Published online Feb 16, 2021. doi: 10.12998/wjcc.v9.i5.1026
Peer-review started: October 11, 2020
First decision: November 29, 2020
Revised: December 13, 2020
Accepted: December 23, 2020
Article in press: December 23, 2020
Published online: February 16, 2021
Processing time: 111 Days and 2.8 Hours
pT2+ prostate cancer (PCa), a term first used in 2004, refers to organ-confined PCa characterized by a positive surgical margin (PSM) without extracapsular extension. Patients with a PSM are vulnerable to biochemical recurrence (BCR) following radical prostatectomy (RP); however, whether adjuvant radiotherapy (aRT) is imperative to PSM after RP remains controversial. This study had the longest follow-up on pT2+ PCa after robotic-assisted RP since 2004. Moreover, we discussed our viewpoints on pT2+ PCa based on real-world experiences.
To conclude a 10-year surveillance on pT2+ PCa and compare our results with those of the published literature.
Forty-eight patients who underwent robotic-assisted RP between 2008 and 2011 were enrolled. Two serial tests of prostate specific antigen (PSA) ≥ 0.2 ng/mL were defined as BCR. Various designed factors were analyzed using statistical tools for BCR risk. SAS 9.4 was applied and significance was defined as P < 0.05. Univariate, multivariate, linear regression, and receiver operating characteristic (ROC) curve analyses were performed for statistical analyses.
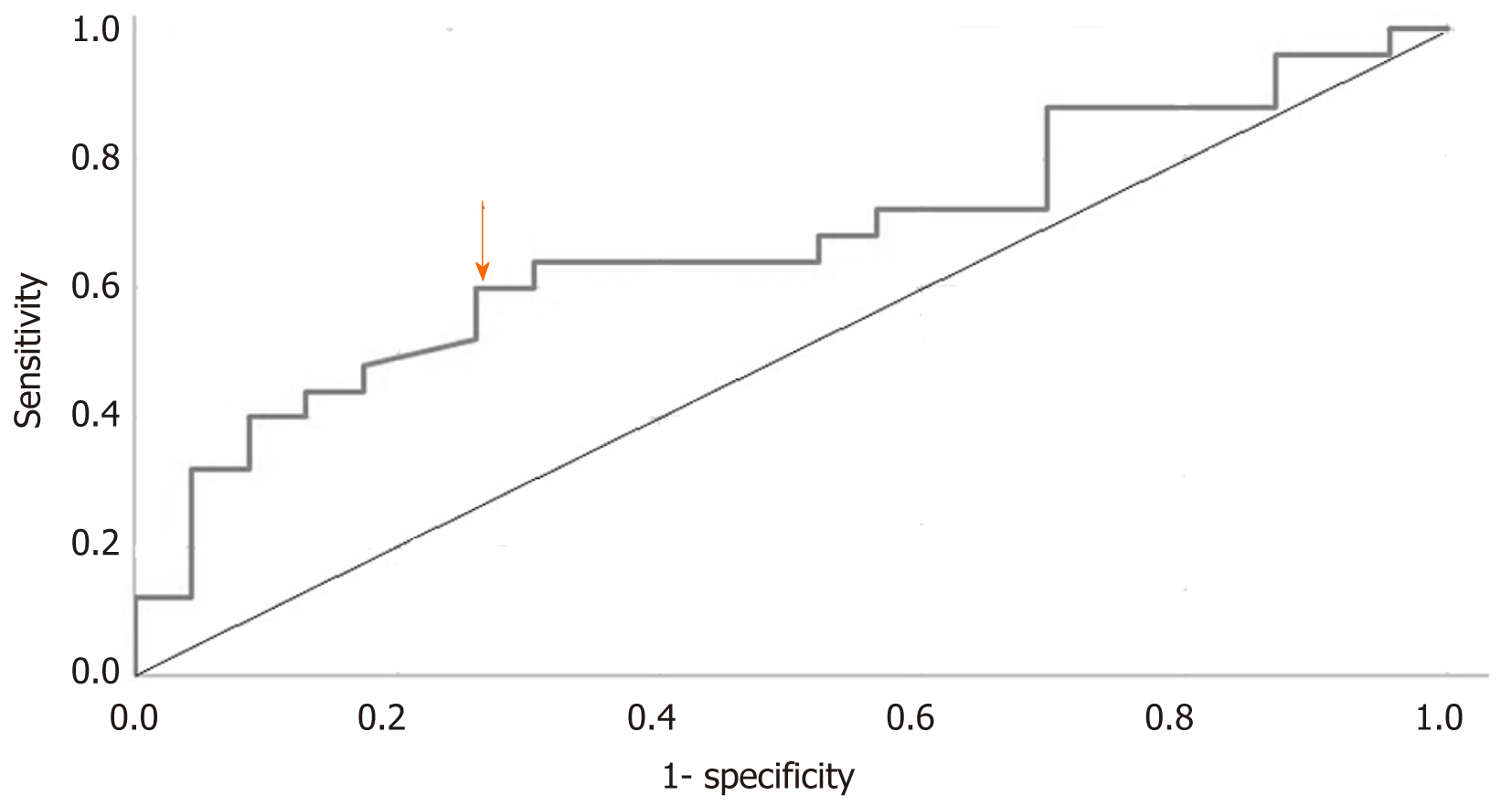
With a median follow-up period of 9 years, 25 (52%) patients had BCR (BCR group), and the remaining 23 (48%) patients did not (non-BCR group). The median time for BCR test was 4 years from the first postoperative PSA nadir. Preoperative PSA was significantly different between the BCR and non-BCR groups (P < 0.001), and ROC curve analysis of preoperative PSA suggested a cut-off value of 19.09 ng/mL (sensitivity, 0.600; specificity: 0.739). The linear regression analysis showed no correlation between time to BCR and preoperative PSA (Pearson’s correlation, 0.13; adjusted R2 = 0.026).
Robotic-assisted RP in pT2+ PCa of worse conditions can provide better BCR-free survival. A surgical technique limiting the PSM in favorable situations is warranted to lower the pT2+ PCa BCR rate. Preoperative PSA cut-off value of 19.09 ng/mL is a predictive factor for BCR. Based on our experiences and review of the literature, we do not recommend routine aRT for pT2+ PCa.
Core Tip: The term pT2+ is coined in 2004 and for prostate cancer (PCa) with a positive surgical margin (PSM) but without extracapsular extension. Although PSM is deemed to be an adverse effect, it is inconclusive whether adjuvant radiotherapy (aRT) is imperative. From this real-world experience, we conclude that robotic-assisted approach can benefit the patients of worse conditions with a non-inferior prognosis, and preoperative prostate specific antigen cut-off value of 19.09 ng/mL can be utilized as a predictive factor for biochemical recurrence after surgery. At the same time, we are not in favor of routine aRT for pT2+ PCa.
- Citation: Yang CH, Lin YS, Ou YC, Weng WC, Huang LH, Lu CH, Hsu CY, Tung MC. Biochemical recurrence of pathological T2+ localized prostate cancer after robotic-assisted radical prostatectomy: A 10-year surveillance. World J Clin Cases 2021; 9(5): 1026-1036
- URL: https://www.wjgnet.com/2307-8960/full/v9/i5/1026.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i5.1026
According to the World Health Organization (WHO) Classification of Tumors in 2004, pathological T2+ (pT2+; pT2R1) is not an officially recognized category that describes organ-confined (OC; pT2) prostate cancer (PCa) with extension to inked surgical margins but without extracapsular extension (ECE; pT3a) or seminal vesicle invasion (SVI; pT3b). One year prior to this statement, the data of all kinds of positive surgical margins (PSMs) and locally advanced PCa were collected in one meta-analysis from the SEARCH database[1], and the authors found out that the prognosis assessment should consider pathological results along with the Tumor-Node-Metastasis (TNM) stage for obviously distinct biochemical recurrence (BCR) characteristics among pT2R1, pT3a with or without PSM, and pT3b with or without PSM.
Radical prostatectomy (RP), which has a cure rate of up to 70% in PCa, has been shown to be beneficial to survival among patients with localized PCa with an average of 2.9 years of life longer than that with conservative observation[2-7]. Across all stages of PCa, one of the aims of RP is to completely remove the whole specimen with a negative margin, achieving > 0.1 mm under pathological analysis. Otherwise, it will increase the likelihood of BCR[8-10]. Clinically localized PCa with a PSM after RP can increase BCR risk by two- to four-fold; however, interestingly, it does not increase cancer-specific mortality. Robotic-assisted radical prostatectomy (RARP) achieves improved cancer control and perioperative outcome by facilitating more sophisticated dissection and more effective preservation of nerves than open or laparoscopic methods, emerging as the first operative choice worldwide. Nevertheless, if patients with OC PCa own a PSM after RARP, they will have a similar BCR rate to those with ECE with or without a PSM[1,9-12].
In general terms, PSM will significantly increase the risk of BCR. In particular, if we studied further details, such as OC with PSM, ECE without PSM, ECE with PSM, or SVI with PSM, they will have different effects on BCR rates. The rate of BCR resulting from PSM has a wide range spanning from 25% to 80%[1,13]. A pT2+ specimen, which has the same risk hypothesis as that of ECE, will influence BCR-free survival after RP. Approximately 60% of patients after RP with a PSM will have no risk of cancer recurrence[8]. However, in terms of metastasis-free survival and local recurrence-free survival, postoperative BCR or not is a decisive factor[14-17].
With regard to predictive factors, localized PCa with a surgical Gleason score above 7, unknown lymph node status, and high preoperative prostate specific antigen (PSA) values are factors associated with an increased risk of BCR[3-7]. Under the modern trend of RARP, high D’Amico risk poses a threat to both PSM and BCR[18]. Some pT2+ patients have been studied on such issues, mostly under the settings of open RP or laparoscopic RP with large-scale analysis. On the basis that BCR can determine further local recurrence and metastatic disease after RP, we analyzed clinical measurements to identify who, among patients with pT2+ after RARP, are vulnerable to BCR and observe if any BCR behavior difference would be achieved by RARP surgery. Thus, we retrospectively studied our patients during a 7-10-year follow-up period. The cases of these PCa patients with a PSM but without ECE or SVI after RARP were analyzed to understand which factors can be linked to higher BCR rate among them during the follow-up and compare our findings with the published literature.
From 2008 to 2011, 48 men diagnosed with TNM clinical stages T1c, T2a, T2b, T2c, T3a, and T3b, were included. They were found to have localized PCa before the surgery. The analyzed variables were included after obtaining informed consent from all qualified patients. From 2008 to 2011, they were categorized into recurrent risk groups as described in the National Comprehensive Cancer Network (NCCN) guidelines for PCa. Low-risk, intermediate-risk, high-risk, and locally advanced PCas were treated according to the NCCN guidelines. RARP was carefully assessed and selected after shared decision-making with the patients. Included patients had to meet the undetectable PSA (PSA < 0.008 ng/mL) at the third postoperative follow-up. Clinically, if patients failed to reach the undetectable PSA level, we closely monitored them for timely adjuvant therapies. The data of patients who needed tailored surveillance strategies was not considered in analysis. Ethical approval was granted by the local ethics committee of Tung’s Taichung MetroHarbor Hospital and informed consent was waived for retrospective nature of the study and all procedures being performed were part of the routine care.
All patients underwent RARP that was performed by a single surgeon. The surgical procedure was the same as that in a previously published article[18]. Transperitoneal RARP was performed using a four-arm system and six ports. Other surgical details are categorized in Table 1. The WHO pathological classification for urinary system and male genital organs (2004) was used to review these specimens. The surgical specimens that had 3-5-mm transverse intervals were all postoperatively examined by pathologists to determine that they were all PSM and had no extension to the extra-prostate tissue or seminal vesicle.
| BCR group, n = 25 (52%) | Non-BCR group, n = 23 (48%) | P value | |
| Age (yr) (mean ± SD) | 66.5 (SD: 4.7) | 69 (SD: 5.4) | 0.102 |
| Body weight (kg) (mean ± SD) | 70.8 (SD: 7.5) | 71.1 (SD: 6.8) | 0.711 |
| Body height (cm) (mean ± SD) | 167.5 (SD: 3.6) | 166.9 (SD: 3.3) | 0.718 |
| ASA grade (n) | 0.818 | ||
| I | 5 (20%) | 4 (17.4%) | |
| II | 20 (80%) | 19 (82.6%) | |
| Surgical method: RARP with: | |||
| BPLND | 24 (96%) | 22 (96%) | 0.954 |
| Bilateral NVB preservation | 20 (80%) | 19 (82.6%) | 0.818 |
| DVC ligation | 9 (36%) | 7 (30.4%) | 0.681 |
| Bladder neck sparing | 25 (100%) | 23 (100%) | |
| Puboprostatic ligament preservation | 0.705 | ||
| 1 | 3 (12%) | 2 (8.7%) | |
| 2 | 22 (88%) | 21 (91.3%) | |
| Lymph node yield number (n) (mean ± SD) | 11.6 (SD: 6.3) | 9.8 (SD: 4.5) | 0.143 |
| Vesicourethral anastomosis | 17.2 (SD: 4.0) | 17.4 (SD: 5.9) | 0.447 |
| Time (min) (mean ± SD) | 109.2 (SD: 24.7) | 111.4 (SD: 24.5) | 0.386 |
| Operation time (min) (mean ± SD) | 82.7 (SD: 66.2) | 102.17 (SD: 112.0) | 0.242 |
| Blood loss (mL) (mean ± SD) | 51.0 (SD: 36.3) | 41.7 (SD: 11.5) | 0.116 |
| Specimen volume (g) (mean ± SD) | 7.0 (SD: 5.7) | 4.9 (SD: 3.4) | 0.074 |
| Tumor volume (cm3) (mean ± SD) | 15.1 (SD: 9.8) | 12.8 (SD: 9.5) | 0.203 |
| Tumor percentage (%) (mean ± SD) | |||
| PSM location: | 13 (52%) | 10 (43.5%) | 0.848 |
| Anterior | 3 (12%) | 3 (13.0%) | |
| Lateral | 1 (4%) | 3 (13.0%) | |
| Posterolateral | 7 (28%)1 | 6 (26.1%)2 | |
| Apex | 1 (4%) | 1 (4.4%) | |
| Posterior | 3.6 (SD: 0.9) | 4.3 (SD: 2.0) | 0.080 |
| Postoperative stay (d) (mean ± SD) | 7.0 (SD: 0.2) | 7.2 (SD: 0.7) | 0.119 |
| Foley catheter (d) (mean ± SD) | 28.6 (SD: 19.9) | 17.0 (SD: 11.3) | < 0.001 |
| Preoperative PSA (ng/mL) (mean ± SD) | |||
| Preoperative PSA distribution (n) | 3 (12%) | 6 (26.1%) | |
| < 10 ng/mL | 9 (3%) | 11 (47.8%) | 0.157 |
| ≥ 10 ng/mL, < 20 ng/mL | 13 (52%) | 6 (26.1%) | |
| ≥ 20 ng/mL | 4.3 (SD: 2.8)/10.1 (SD: 2.0) | 4.1 (SD: 2.5)/10.5 (SD: 1.9) | |
| Positive biopsy cores (mean ± SD) | 0.832 | ||
| Biopsy tumor percentage (%) (mean ± SD) | 26.5 (SD: 21.2) | 24.0 (SD: 20.3) | 0.686 |
| Biopsy core | 0.839 | ||
| < 3 fragments | 7 (31.8%) | 5 (23.8%) | |
| ≥ 3 fragments, < 50% | 12 (54.6%) | 13 (61.9%) | |
| ≥ 50% | 3 (13.6%) | 3 (14.3%) | |
| Biopsy Gleason group | 0.626 | ||
| 1 | 12 (48.0%) | 10 (43.5%) | |
| 2 | 4 (16.0%) | 4 (17.4%) | |
| 3 | 1 (4.0%) | 4 (17.4%) | |
| 4 | 3 (12.0%) | 2 (8.7%) | |
| 5 | 5 (20.0%) | 3 (13.0%) | |
| Clinical stage | 0.445 | ||
| cT1 | 4 (16%) | 3 (13.0%) | |
| cT2a | 2 (8.0%) | 1 (4.4%) | |
| cT2b | 7 (28%) | 5 (21.7%) | |
| cT2c | 8 (32%) | 13 (56.5%) | |
| cT3 | 4 (16%) | 1 (4.4%) | |
| Risk category | 0.493 | ||
| Very low and low | 1 (4.0%) | 4 (17.4%) | |
| Favored-intermediate | 8 (32.0%) | 6 (26.1%) | |
| Unfavored-intermediate | 2 (8.0%) | 4 (17.4%) | |
| High | 10 (40.0%) | 7 (30.4%) | |
| Very high | 4 (16.0%) | 2 (8.7%) | |
| PNI | 0.061 | ||
| Positive | 22 (88.0%) | 15 (65.2%) | |
| Negative | 3 (12.0%) | 8 (34.8%) | |
| ALI | N/A | ||
| Positive | 2 (8.0%) | 0 (0.0%) | |
| Negative | 23 (92.0%) | 23 (100%) | |
| Pathological Gleason group | 0.265 | ||
| < 3 | 17 (73.9%) | 20 (87%) | |
| ≥ 3 | 6 (26.1%) | 3 (13.0%) | |
| Pathological primary Gleason score | 0.265 | ||
| < 3 | 17 (73.9%) | 20 (87%) | |
| ≥ 3 | 6 (26.1%) | 3 (13.0%) | |
| Change of Gleason group | 0.943 | ||
| Upgrade | 9 (39.1%) | 8 (34.8%) | |
| Same | 6 (26.1) | 6 (26.1%) | |
| Downgrade | 8 (34.8%) | 9 (39.1%) |
Follow-ups at outpatient department offices were assigned at the 1st week, 6th week, 3rd month, 6th month, and 12th month in the first year, and every 6 mo after the second year. Only the data of patients who achieved undetectable PSA, which was < 0.008 in our institute, at the 3rd week after RARP were recruited. We recorded 7-10-year follow-ups with the primary endpoint as BCR, defined as two consecutive PSA values of ≥ 0.2 ng/mL. All patients received neither neoadjuvant nor adjuvant therapies. The patients had no lymph node involvement (pT2R1N0).
Initially, we subdivided the patients into two groups: Patients with and without BCR (BCR and non-BCR groups, respectively). Basic characteristics such as age, body height, body weight, body mass index, American Society of Anesthesiologists (ASA) classification, total resected specimen volume, percentage of tumor volume, and positive chip ratio were statistically compared. Subsequently, we compared several parameters to determine any significant differences. We used the following parameters: (1) Biopsy cores; (2) Preoperative PSA; (3) Preoperative PSA interval; (4) Biopsy grade group; (5) Clinical stage; (6) Risk category; (7) Pathological grade group; (8) Pathological primary Gleason score; (9) Perineural invasion (PNI); and (10) Angiolymphatic invasion (ALI). Besides absolute digits, preoperative PSA was attributed to an interval of ≤ 10, 10-20, or > 20 ng/mL.
As for statistical analyses, Student’s t-test (continuous variables between groups), Fisher’s exact test (categorical variables), and chi-square test (categorical variables) were used to analyze the baseline characteristics and subgroup differences between the BCR and non-BCR groups, respectively. If any factor was meaningful under univariate analysis, further multivariate analysis was conducted. Pearson’s correlation was used to establish the correlation between preoperative PSA and time to BCR, and receiver operating characteristic (ROC) curve analysis was used to obtain a meaningful cut-off value. SAS 9.4 was used as the statistical software and a P value of < 0.05 was considered statistically significant.
Of 48 men, 25 (52%) experienced BCR during postoperative follow-ups for a median of 9 years (range: 7-10 years) and the remaining 23 (48%) men did not. Basic characteristics, surgical procedures, PSM location, and clinical and pathological parameters are listed in Table 1.
Three and two patients in the BCR and non-BCR groups, respectively, had incomplete biopsy core data and were thus excluded from analysis. The specimen of one patient in the BCR group was unrecorded, and another in the same group had carcinoid tumor. No patients had a surgical specimen ranked as Gleason group 4. No patients had ALI in the non-BCR group; thus, this category was not assessed.
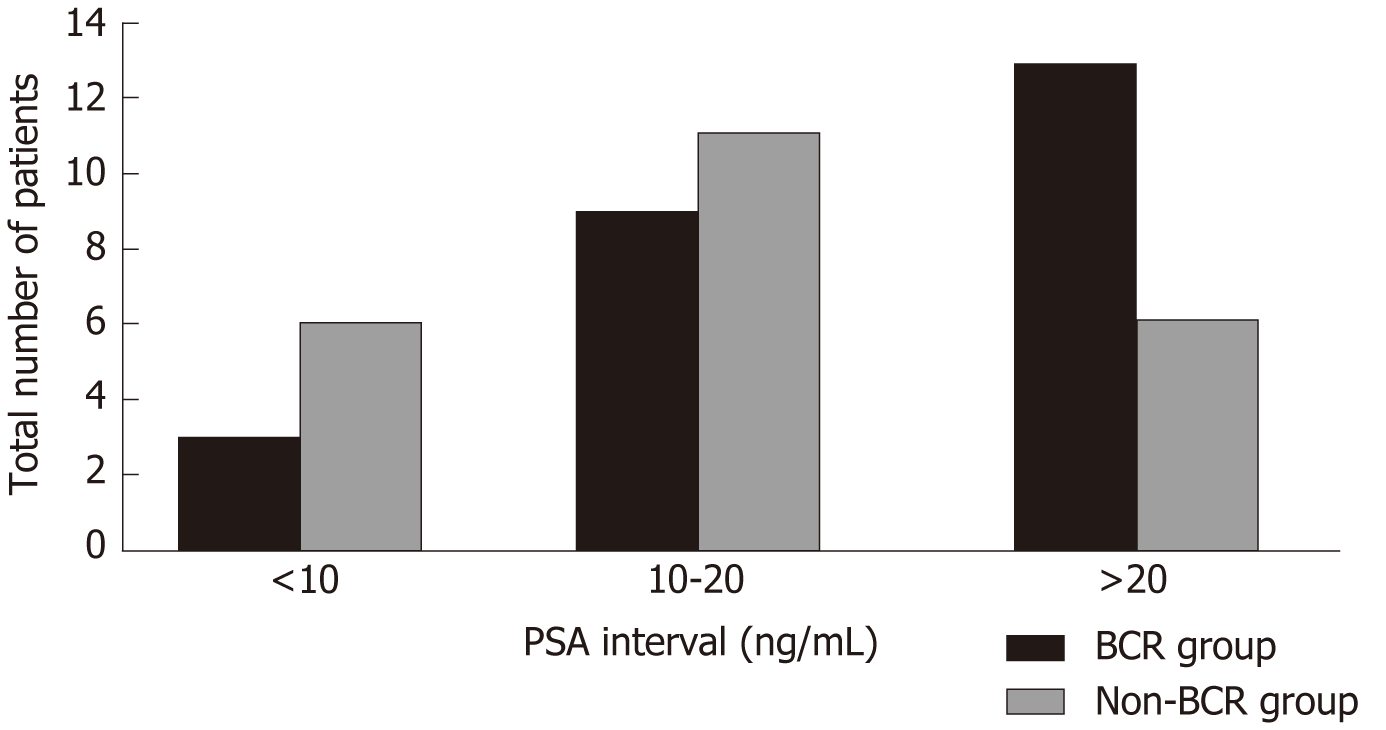
One patient in the non-BCR group had multiple PSM, but only the length of the site of the apex was described. One patient in the BCR group had a PSM of 2 mm at the apex, and the remaining patients had a PSM of ≤ 1 mm. The observed trend was that patients with BCR were prone to have a high tumor volume and PNI, but without significance. Only PSA made a significant difference between these two groups (P < 0.001). ROC curve analysis of PSA values suggested a cut-off value of 19.09 ng/mL (sensitivity: 0.600; specificity: 0.739; Figure 1). Although no significant differences were observed in PSA distribution (P = 0.157) between the two groups, twice the percentage in the BCR group in the range > 20 ng/mL (BCR: 52% vs non-BCR: 26.1%) and half the percentage in the BCR group compared with the non-BCR group in the range of < 10 ng/mL were observed (BCR: 12% vs non-BCR: 26.1%, Figure 2). According to the cut-off value of PSA, we found that the difference in PSA percentage range > 20 ng/mL between the two groups was close to significance (P = 0.067).
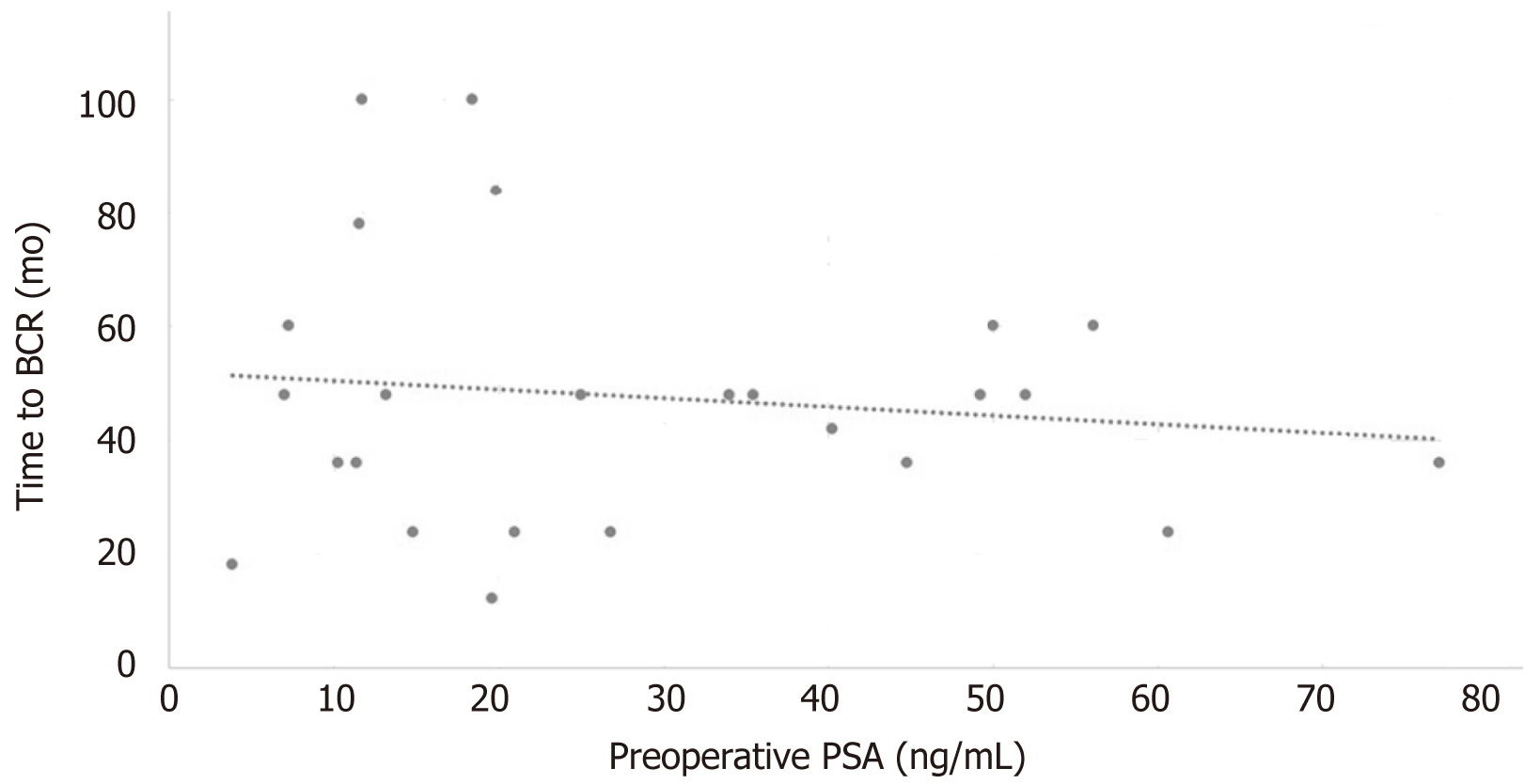
Overall, in the BCR group, the mean time to experience BCR after postoperative nadir was 47.6 mo (SD: 22.93 mo; median: 48 mo). Linear regression trend line was evaluated, but no reliable correlations were established using the preoperative PSA level (Pearson’s correlation: 0.13; adjusted R2 = 0.026; Figure 3).
PSM itself acts as a risk factor for BCR among OC PCa. According to the previously published literature[15], the occurrence rate of PSM falls from 6.5% to 32% across all stages after RP, predisposing individuals to BCR, local recurrence, and metastatic disease. In this study on pT2+ PCa with a median 9-year surveillance and BCR rates of 52%, 21 (84%) patients from the BCR group developed BCR in the first 5 years after RARP, and a median of 4 years after first postoperative PSA nadir was detected.
The related published literature has been searched using PubMed, and information on pT2+ and their BCR is summarized in Table 2[1,12,13,19]. Our operative method consisted of RARP, mostly with neurovascular bundle (NVB) preservation and with bladder neck sparing (BNS). A prospective study[20] stated that NVB preservation can help improve potency and continence after RARP without affecting PSM and BCR. BNS was proven to have short-term benefits in removing the urethral catheter without posing a risk to PSM or BCR[21]. Our study groups had PSM in a favorable single pattern within a length of 1 mm, except for one with a multifocal finding. This condition indicates a better result than the unfavorable (multifocal or single but > 3 mm) PSM[22]. Non-apical PSM, which constituted 73% of our cases, was meta-analyzed and was found to increase the likelihood of BCR[23] and negatively affect the BCR rate to a certain degree.
| Year | Sample size | Surgical method | PSA level (ng/mL) | Clinical TNM stage | Pathology Gleason group | BCR rate | |
| Freedland et al[1] | 2003 | Retrospective, 214 men, SEARCH Database | RP (no mention of open RP or RARP) | Mean: 10.5 ± 8.6, median: 8.0 | T1: 97 (46%); T2: 110 (53%); T3: 3 (1%) | 1: 105 (51%); 2 and 3: 89 (43%); 4 and 5: 14 (7%) | 6-yr BCR rate 50% |
| Leite et al[19] | 2014 | Retrospective, 58 men | Open RP | N/M | N/M | N/M | Mean follow-up: 35.9 ± 23.1 mo; BCR rate: 31% |
| Hashimoto et al[12] | 2015 | Retrospective, 837 men | RARP | 6.90, range: 3-47.4 | T1c: 634 (75.7%); T2a: 111 (13.3%); T2b: 46 (5.5%); T2c: 39 (4.7%); T3: 7 (0.8%) | 1: 65 (7.8%); 2: 377 (45%); 3: 230 (26.2%); 4 and 5: 165 (19.7%) | 5-yr BCR free-survival: 62.4% |
| Karl et al[13] | 2015 | Retrospective, 956 men | Retropubic RP: 88%; Laparoscopic RP: 6%; RARP: 4%; Perineal RP: 3% | < 4: 13%; 4-9.9: 65%; 10-19.9: 18%; ≥ 20: 4% | N/M | 1: 39%; 2: 52%; 3: 7%; 4 and 5: 2% | Mean follow-up: 48 mo; BCR rate: 25.4% |
Our BCR rate (52% in the 9-year follow-up period) is comparable to that recorded in a meta-analysis in 2003 using the SEARCH database (6-year BCR rate approximately 50%)[1]. However, our study had beneficial effects with three more years of follow-up. This result may be explained by the benefits of the surgical method of RARP[18,20] compared to methods without RARP. Our BCR rate appeared to be higher than those of three other published studies. Nonetheless, an investigation into the patient group of a multicenter retrospective study from German[13] showed that the mean PSA, whether with BCR or not, was 23.12 (SD: 17.10) ng/mL, which falls in the top 4% of their range. Moreover, nearly 75% of our pathology specimens consisted of Gleason group > 1, and almost half of the pathological specimens were ranked as high-risk groups; only 61% of their data had Gleason group > 1. Furthermore, 18 (37.5%) patients in our BCR group had their BCR within 48 mo. Overall, our pT2+ after RARP provides a reasonable BCR rate to those with worse conditions, such as higher PSA, worse Gleason group, or high-risk patients, compared with PCa of initial occurrence by open RP[13,19].
Only one of these four listed articles[12] is similar to ours, which used RARP as the primary surgical method. Their recruited data were mostly composed of initial low-risk group, low PSA level (< 10 ng/mL), and up to 75% in the T1c stage compared with the PSA 23.12 ng/mL and only 29% of T1c in our study. Twenty-one (43.75%) men in our analysis experienced BCR during the 5-year follow-up. This 6% gap may be attributed to study group bias, sample size, and massive non-apical PSM in our patients.
Of all these designed factors, we disclosed only the relationship of preoperative PSA levels with BCR in these pT2+ patients, which was consistent with other studies[1,12,13,19] of pT2+ under univariate or multivariate analysis. The high preoperative level, 19.09 ng/mL, would indicate the likelihood of postoperative BCR development. Nonetheless, when we attempted to establish a linear regression model to predict the time to BCR, we obtained no correlation on the basis of preoperative PSA.
However, we observed that the BCR group tended to have bulkier tumors and more PNI occurrence than the non-BCR group. Univariate analysis of many studies found that tumor volume is linked with BCR[13,19] in this pT2+ situation. A former retrospective analysis[24] revealed that PNI was an independent histological factor in BCR after RP, and this correlation was also verified in pT2+ PCa after RARP[12] using univariate analysis.
This study is one of few studies that compared BCR of pT2+ PCa with other publications and simultaneously analyzed predictive factors. Despite ongoing debates on whether regular adjuvant radiation therapy (aRT) should be performed[25,26] on PSMs of all types, we conclude from other studies and filter the related factors to predict the upcoming BCR effects after RARP with pT2+ PCa. Based on this rate (5-year BCR rate, 44%; 10-year BCR rate, 52%), we do not recommend regular aRT on all pT2+ conditions, for only half of them will experience BCR in 10 years following RARP. In addition to marginal benefits from aRT and controversies on improving cancer specific-free survival[26], the costs may be a bother to the other half with no recurrence[27].
This study features the longest follow-up, 10 years, among the studies with a similar topic. Moreover, all these cases were operated by a single surgeon, minimizing technique-related bias. Furthermore, the chosen skilled surgeon in this study has handled more than 2000 RARP cases and can guarantee the quality of the surgery and eliminate episodic complications and adverse effects.
Our analysis has several limitations. The most important point is the study group size, which consequently leads to a bias in other published reports. Moreover, some of our designs are so sophisticated that they will leave a number of zero in a subgroup, especially on the basis of such a small data size, thus hindering subsequent statistical analysis. For example, the PNI subgroup and PSA distribution showed a close tendency to be statistically significant. Meanwhile, a complete blank was left in the ALI category, which turned into an obstacle for further analysis. According to other published data and experiences, these two factors may have prognosis effects. We may be able to derive their predictive role after further expanding the data size.
RARP can benefit pT2+ PCa of worse conditions with better BCR-free survival than open RP. The 5-year and 10-year BCR rates of pT2+ PCa after RARP were estimated to be 43.75% and 52%, respectively. Most of them experienced BCR in the first 5 years after RARP, and median time to BCR was 4 years after first PSA nadir. Based on this rate, we are not in favor of routine aRT on all pT2+ conditions. The adverse effects during operation, such as non-apical PSM or unfavorable PSM status, and certain clinical statuses, such as high preoperative PSA or more advanced stage, will make patients more vulnerable to BCR. According to this analysis and literature review, a skilled surgical technique limiting PSM in a favorable situation is warranted to lower the BCR rate of pT2+ PCa. Other possible tendencies include bulky tumor volume and PNI; however, a larger sample size and well-designed analyses are required to determine their definite roles.
Prostate cancer (PCa) is overwhelmingly prevalent in Western countries, and also seen with an escalating trend in Asia. Its oncological outcome is affected by many factors, and pathological status is one of the essential ones. In the early results of studies with open radical prostatectomy (RP), pathologically localized organ-confined PCa with a positive surgical margin (PSM) but without extracapsular extension (ECE) (pT2+; pT2R1) was equal to that of ECE with or without PSM in terms of oncological outcomes.
Nowadays, robotics-assisted RP (RARP) is proved to provide better functional and oncological outcomes to men with PCa, and it herein emerges as the first choice to surgeons intending to perform RP. The most pivotal and large-scale analysis on this pathological topic was issued in 2003 only with open RP, and the present reports regarding it were few and only with a short surveillance. Hence, we conducted an analysis with RARP and a long duration of follow-up.
To examine the oncological outcomes of localized pT2+ PCa after RARP in a 10-year surveillance and to address our contemporary viewpoints based on our real-world experiences.
We enrolled the data of 48 men from 2008 to 2011 with localized pT2+ PCa after RARP, and recorded their pathological status and postoperative follow-up in detail. Postoperative visits were scheduled at the 1st week, 6th week, 3rd month, 6th month, and 12th month in the first year, and every 6 mo after the second year. The included men needed to have their postoperative prostate specific antigen (PSA) detected at nadir (PSA < 0.008 ng/mL) in 3 mo after RARP, or they would be excluded from this analysis. The patients were divided into two groups, with biochemical recurrence (BCR) or without BCR. BCR was defined as serial PSA that was tested above 0.2 ng/mL. Characteristics of these two groups were compared using corresponding statistical methods.
RARP was successfully performed without any major complication or intraoperative conversion. In a median follow-up of 9 years, BCR occurred in 25 (52%) men, and most of them experienced it in the first 5-year surveillance. Our data seemed to be similar to that of open RP, but ours consisted of a longer duration of surveillance. Compared to similar reports, the unfavored margin status and initially worse presentation of our included patients made our data inferior on the surface. Of all analyzed predicted factors, preoperative PSA was the only meaningful one, with a cut-off value of 19.09 ng/mL (sensitivity: 0.600; specificity: 0.739).
RARP can provide better BCR-free survival to those with localized pT2+ PCa than open RP. Preoperative PSA can act as an auxiliary parameter to predict the coming of BCR. Skilled surgical techniques can help to minimize unfavorable margin status, and furthermore lower the BCR rate.
This study is retrospective with a small sample size. For discussing more aspects in this topic and probing for more meaningful predictive factors, we anticipate inclusions of more data in the future, and also comparing pT2+ with pT3aR0 and pT3aR1 after RARP.
We thank the project managers of Urological Cancer Center of Tungs’ Taichung MetroHarbor Hospital for assistance with data maintenance.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ganeshan D S-Editor: Huang P L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Freedland SJ, Aronson W, Presti JC Jr, Kane CJ, Terris MK, Elashoff D, Amling CL; SEARCH Database Study Group. Should a positive surgical margin following radical prostatectomy be pathological stage T2 or T3? J Urol. 2003;169:2142-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Mohler JL, Antonarakis ES, Armstrong AJ, D'Amico AV, Davis BJ, Dorff T, Eastham JA, Enke CA, Farrington TA, Higano CS, Horwitz EM, Hurwitz M, Ippolito JE, Kane CJ, Kuettel MR, Lang JM, McKenney J, Netto G, Penson DF, Plimack ER, Pow-Sang JM, Pugh TJ, Richey S, Roach M, Rosenfeld S, Schaeffer E, Shabsigh A, Small EJ, Spratt DE, Srinivas S, Tward J, Shead DA, Freedman-Cass DA. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:479-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 911] [Article Influence: 182.2] [Reference Citation Analysis (0)] |
| 3. | Bill-Axelson A, Holmberg L, Garmo H, Taari K, Busch C, Nordling S, Häggman M, Andersson SO, Andrén O, Steineck G, Adami HO, Johansson JE. Radical Prostatectomy or Watchful Waiting in Prostate Cancer - 29-Year Follow-up. N Engl J Med. 2018;379:2319-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 310] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 4. | Bill-Axelson A, Holmberg L, Garmo H, Rider JR, Taari K, Busch C, Nordling S, Häggman M, Andersson SO, Spångberg A, Andrén O, Palmgren J, Steineck G, Adami HO, Johansson JE. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370:932-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 678] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 5. | Bill-Axelson A, Holmberg L, Filén F, Ruutu M, Garmo H, Busch C, Nordling S, Häggman M, Andersson SO, Bratell S, Spångberg A, Palmgren J, Adami HO, Johansson JE; Scandinavian Prostate Cancer Group Study Number 4. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian prostate cancer group-4 randomized trial. J Natl Cancer Inst. 2008;100:1144-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 400] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 6. | Bill-Axelson A, Holmberg L, Ruutu M, Häggman M, Andersson SO, Bratell S, Spångberg A, Busch C, Nordling S, Garmo H, Palmgren J, Adami HO, Norlén BJ, Johansson JE; Scandinavian Prostate Cancer Group Study No. 4. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352:1977-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 865] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 7. | Holmberg L, Bill-Axelson A, Helgesen F, Salo JO, Folmerz P, Häggman M, Andersson SO, Spångberg A, Busch C, Nordling S, Palmgren J, Adami HO, Johansson JE, Norlén BJ; Scandinavian Prostatic Cancer Group Study Number 4. A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. N Engl J Med. 2002;347:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 501] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 8. | Yossepowitch O, Briganti A, Eastham JA, Epstein J, Graefen M, Montironi R, Touijer K. Positive surgical margins after radical prostatectomy: a systematic review and contemporary update. Eur Urol. 2014;65:303-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 299] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 9. | Karakiewicz PI, Eastham JA, Graefen M, Cagiannos I, Stricker PD, Klein E, Cangiano T, Schröder FH, Scardino PT, Kattan MW. Prognostic impact of positive surgical margins in surgically treated prostate cancer: multi-institutional assessment of 5831 patients. Urology. 2005;66:1245-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 220] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Izard JP, True LD, May P, Ellis WJ, Lange PH, Dalkin B, Lin DW, Schmidt RA, Wright JL. Prostate cancer that is within 0.1 mm of the surgical margin of a radical prostatectomy predicts greater likelihood of recurrence. Am J Surg Pathol. 2014;38:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Stephenson AJ, Eggener SE, Hernandez AV, Klein EA, Kattan MW, Wood DP Jr, Rabah DM, Eastham JA, Scardino PT. Do margins matter? Eur Urol. 2014;65:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Hashimoto T, Yoshioka K, Horiguchi Y, Inoue R, Yoshio O, Nakashima J, Tachibana M. Clinical effect of a positive surgical margin without extraprostatic extension after robot-assisted radical prostatectomy. Urol Oncol 2015; 33: 503.e1-503. e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Karl A, Buchner A, Tympner C, Kirchner T, Ganswindt U, Belka C, Ganzer R, Burger M, Eder F, Hofstädter F, Schilling D, Sievert K, Stenzl A, Scharpf M, Fend F, Vom Dorp F, Rübben H, Schmid K, Porres-Knoblauch D, Heidenreich A, Hangarter B, Knüchel-Clarke R, Rogenhofer M, Wullich B, Hartmann A, Comploj E, Pycha A, Hanspeter E, Pehrke D, Sauter G, Graefen M, Stief C, Haese A. The natural course of pT2 prostate cancer with positive surgical margin: predicting biochemical recurrence. World J Urol. 2015;33:973-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Alkhateeb S, Alibhai S, Fleshner N, Finelli A, Jewett M, Zlotta A, Nesbitt M, Lockwood G, Trachtenberg J. Impact of positive surgical margins after radical prostatectomy differs by disease risk group. J Urol. 2010;183:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Aoun F, Albisinni S, Henriet B, Tombal B, Van Velthoven R, Roumeguère T. Predictive factors associated with biochemical recurrence following radical prostatectomy for pathological T2 prostate cancer with negative surgical margins. Scand J Urol. 2017;51:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, Lin X, Greenfield TK, Litwin MS, Saigal CS, Mahadevan A, Klein E, Kibel A, Pisters LL, Kuban D, Kaplan I, Wood D, Ciezki J, Shah N, Wei JT. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1715] [Cited by in RCA: 1733] [Article Influence: 101.9] [Reference Citation Analysis (0)] |
| 17. | Brenner DJ, Curtis RE, Hall EJ, Ron E. Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer. 2000;88:398-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Ou YC, Yang CK, Wang J, Hung SW, Cheng CL, Tewari AK, Patel VR. The trifecta outcome in 300 consecutive cases of robotic-assisted laparoscopic radical prostatectomy according to D'Amico risk criteria. Eur J Surg Oncol. 2013;39:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Leite KR, Hartmann C, Reis ST, Viana N, Dall'Oglio MF, Sant'Anna AC, Nesrallah A, Nesrallah L, Antunes AA, Camara-Lopes LH, Srougi M. Biochemical recurrence rates are similar for pT2-positive surgical margins and pT3a. Int Braz J Urol. 2014;40:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Ou YC, Yang CK, Kang HM, Chang KS, Wang J, Hung SW, Tung MC, Tewari AK, Patel VR. Pentafecta Outcomes of 230 Cases of Robotic-assisted Radical Prostatectomy with Bilateral Neurovascular Bundle Preservation. Anticancer Res. 2015;35:5007-5013. [PubMed] |
| 21. | Preisser F, Busto Martin L, Pompe RS, Heinze A, Haese A, Graefen M, Tilki D. Effect of bladder neck sparing at robot-assisted laparoscopic prostatectomy on postoperative continence rates and biochemical recurrence. Urol Oncol 2020; 38: 1.e11-1. e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Martini A, Gandaglia G, Fossati N, Scuderi S, Bravi CA, Mazzone E, Stabile A, Scarcella S, Robesti D, Barletta F, Cucchiara V, Mirone V, Montorsi F, Briganti A. Defining Clinically Meaningful Positive Surgical Margins in Patients Undergoing Radical Prostatectomy for Localised Prostate Cancer. Eur Urol Oncol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 23. | Røder MA, Kawa S, Scheike T, Toft BG, Hansen JB, Brasso K, Vainer B, Iversen P. Non-apical positive surgical margins after radical prostatectomy for pT2 prostate cancer is associated with the highest risk of recurrence. J Surg Oncol. 2014;109:818-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Zhang LJ, Wu B, Zha ZL, Qu W, Zhao H, Yuan J, Feng YJ. Perineural invasion as an independent predictor of biochemical recurrence in prostate cancer following radical prostatectomy or radiotherapy: a systematic review and meta-analysis. BMC Urol. 2018;18:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Hervás A, Gómez-Caamaño A, Casaña M, Gómez-Iturriaga A, Pastor J, Jove J, Mengual JL, Gónzalez-San Segundo C, Muñoz J. Adjuvant versus salvage radiotherapy in prostate cancer: multi-institutional retrospective analysis of the Spanish RECAP database. Clin Transl Oncol. 2018;20:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Kvåle R, Myklebust TÅ, Fosså SD, Aas K, Ekanger C, Helle SI, Honoré A, Møller B. Impact of positive surgical margins on secondary treatment, palliative radiotherapy and prostate cancer-specific mortality. A population-based study of 13 198 patients. Prostate. 2019;79:1852-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Martini A, Marqueen KE, Falagario UG, Waingankar N, Wajswol E, Khan F, Fossati N, Briganti A, Montorsi F, Tewari AK, Stock R, Rastinehad AR. Estimated Costs Associated With Radiation Therapy for Positive Surgical Margins During Radical Prostatectomy. JAMA Netw Open. 2020;3:e201913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |