Published online Feb 6, 2021. doi: 10.12998/wjcc.v9.i4.976
Peer-review started: November 9, 2020
First decision: December 8, 2020
Revised: December 9, 2020
Accepted: December 23, 2020
Article in press: December 23, 2020
Published online: February 6, 2021
Processing time: 77 Days and 2.3 Hours
Squamous cell carcinoma (SCC) of bone is usually caused by metastasis from the lungs, bladder, or other sites. Primary SCC of bone most frequently involves the skull bones, and primary involvement of other sites in the skeletal system is extremely rare. To date, only three such cases have been reported, which makes the diagnosis, treatment, and prognosis of this disease a challenge.
A 76-year-old Chinese man presented to our hospital with nonspecific pain and limited mobility in the right shoulder for 4 mo. He underwent three-dimensional computed tomography reconstruction and magnetic resonance imaging of the right shoulder, which revealed an osteolytic destructive lesion in the right scapula with invasion into the surrounding muscles and soft tissues. Ultrasound-guided core needle biopsy detected a malignant tumor, and immunohistochemical analysis revealed a poorly differentiated SCC. Wide excision of the right scapular bone was performed, and pathological examination of the surgical specimen confirmed the diagnosis. At the last follow-up examination within 2 years, the patient was doing well with the pain significantly relieved in the right shoulder.
Primary SCC of bone is extremely rare at sites other than the skull. Clinicians must exhaust all available means for the diagnosis of primary SCC of the bone, so greater attention can be paid to its timely and effective management. Regular and adequate follow-up is essential to help rule out metastasis and judge the prognosis.
Core Tip: To the best of our knowledge, the present case represents the fourth case of primary squamous cell carcinoma (SCC) of a bone outside the skull and the first case of primary nonkeratinizing SCC of the scapular bone. Our findings suggest the need to improve the techniques used for the diagnosis of primary SCC of bones outside the skull, so greater attention can be paid to timely and effective management. Moreover, it is necessary to rule out metastasis and judge the prognosis with regular and adequate follow-up.
- Citation: Li Y, Zuo JL, Tang JS, Shen XY, Xu SH, Xiao JL. Primary nonkeratinizing squamous cell carcinoma of the scapular bone: A case report. World J Clin Cases 2021; 9(4): 976-982
- URL: https://www.wjgnet.com/2307-8960/full/v9/i4/976.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i4.976
Squamous cell carcinoma (SCC) is the second most common non-melanoma skin cancer. Although SCC can metastasize to other organs such as the bones[1-4], primary SCC of the bone is rare due to the absence of native squamous epithelium in osseous tissue[5]. When primary SCC does affect the bones, the most common site of involvement is the skull. Indeed, only three cases of primary SCC at other sites in the skeletal system, namely, the iliac bone, distal tibia, and tarsal bone, have been reported in the English literature[5-7]. Herein, we report the fourth case of primary SCC of a non-skull bone, which is also the first case of primary nonkeratinizing SCC of the scapula.
A 76-year-old Chinese man experienced severe pain in the right shoulder for 4 mo, along with limitation of joint mobility.
The patient suffered pain and limited mobility in the right shoulder for 4 mo, without an obvious cause. Conservative treatment with oral analgesics and rest was taken by the patient; however, its effect was poor, and the pain in the right shoulder worsened. In October 2018, the patient was referred to our department for therapy.
The patient had a free previous medical history.
The patient was a non-smoker, without relevant family history.
A physical examination revealed significant tenderness in the right scapula. The muscle strength of the right upper limb was grade II according to the manual muscle test classification. The range of motion of the right shoulder could not be assessed due to severe pain.
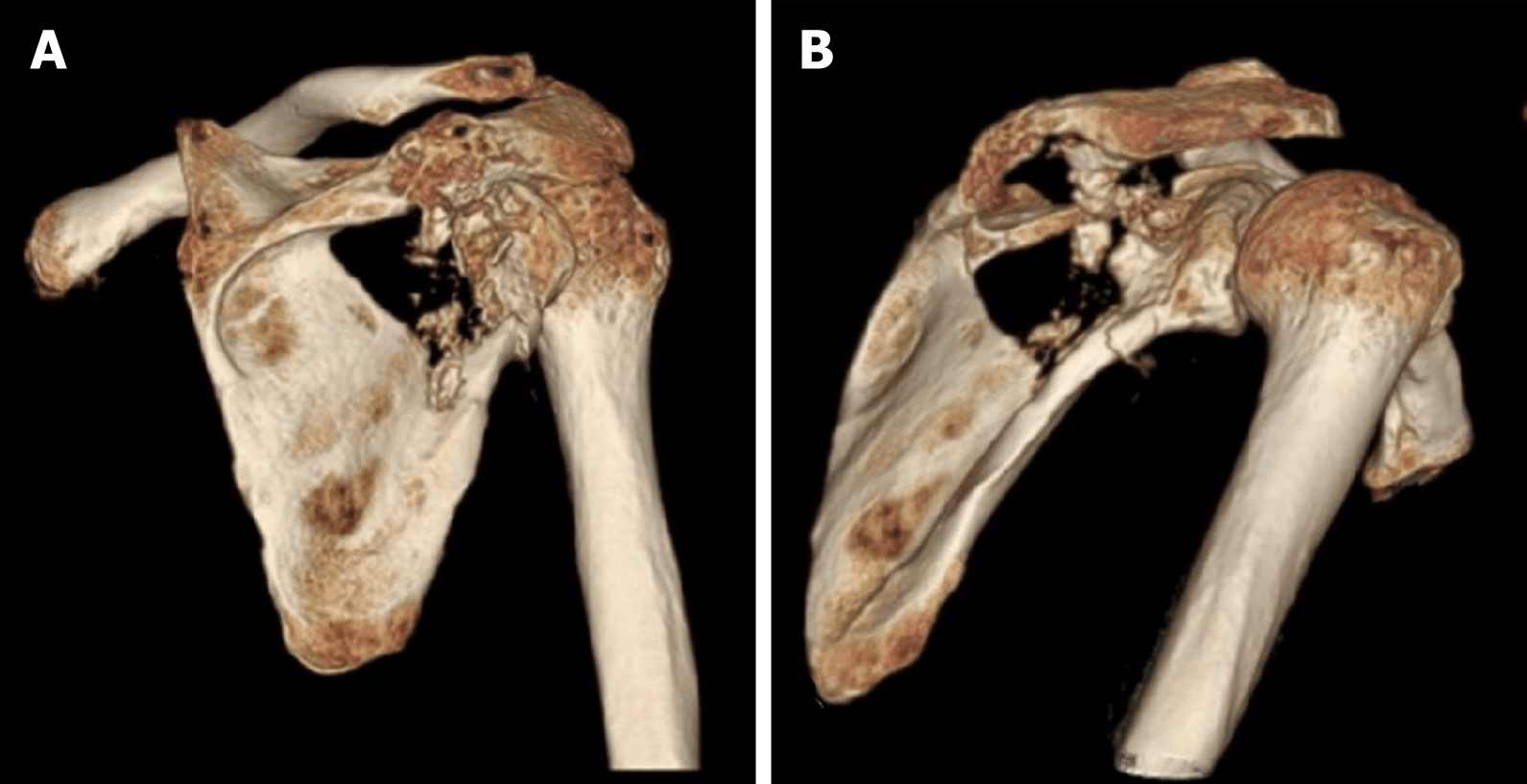
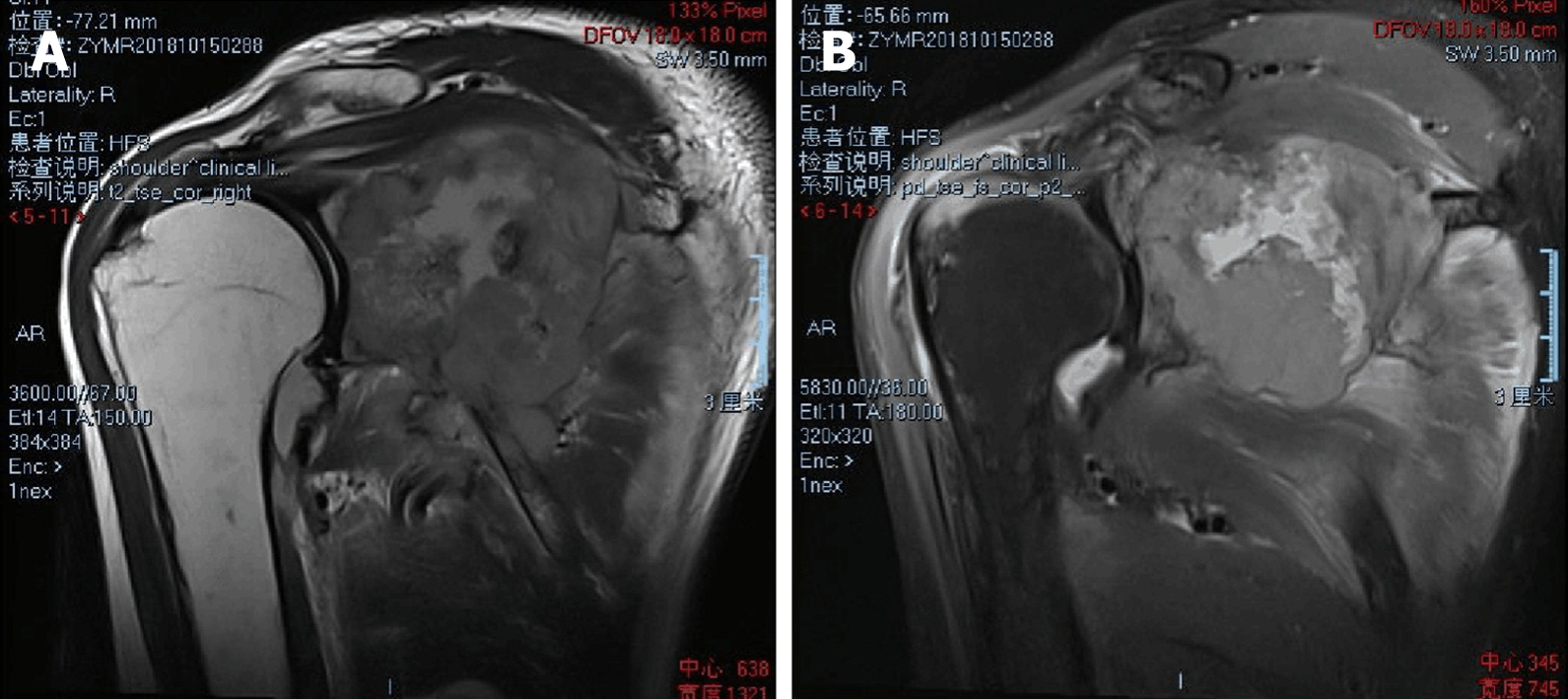
Three-dimensional computed tomography (CT) reconstruction (Figure 1) and magnetic resonance imaging revealed an osteolytic destructive lesion of the right scapula with invasion into the surrounding muscles and soft tissues (Figure 2).
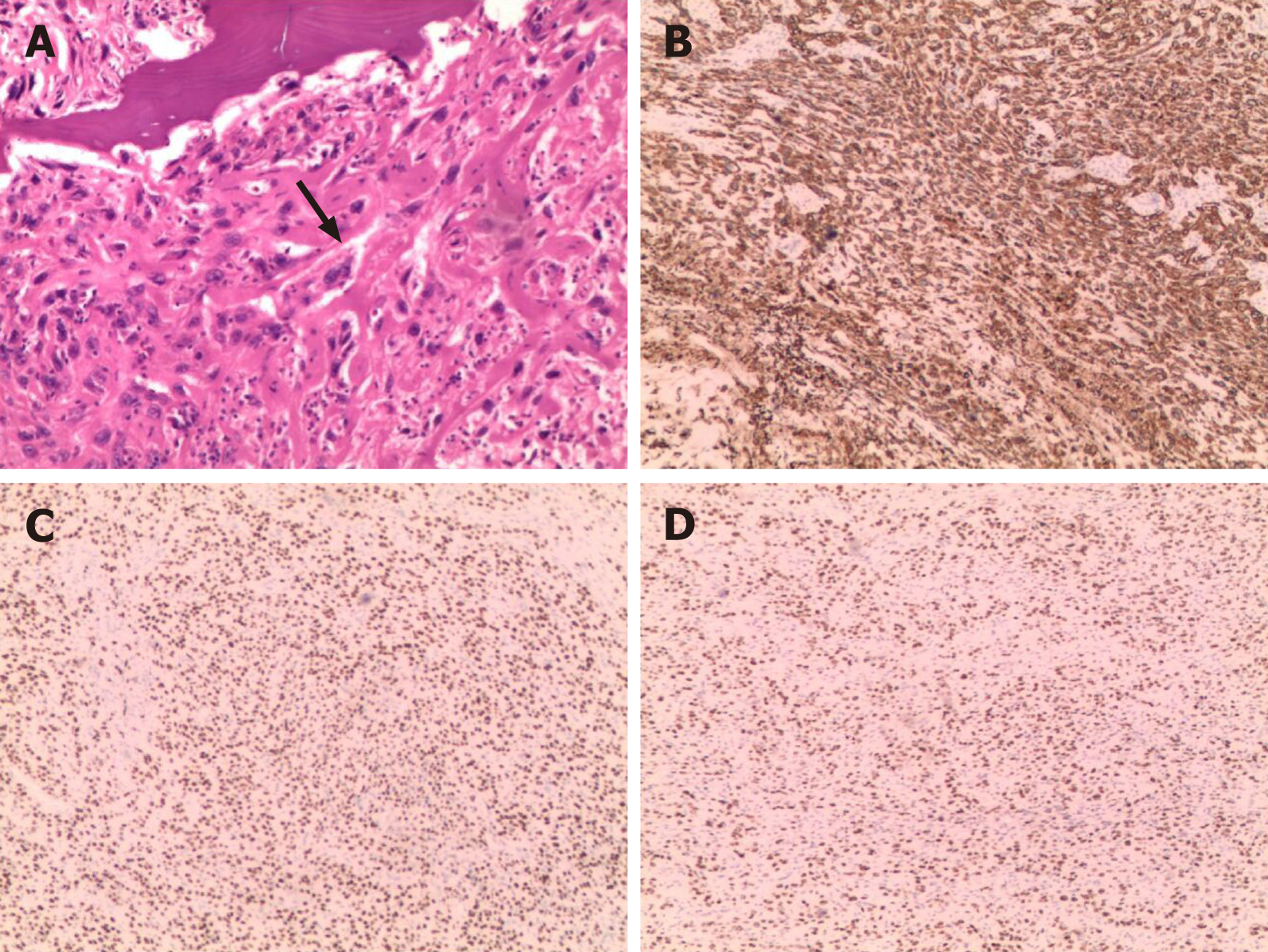
After obtaining informed consent from the patient, we performed an ultrasound-guided core needle biopsy. Histopathological examination of the core biopsy specimen revealed that it consisted of purely malignant squamous cells along. However, no typical keratin pearls were seen, as the malignant squamous cells were poorly differentiated. Therefore, a diagnosis of nonkeratinizing SCC was made. Immunohistochemical analysis showed that the tumor cells were reactive to cytokeratin 5/6, p63, p40, and vimentin (Figure 3). Furthermore, CT scans of the lungs, skull, and abdomen, single-photon emission CT-CT, and positron-emission tomography-CT confirmed that there were no other lesions outside the right scapular bone, which indicated that this was a rare presentation of a primary SCC involving the scapular bone. Therefore, the final diagnosis was primary nonkeratinizing SCC of the right scapular bone.
Primary nonkeratinizing SCC of the right scapular bone.
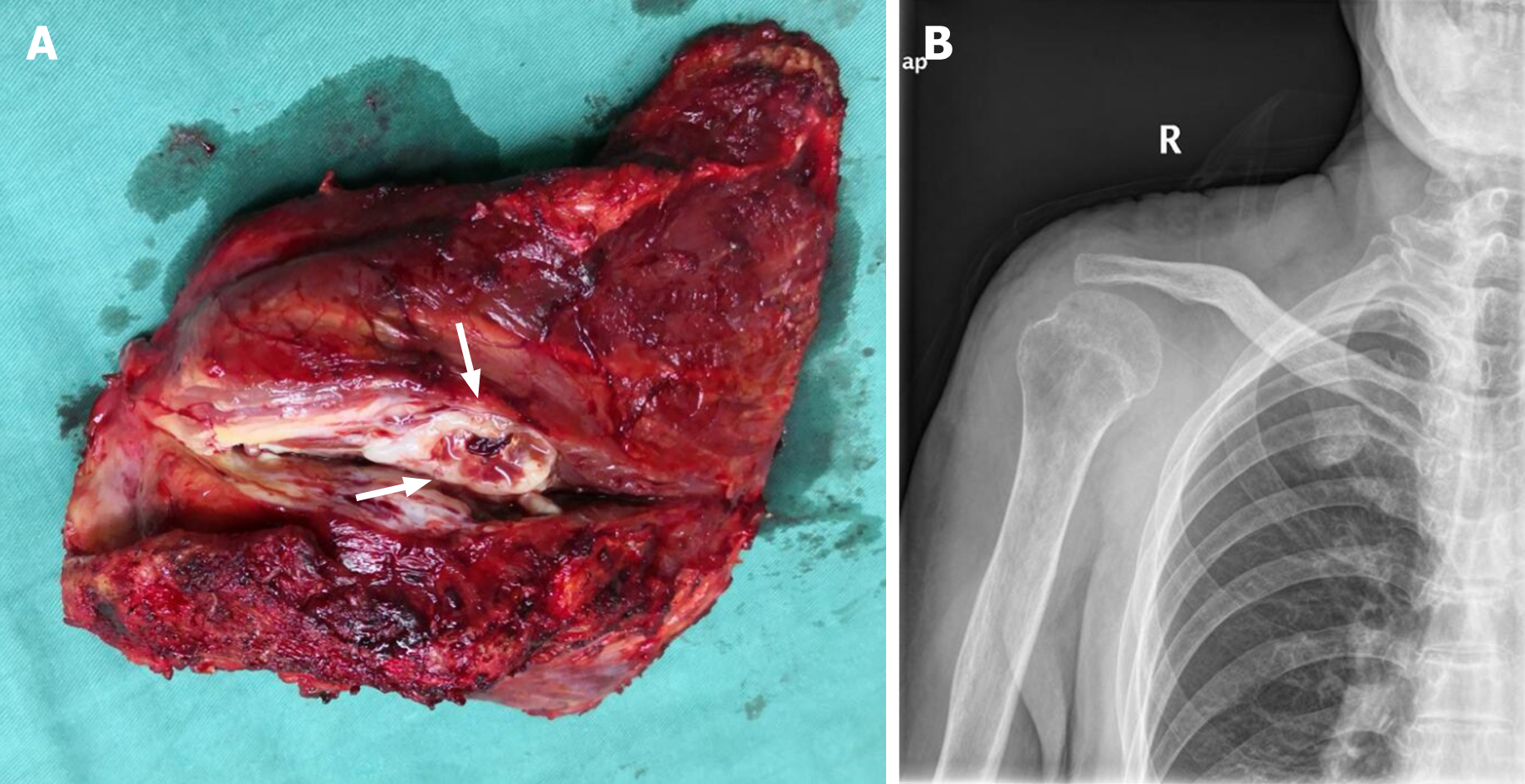
The patient underwent wide excision of the right scapular bone and reconstruction of the resulting defect with the right humeral head, right collarbone, and surrounding muscles. The whole gross specimen measured 7.0 cm × 6.5 cm × 5 cm. The lesion itself was a dark red ovoid mass that originated from the right scapular bone and appeared soft and creamy-white on cross section (Figure 4). No connection to the epidermis was identified. The histopathological examination and immunohistochemical analysis of the resected specimen confirmed that it is composed entirely of malignant squamous cells. Therefore, the diagnosis was primary nonkeratinizing SCC of the right scapular bone.
Regular follow-up was continued after surgery. The postoperative course was quite good. Neither recurrence nor metastasis was found during the last 2 years of follow-up. The severe pain in the right shoulder was significantly relieved, and the mobility and function of the right shoulder were improved.
SCC is a tumor of the epithelial tissue that typically originates from the epithelial linings of the skin, respiratory tract, digestive tract, and reproductive tract; thus, SCC can involve the head and neck, esophagus, lungs, cervix, and genital area[8]. Epithelial linings can be divided into layered squamous epithelium and non-squamous epithelium. The squamous differentiation phenotype of SCC depends on the type of oncogenic mutation involved and the cell of tumor origin, and this phenotype determines the degree of differentiation and therefore, the aggressiveness and invasiveness of these tumors[9]. In the case of most cancers, the initial target cells of the oncogenic mutations as well as the number of cancer stem cells in the tumor are unknown[10]. Therefore, it is difficult to know the source cells for primary SCC in the bones. SCC-derived cells share a common feature, in that they originate due to the mutation of proliferative basal cells, which are characterized by their ability to self-renew and produce terminally differentiated cells. Under the influence of oncogenic genes, both stem and progenitor cells can act as the origin cells of cancer[8]. A comparison of different SCCs shows that they are characterized by very similar mutant genes, including TP53, SOX2, TP63, CDNK2A (P16-INK4A), NOTCH1, KMT2D, PIK3CA, and PTEN[9].
Primary SCC of bone is commonly seen in the head and neck region[11-15], and it is rarely found elsewhere in the skeletal system. According to the literature, the present case is only the fourth case ever reported of primary SCC of a bone outside the skull and the first case of a primary nonkeratinizing SCC of the scapula (Table 1). It is not easy to make a diagnosis of primary SCC of a non-skull bone, as this depends not only on pathological and immunohistochemical examinations but also requires extensive workup to rule out metastasis. In addition to metastasis, the differential diagnosis of primary SCC should also include SCC caused by chronic osteomyelitis[16-18]. Keratin pearls are the pathological features of highly differentiated SCCs, and their presence in histopathological sections of well-differentiated SCCs is a common phenomenon[19]. Unlike the three cases of primary SCC of a non-skull bone reported previously, our case was unique in that it had no keratin pearls. This is because our patient had a poorly differentiated SCC, while the previous three patients had well-differentiated SCCs with keratin pearl formation. The immunohistochemical features of the previous three cases were also similar to those of our case[5-7], in that the tumor cells were reactive to cytokeratin 5/6, p63, and p40[20-22]. However, in our case, the tumor cells were also reactive to vimentin, which may be related to the metastasis capability and invasiveness of the primary SCC[23]. In our patient, the final diagnosis of a primary SCC of the bone was supported by the immunohistochemical findings, the extensive workup for the identification of a primary source, and the fact that the patient remained disease-free during a 2-year follow-up period.
| Age/Sex | Location | Clinicopathological features | Treatment | Follow-up | Outcome | |
| Gangopadhyay et al[6] | 20/F | Left iliac bone | Cytokeratin (5/6), p63, p40, and keratin pearls (+) | Radiotherapy alone | 10 mo | Good |
| Abbas et al[7] | 47/F | Distal tibia | Cytokeratin (5/6), p63, p40, and keratin pearls (+) | Below-knee amputation | Not reported | Unidentified |
| Gaston et al[5] | 57/M | Tarsal bone | Cytokeratin (5/6), p63, p40, and keratin pearls (+) | Wide excision of the first cuneiform | 26 mo | Good |
| Current study | 76/M | Right scapular bone | Cytokeratin (5/6), p63, p40, vimentin (+), and no keratin pearls (-) | Wide excision of the right scapula | 2 yr | Good |
For patients with primary SCC, the choice of treatment depends on the specific tumor characteristics, and an effective personalized treatment strategy must be devised. For most patients with primary SCC of the bone without metastasis, a negative tumor margin of at least 2 cm must be achieved during surgery[24]. For patients with SCC of the temporal bone, this margin may be difficult to achieve, as many important structures are located nearby, and the anatomical structure of the temporal bone is complex[25]; in such patients, adjuvant radiotherapy may help control minimal residual disease[26]. In our patient, as the SCC originated from the relatively independent anatomical structure of the scapula[27], it was easier to achieve complete resection of the tumor. Surgical resection is the most important treatment method for this type of tumor, and postoperative adjuvant treatment can be individualized according to the immunohistochemical characteristics of the tumor. For example, cetuximab is a monoclonal antibody that targets the epidermal growth factor receptor, and compared with conventional radiotherapy, cetuximab combined with radio-therapy may help achieve good outcomes in some patients with SCC[28]. In addition, cisplatin, 5-fluorouracil, and docetaxel is an effective combination chemotherapy regimen[29]. These adjuvant treatments can improve local control and reduce the mortality of advanced head and neck cancers, but due to the paucity of reports of primary SCCs outside the head and neck, there is still a lack of effective clinical research evidence.
To the best of our knowledge, this report is the first to describe primary non-keratinizing SCC of the scapular bone. However, there is still no consensus on the standard treatment method or prognosis of primary SCC of non-skull bones because of its rarity, with only a few cases having been reported and followed up. Our findings demonstrate that clinicians must exhaust all available means for the diagnosis of primary SCC of bones, so greater attention can be paid to timely treatment and effective management. Regular and adequate follow-up is essential to help rule out metastasis and judge the prognosis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kao NH S-Editor: Huang P L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Waldman A, Schmults C. Cutaneous Squamous Cell Carcinoma. Hematol Oncol Clin North Am. 2019;33:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 208] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 2. | Suzuki A, Kashiwagi N, Doi H, Ishii K, Doi K, Kitano M, Kozuka T, Hyodo T, Tsurusaki M, Yagyu Y, Nakanishi K. Patterns of bone metastases from head and neck squamous cell carcinoma. Auris Nasus Larynx. 2020;47:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Griffin LL, Ali FR, Lear JT. Non-melanoma skin cancer. Clin Med (Lond). 2016;16:62-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Kallini JR, Hamed N, Khachemoune A. Squamous cell carcinoma of the skin: epidemiology, classification, management, and novel trends. Int J Dermatol. 2015;54:130-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 5. | Gaston CL, Vergel de Dios AM, Dela Rosa TL, Wang EH. Case report: Primary squamous cell carcinoma of a tarsal bone. Clin Orthop Relat Res. 2009;467:3346-3350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Gangopadhyay S, Saha S. Primary squamous cell carcinoma of bone. J Indian Med Assoc. 1997;95:521, 523. [PubMed] |
| 7. | Abbas A, Bromage JD, Stocks PJ, Al-Sarireh B. Case of the Conference: Primary squamous cell carcinoma in a long bone. J Bone Joint Surg Br. 2005;87 suppl 1:6. |
| 8. | Guan Y, Wang G, Fails D, Nagarajan P, Ge Y. Unraveling cancer lineage drivers in squamous cell carcinomas. Pharmacol Ther. 2020;206:107448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 9. | Sánchez-Danés A, Blanpain C. Deciphering the cells of origin of squamous cell carcinomas. Nat Rev Cancer. 2018;18:549-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 188] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 10. | Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1024] [Cited by in RCA: 926] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 11. | Bodner L, Manor E, Shear M, van der Waal I. Primary intraosseous squamous cell carcinoma arising in an odontogenic cyst: a clinicopathologic analysis of 116 reported cases. J Oral Pathol Med. 2011;40:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Abdelkarim AZ, Elzayat AM, Syed AZ, Lozanoff S. Delayed diagnosis of a primary intraosseous squamous cell carcinoma: A case report. Imaging Sci Dent. 2019;49:71-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Lovin BD, Gidley PW. Squamous cell carcinoma of the temporal bone: A current review. Laryngoscope Investig Otolaryngol. 2019;4:684-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Kikuchi K, Ide F, Takizawa S, Suzuki S, Sakashita H, Li TJ, Kusama K. Initial-Stage Primary Intraosseous Squamous Cell Carcinoma Derived from Odontogenic Keratocyst with Unusual Keratoameloblastomatous Change of the Maxilla: A Case Report and Literature Discussion. Case Rep Otolaryngol. 2018;2018:7959230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Allanson BM, Low TH, Clark JR, Gupta R. Squamous Cell Carcinoma of the External Auditory Canal and Temporal Bone: An Update. Head Neck Pathol. 2018;12:407-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Akoh CC, Chang J, Buckwalter J. Marjolin's Ulcer of the Tibia With Pelvic Lymph Node Metastasis. Iowa Orthop J. 2017;37:133-138. [PubMed] |
| 17. | Li Q, Cui H, Dong J, He Y, Zhou D, Zhang P, Liu P. Squamous cell carcinoma resulting from chronic osteomyelitis: a retrospective study of 8 cases. Int J Clin Exp Pathol. 2015;8:10178-10184. [PubMed] |
| 18. | Alami M, Mahfoud M, El Bardouni A, Berrada MS, El Yaacoubi M. Squamous cell carcinoma arising from chronic osteomyelitis. Acta Orthop Traumatol Turc. 2011;45:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Sarode SC, Sarode GS, Sengupta N, Sharma NK, Patil S. Calcified keratin pearls in oral squamous cell carcinoma. Oral Oncol. 2020;109:104681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Martínez-Martínez M, Mosqueda-Taylor A, Delgado-Azañero W, Rumayor-Piña A, de Almeida OP. Primary intraosseous squamous cell carcinoma arising in an odontogenic keratocyst previously treated with marsupialization: case report and immunohistochemical study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121:e87-e95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Tatsumori T, Tsuta K, Masai K, Kinno T, Taniyama T, Yoshida A, Suzuki K, Tsuda H. p40 is the best marker for diagnosing pulmonary squamous cell carcinoma: comparison with p63, cytokeratin 5/6, desmocollin-3, and sox2. Appl Immunohistochem Mol Morphol. 2014;22:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Affandi KA, Tizen NMS, Mustangin M, Zin RRMRM. p40 Immunohistochemistry Is an Excellent Marker in Primary Lung Squamous Cell Carcinoma. J Pathol Transl Med. 2018;52:283-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Dong Y, Zheng Y, Wang C, Ding X, Du Y, Liu L, Zhang W, Zhang W, Zhong Y, Wu Y, Song X. MiR-876-5p modulates head and neck squamous cell carcinoma metastasis and invasion by targeting vimentin. Cancer Cell Int. 2018;18:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Caruso G, Gerace E, Lorusso V, Cultrera R, Moretti L, Massari L. Squamous cell carcinoma in chronic osteomyelitis: a case report and review of the literature. J Med Case Rep. 2016;10:215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | da Silva AP, Breda E, Monteiro E. Malignant tumors of the temporal bone - our experience. Braz J Otorhinolaryngol. 2016;82:479-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Sun HY, Tsang RK. Squamous cell carcinoma of the temporal bone in 30 patients: Difference in presentation and treatment in de novo disease vs radiation associated disease. Clin Otolaryngol. 2017;42:1414-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Mimata Y, Nishida J, Nagai T, Tada H, Sato K, Doita M. Importance of latissimus dorsi muscle preservation for shoulder function after scapulectomy. J Shoulder Elbow Surg. 2018;27:510-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Ebisumoto K, Okami K, Hamada M, Maki D, Sakai A, Saito K, Shimizu F, Kaneda S, Iida M. Cetuximab with radiotherapy as an alternative treatment for advanced squamous cell carcinoma of the temporal bone. Auris Nasus Larynx. 2018;45:637-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Shinomiya H, Hasegawa S, Yamashita D, Ejima Y, Kenji Y, Otsuki N, Kiyota N, Sakakibara S, Nomura T, Hashikawa K, Kohmura E, Sasaki R, Nibu K. Concomitant chemoradiotherapy for advanced squamous cell carcinoma of the temporal bone. Head Neck. 2016;38 Suppl 1:E949-E953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |