Ind Health. 2014;30:475-479. [PubMed] [DOI] [Full Text] |
| 7. | Bridget K, Megan J, Chevy C, Cynthia CD. Chloracne in a suburban landscaper: A case report. J Am Acad Dermatol. 2016;74:3594. [DOI] [Full Text] |
| 8. | Wu N, Hao F, Yu X. Peripheral nerve and skin damage associated with working in a STCP factory: report of four cases. Clin Toxicol (Phila). 2012;50:514-517. [PubMed] [DOI] [Full Text] |
| 9. | Albers JW, Garabrant DH, Mattsson JL, Burns CJ, Cohen SS, Sima C, Garrison RP, Richardson RJ, Berent S. Dose-effect analyses of occupational chlorpyrifos exposure and peripheral nerve electrophysiology. Toxicol Sci. 2007;97:196-204. [PubMed] [DOI] [Full Text] |
| 10. | Richardson RJ. Assessment of the neurotoxic potential of chlorpyrifos relative to other organophosphorus compounds: a critical review of the literature. J Toxicol Environ Health. 1995;44:135-165. [PubMed] [DOI] [Full Text] |
| 11. | Panteleyev AA, Bickers DR. Dioxin-induced chloracne--reconstructing the cellular and molecular mechanisms of a classic environmental disease. Exp Dermatol. 2006;15:705-730. [PubMed] [DOI] [Full Text] |
| 12. | Gawkrodger DJ, Harris G, Bojar RA. Chloracne in seven organic chemists exposed to novel polycyclic halogenated chemical compounds (triazoloquinoxalines). Br J Dermatol. 2009;161:939-943. [PubMed] [DOI] [Full Text] |
| 13. | Liu L, Wang G, Gao TW. Severe 3,5,6-Trichloropyridin-2-ol sodium induced Chloracne. J Dermatol. 2012;39:264. |
| 14. | Chiaro CR, Patel RD, Marcus CB, Perdew GH. Evidence for an aryl hydrocarbon receptor-mediated cytochrome p450 autoregulatory pathway. Mol Pharmacol. 2007;72:1369-1379. [PubMed] [DOI] [Full Text] |
| 15. | Cheon H, Woo YS, Lee JY, Kim HS, Kim HJ, Cho S, Won NH, Sohn J. Signaling pathway for 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced TNF-alpha production in differentiated THP-1 human macrophages. Exp Mol Med. 2007;39:524-534. [PubMed] [DOI] [Full Text] |
| 16. | Bock KW. Toward elucidation of dioxin-mediated chloracne and Ah receptor functions. Biochem Pharmacol. 2016;112:1-5. [PubMed] [DOI] [Full Text] |
| 17. | Yip J, Peppall L, Gawkrodger DJ, Cunliffe WJ. Light cautery and EMLA in the treatment of chloracne lesions. Br J Dermatol. 1993;128:313-316. [PubMed] [DOI] [Full Text] |
| 18. | Furue M, Fuyuno Y, Mitoma C, Uchi H, Tsuji G. Therapeutic Agents with AHR Inhibiting and NRF2 Activating Activity for Managing Chloracne. Antioxidants (Basel). 2018;7. [PubMed] [DOI] [Full Text] |