Published online Feb 6, 2021. doi: 10.12998/wjcc.v9.i4.919
Peer-review started: September 26, 2020
First decision: November 8, 2020
Revised: November 16, 2020
Accepted: November 29, 2020
Article in press: November 29, 2020
Published online: February 6, 2021
Processing time: 121 Days and 8.1 Hours
Occult breast cancer (OBC) is a special type of breast cancer presenting as axillary lymph node metastasis with undetectable primary lesions in the breast. Due to its low incidence and unique clinical manifestations, there is a lack of consensus on the diagnosis and treatment of OBC. We report a case of OBC treated with neoadjuvant chemotherapy combined with anlotinib. The treatment was well tolerated, and the patient achieved a pathologic complete response.
A 53-year-old woman presented with a lump in her right axillary area with no primary lesions in the breast. Pathological biopsy confirmed right axillary metastatic carcinoma. Immunohistochemical staining results were positive for progesterone receptor, cytokeratin 7, specific breast markers GATA3 and gross cystic disease fluid protein-15. Tumor cells were negative for estrogen receptor, human epidermal growth factor receptor-2, cytokeratin 5/6, cytokeratin 20, and villin. The patient was diagnosed with OBC, and she underwent neoadjuvant chemotherapy combined with anlotinib. Mastectomy plus axillary lymph node dissection was performed. The patient achieved pathologic complete response with no residual invasive tumor cells in the breast or axillary lymph nodes. Postoperatively, she received adjuvant radiotherapy and endocrine therapy.
Neoadjuvant chemotherapy and anlotinib had good efficacy and safety in the treatment of OBC and may be a new therapeutic option.
Core Tip: Occult breast cancer is a rare type of breast cancer, and there are some difficulties and confusion in its diagnosis and treatment. Biopsy and immunohi-stochemical analysis can help to make a diagnosis after excluding the possibility of other primary cancers. Because of its low incidence, there is no systematic and standard treatment plan. We first reported the application of neoadjuvant chemotherapy combined with anlotinib in occult breast cancer, and pathologic complete response was obtained after operation. This case may provide a new, suitable treatment option for these patients.
- Citation: Zhang Y, Wu D, Zhao B, Tian XL, Yao TC, Li F, Liu WF, Shi AP. Application of neoadjuvant chemotherapy combined with anlotinib in occult breast cancer: A case report and review of literature. World J Clin Cases 2021; 9(4): 919-926
- URL: https://www.wjgnet.com/2307-8960/full/v9/i4/919.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i4.919
Occult breast cancer (OBC) is defined as breast cancer with axillary lymph node metastasis (or distant metastasis beyond the axillary lymph nodes) as the first manifestation, and physical examination, ultrasound (US) and breast X-ray examination have failed to find the primary breast lesion. Due to the low incidence of OBC, which is approximately 0.3% to 1.0%[1-3], there is a lack of direct evidence-based medical data on the diagnosis and treatment of this disease. OBC is equivalent to stage ІІ-Ш lymph node positive breast cancer, and it is recognized that systemic treatment is carried out according to the molecular classification of breast cancer. Anlotinib is a new oral tyrosine kinase inhibitor, which has the dual effects of antitumor angiogenesis and tumor growth. At present, the application of anlotinib in breast cancer is still in the exploratory stage, and its application in OBC has never been reported. Here, we report a case of OBC in which a pathologic complete response (pCR) was obtained following neoadjuvant chemotherapy (NAC) combined with anlotinib.
A 53-year-old woman was admitted to The First Hospital of Jilin University in September 2019 because of right axillary metastatic carcinoma.
The patient presented with an enlarged lymph node in her right axillary area 2 wk before she visited a local hospital for examination. A needle puncture biopsy of the lymph node indicated right axillary metastatic carcinoma, most likely originating from mammary tissue.
She had no previous history of any illnesses.
The patient had no relevant personal or family history.
Physical examination indicated no lumps in the breasts. A hard, fused and fixed lump 6.0 cm × 3.0 cm in size was identified in her right axillary area.
There was no abnormality.
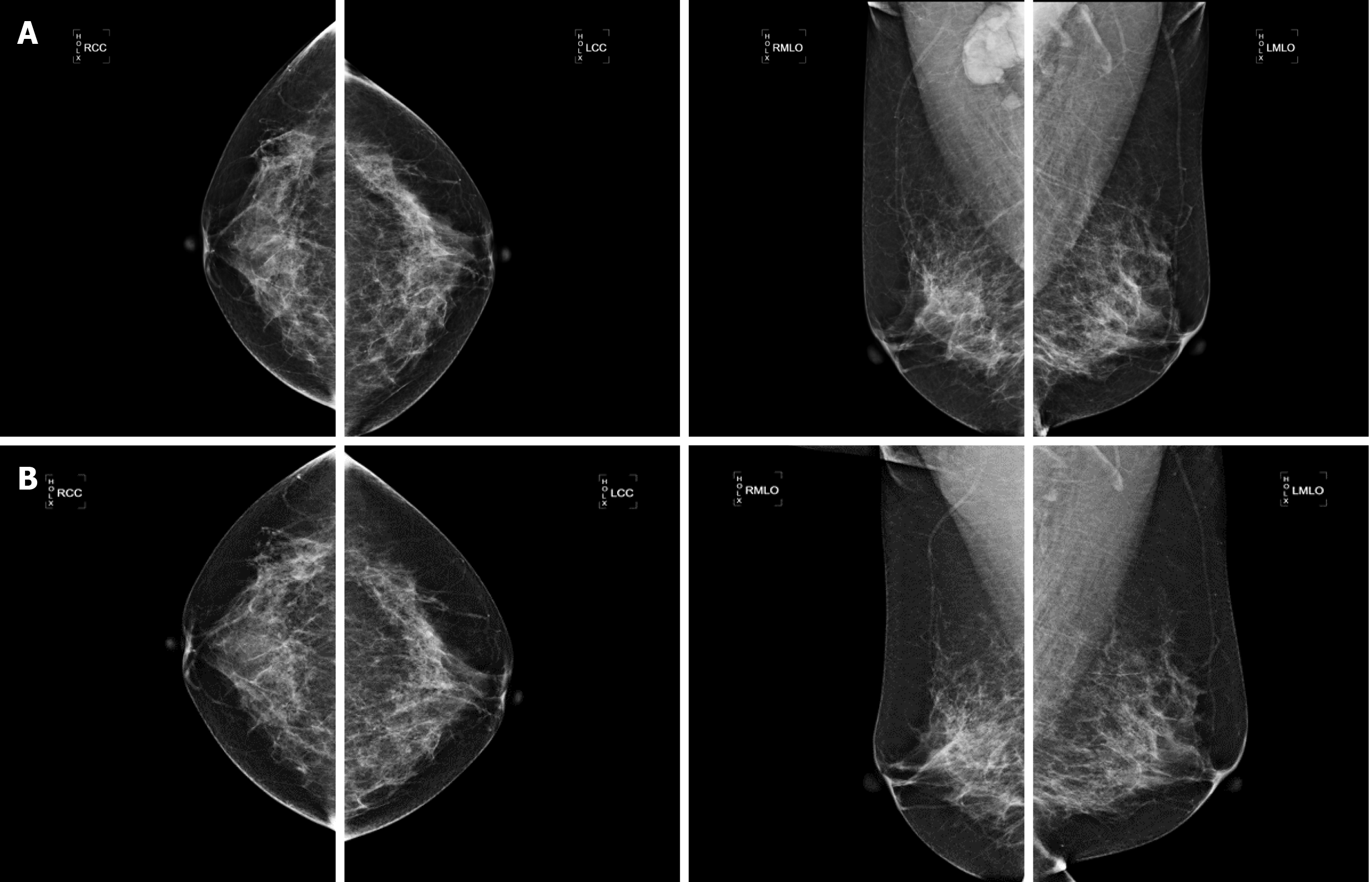
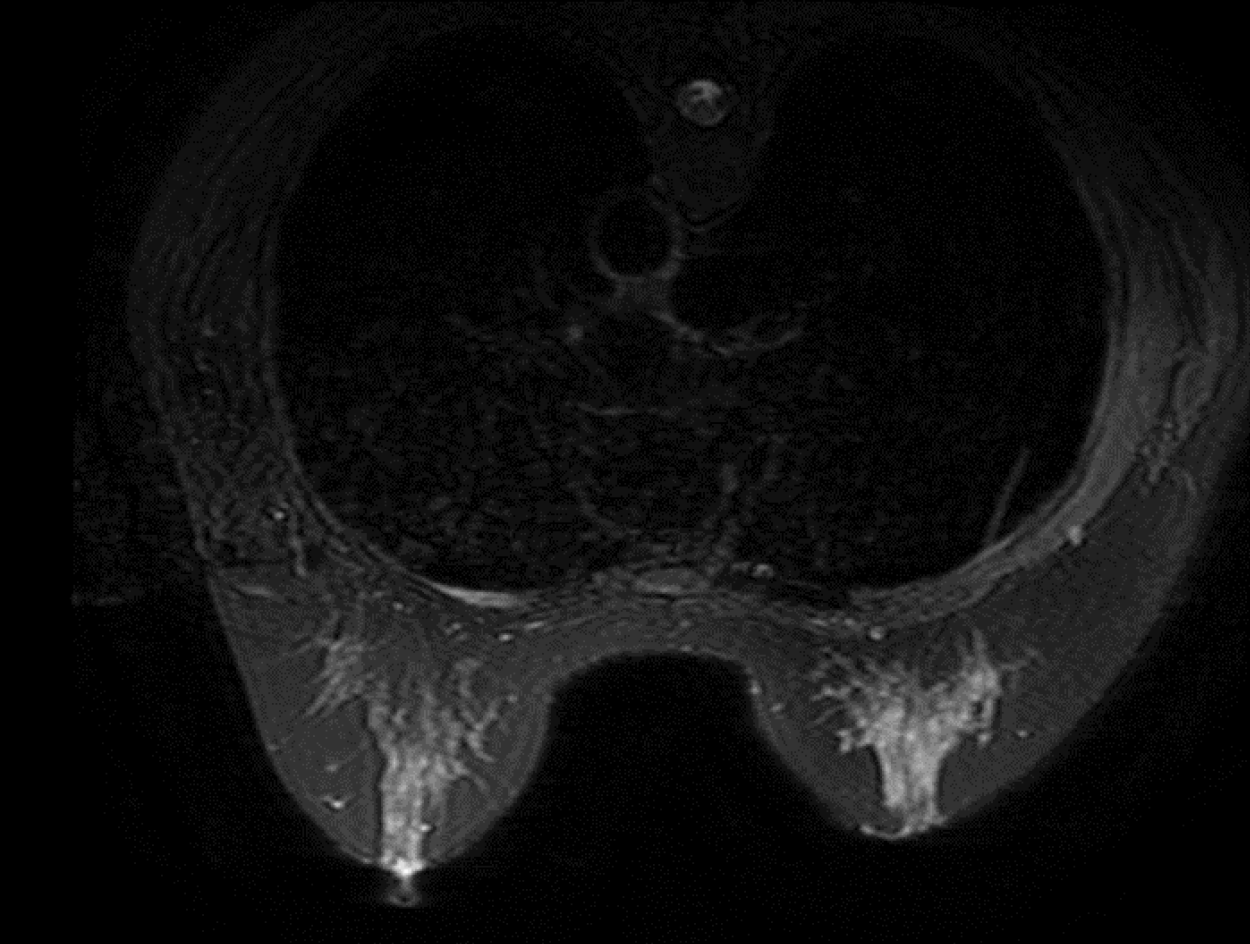
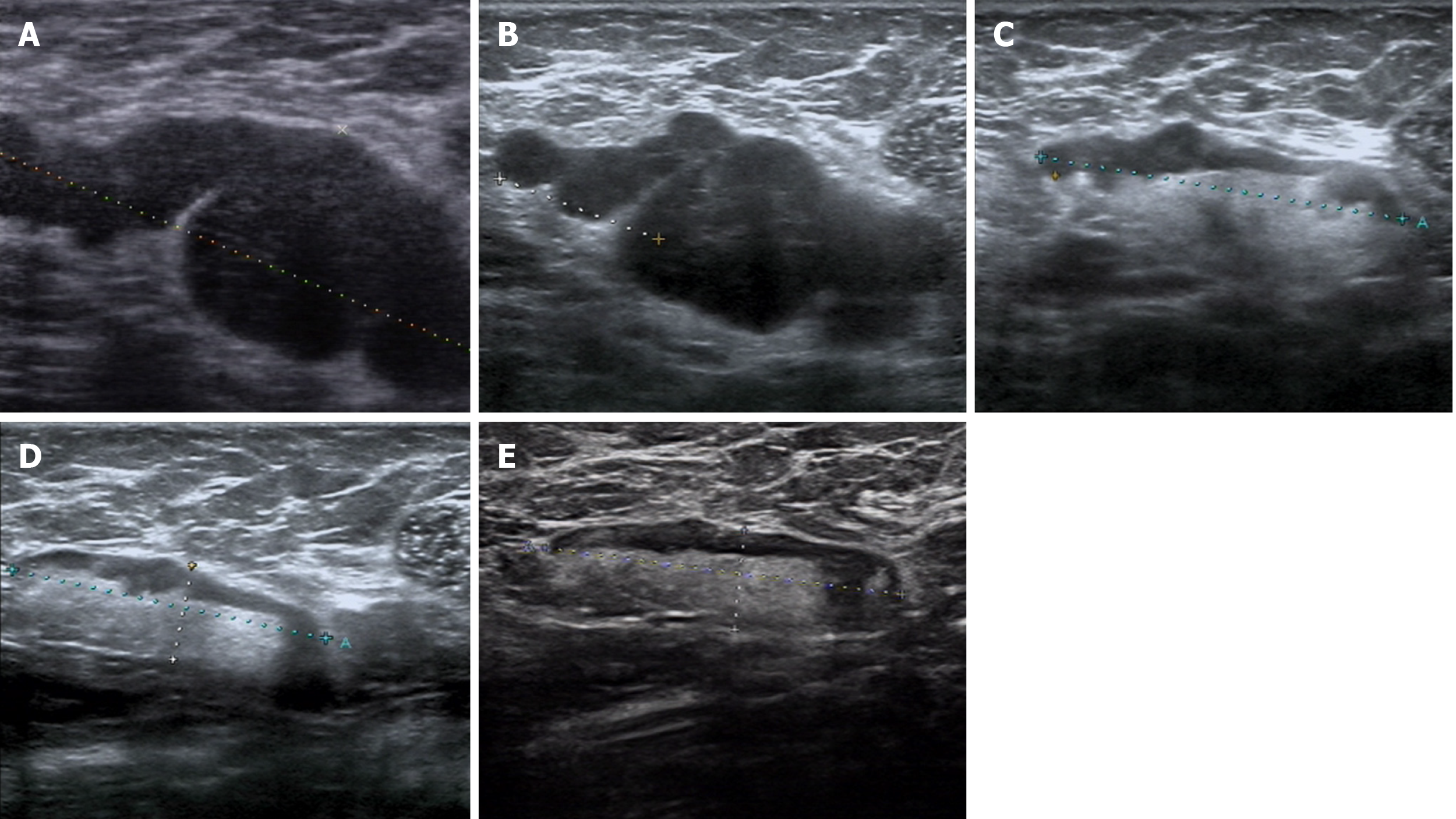
US revealed a hypoechoic mass 6.0 cm × 3.2 cm × 2.0 cm in size in the right axilla, which had internal vascularity. Breast lesions were not identified by US, mammography (Figure 1) or magnetic resonance imaging (Figure 2). There was no evidence of a malignant primary lesion or distant metastasis on computed tomography scans of the thorax, abdomen and bone. Immunohistochemical staining results were positive for progesterone receptor (PR), cytokeratin (CK) 7 and specific breast markers GATA3 and gross cystic disease fluid protein-15. Tumor cells were negative for estrogen receptor (ER), human epidermal growth factor receptor-2 (HER-2), CK5/6, CK20 and villin.
The final diagnosis is OBC.
After signing an informed consent form, the patient received four 3-wk cycles of 35 mg/m2 pegylated liposomal doxorubicin plus 600 mg/m2 cyclophosphamide followed by five 3-wk cycles of 260 mg/m2 nab-paclitaxel. As the clinical response of axillary lymph nodes to NAC was unsatisfactory, anlotinib (12 mg/d) was added to the treatment in the last four cycles of chemotherapy. The targeted lymph node was significantly smaller on US following treatment with anlotinib and nab-paclitaxel. The patient received four more cycles of nab-paclitaxel and anlotinib until the size of the lymph node was stable (Table 1 and Figure 3).
| Date | Size of targeted lymph node |
| September 6, 2019 | 60.0 mm × 32.0 mm |
| October 14, 2019 | 56.9 mm × 22.7 mm |
| October 31, 2019 | 56.5 mm × 23.9 mm |
| November 24, 2019 | 52.7 mm × 24.9 mm |
| December 13, 2019 | 55.4 mm × 23.4 mm |
| January 6, 2020 | 42.9 mm × 15.4 mm |
| February 3, 2020 | 41.8 mm × 12.5 mm |
| March 27, 2020 | 33.2 mm × 10.4 mm |
| April 17, 2020 | 40.9 mm × 11.4 mm |
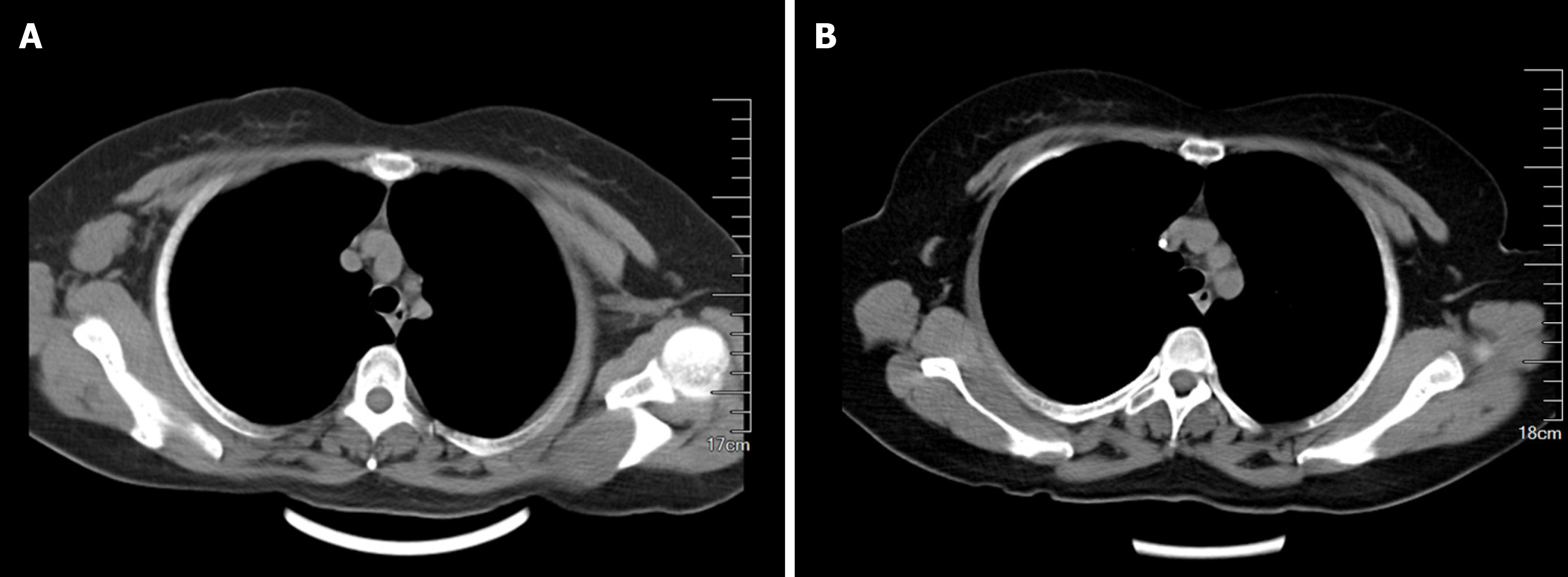
After the completion of NAC, the axillary lymph node was significantly smaller on imaging examination (Figures 1B and 4). On April 20, 2020, the patient underwent right modified radical mastectomy. Histopathology of the specimen from the right breast and axilla revealed low-grade ductal carcinoma in situ (DCIS) in the outer upper quadrant of the breast, and the maximum diameter was about 0.6 cm. No metastasis was found in the dissected axillary lymph nodes, and the patient recovered well. The pathological stage was ypTis(DCIS)ypN0M0. The breast tumor was positive for ER, PR, p63, E-cadherin and GATA3 and negative for HER-2, CK5/6, synaptophysin and thyroid transcription factor 1.
According to the pathological results, the patient received radiotherapy and endocrine therapy after surgery. Anlotinib was not included in adjuvant therapy. During a follow-up period of 5 mo, there were no signs of recurrence or metastasis.
OBC is a special type of breast cancer defined by the presence of axillary lymph node metastases or distant metastasis without evidence of a primary breast tumor. As OBC is rare and the current literature is limited to small retrospective reviews or case reports, there is controversy over its diagnosis and treatment. For suspicious OBC with a swollen axillary lymph node, pathological examinations and immunohistochemical indices, including ER, PR, HER-2, gross cystic disease fluid protein-15, CK7, GATA3, E-cadherin, CK5/6 and CK20, are of great significance in determining the source of the primary tumor and confirming the diagnosis. When OBC is suspected, magnetic resonance imaging is one of the most sensitive methods to evaluate occult lesions that are not found on physical examination, in addition to US and mammography. In our case, magnetic resonance imaging failed to detect suspicious primary breast lesions. Based on the results of immunohistochemistry and systemic examinations, the patient was finally diagnosed with OBC.
There is no uniform standard treatment for OBC. OBC is defined as TxN1-3M0 in the American Joint Committee on Cancer staging. Treatments can be based on the status of lymph node metastasis with reference to the non-OBC stage II and III treatment guidelines for patients with OBC. NAC is used as a standard therapy for inoperable locally advanced breast cancers, which can reduce tumor stage and increase the rate of surgical resection. A pCR after NAC has been associated with a favorable clinical outcome in breast cancer patients[4]. However, there are few studies on the use of NAC in treating OBC. Yang et al[5] reported five cases of OBC, all of which were treated with NAC and four out of five patients achieved a pCR. A similar pCR rate was reported by Rueth et al[6] in 15 of 25 OBC patients treated with NAC. A recent study evaluated 214 patients with OBC who underwent NAC, and 26% of these patients achieved a pCR[7].
In our case, the patient was not expected to achieve a pCR with five cycles NAC alone based on a slight reaction in the targeted lymph node. A pCR was achieved after the completion of nine cycles of NAC in combination with anlotinib. Compared with non-OBC, the higher pCR rate in OBC may indicate that OBC, as a special type of breast cancer, might have a better response to NAC. Previous studies have shown that in ER+/HER-2- cancers and triple negative breast cancers, lymph nodes are more sensitive to NAC than breast lesions, which might contribute to this optimistic consequence[8]. However, the limitations were that the case series of OBC was small with a short follow-up period. It is believed that patients with triple-negative and HER-2 positive breast cancers are more likely to achieve a pCR[9,10]. However, the relationship between molecular type and pCR rate in OBC patients was not analyzed in these studies. In order to determine which patients with OBC benefit from NAC needs to be confirmed with a larger sample size in randomized controlled trials.
Anlotinib is a novel oral multitargeted receptor tyrosine kinase inhibitor, which inhibits vascular endothelial growth factor/vascular endothelial growth factor receptor (VEGF/VEGFR) signal transduction by targeting VEGFR1/2/3 and fibroblast growth factor receptor 1/2/3/4. In addition, it suppresses the activity of platelet-derived growth factor receptor and c-Kit, resulting in its dual effects on antitumor angiogenesis and tumor growth[11,12]. Anlotinib has exceptional efficacy and acceptable toxicity in the treatment of a variety of solid tumors, especially in patients with advanced non-small cell lung cancer who have progressed after at least two previous lines of treatment. Tumor angiogenesis plays an important role in tumor growth and lymph node metastasis in breast cancer[13]. This suggests that anti-angiogenic therapy may be effective for OBC with lymph node metastasis as the only clinical manifestation. Previous studies have shown that the combination of bevacizumab, an anti-angiogenic monoclonal antibody against VEGF, and NAC can increase the rate of pCR in breast cancer patients[14-16]. Nevertheless, there are few studies on the efficacy and safety of anlotinib combined with chemotherapy in the treatment of solid tumors. Han et al[17] showed the potential effect and manageable safety profile of anlotinib combined with chemotherapy in patients with previously untreated EGFR/ALK/ROS1 negative advanced non-small cell lung cancer.
In a retrospective study, chemotherapy combined with anlotinib plus anlotinib maintenance therapy showed good efficacy and resulted in more favorable survival with good tolerance in advanced soft tissue sarcoma[18]. For the first time, we report the effects of NAC combined with anlotinib in the treatment of OBC. The patient achieved a pCR with no residual invasive tumor cells in the breast or axillary lymph nodes. Due to a lack of evidence related to the use of anlotinib in adjuvant therapy for breast cancer, and considering its toxicity, anlotinib was not used in postoperative treatment. As shown in other clinical trials, the most common adverse reaction to anlotinib is hypertension, which occurred in our patient[19]. After appropriate medical management, the drug was well tolerated. Due to its manageable toxicity, anlotinib can be used in combination with conventional chemotherapy.
Mastectomy with axillary lymph node dissection (ALND) has traditionally been the recommended treatment for OBC. In recent years, many studies have shown that breast conservation with ALND plus radiotherapy is also a feasible option for OBC. Breast conservation includes resection of the upper outer quadrant or observation only without surgical treatment. Due to the low detection rate of primary tumor after quadrant resection, breast observation plus ALND are more commonly used in breast conservation at present[20]. Compared with patients who only underwent ALND, patients who underwent mastectomy or breast conservation plus radiotherapy had significantly improved survival benefits[21]. However, studies showed no significant survival difference between patients who underwent mastectomy and those who underwent breast conservation[20-24]. In addition, the local recurrence rate of both treatments was very low (13%-15%)[20,25]. The reported detection rate of primary tumors in mastectomy specimens in OBC patients ranged from 31% to 75%[2,21,26,27]. Most of these primary lesions were infiltrative tumors[21,28].
In our case, DCIS was found in the resected breast, which is relatively rare, with a reported incidence in the literature of approximately 10% to 20%[2,21,26]. Immunohi-stochemical results were different between the primary tumor and axillary metastatic carcinoma. ER was negative and PR (30%) expression was low in the axillary lymph nodes. However, both ER (90%) and PR (90%) were highly expressed in the primary breast lesion. As DCIS was found in the breast after NAC, we could not rule out the possibility that invasive cancer may have been present before NAC treatment; thus, endocrine therapy was considered. Obviously, this will result in significant survival benefits in this patient. The identification of primary breast lesions is helpful in defining tumor stage and providing more information for follow-up treatment. Therefore, we propose that mastectomy is the most effective local treatment for patients with OBC.
Despite the fact that OBC is a rare disease and there are few clinical trials on its treatment, previous studies have shown that the prognosis of OBC patients is similar to or better than that of non-OBC patients[23,26,29,30]. In addition, more than four positive lymph nodes may independently predict a poor outcome in patients with OBC[20,21,30]. OBC may have its own pathological and biological characteristics, but little is known about these due to its low incidence. Our case provides an exploratory and successful treatment plan for OBC. Further research is needed to guide clinical diagnosis and to determine a standard and effective treatment for this disease.
This case report, for the first time, describes the effects of NAC plus anlotinib in the treatment of OBC. The treatment was well tolerated and the patient achieved a pCR. NAC and anlotinib may provide an effective and safe treatment choice for OBC.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pop TL S-Editor: Huang P L-Editor: Filipodia P-Editor: Li JH
| 1. | Fitts WT, Steiner GC, Enterline HT. Prognosis of occult carcinoma of the breast. Am J Surg. 1963;106:460-463. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Baron PL, Moore MP, Kinne DW, Candela FC, Osborne MP, Petrek JA. Occult breast cancer presenting with axillary metastases. Updated management. Arch Surg. 1990;125:210-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 118] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Rosen PP. Axillary lymph node metastases in patients with occult noninvasive breast carcinoma. Cancer. 1980;46:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Choi M, Park YH, Ahn JS, Im YH, Nam SJ, Cho SY, Cho EY. Evaluation of Pathologic Complete Response in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy: Experience in a Single Institution over a 10-Year Period. J Pathol Transl Med. 2017;51:69-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Yang H, Li L, Zhang M, Zhang S, Xu S, Ma X. Application of neoadjuvant chemotherapy in occult breast cancer: Five case reports. Medicine (Baltimore). 2017;96:e8200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Rueth NM, Black DM, Limmer AR, Gabriel E, Huo L, Fornage BD, Dogan BE, Chavez-MacGregor M, Yi M, Hunt KK, Strom EA. Breast conservation in the setting of contemporary multimodality treatment provides excellent outcomes for patients with occult primary breast cancer. Ann Surg Oncol. 2015;22:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Cohen BL, Collier AL, Kelly KN, Goel N, Kesmodel SB, Yakoub D, Moller M, Avisar E, Franceschi D, Macedo FI. Surgical Management of the Axilla in Patients with Occult Breast Cancer (cT0 N+) After Neoadjuvant Chemotherapy. Ann Surg Oncol. 2020;27:1830-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Mamtani A, Barrio AV, King TA, Van Zee KJ, Plitas G, Pilewskie M, El-Tamer M, Gemignani ML, Heerdt AS, Sclafani LM, Sacchini V, Cody HS 3rd, Patil S, Morrow M. How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissection in Patients With Histologically Confirmed Nodal Metastases? Ann Surg Oncol. 2016;23:3467-3474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 242] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 9. | Haque W, Verma V, Hatch S, Suzanne Klimberg V, Brian Butler E, Teh BS. Response rates and pathologic complete response by breast cancer molecular subtype following neoadjuvant chemotherapy. Breast Cancer Res Treat. 2018;170:559-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 210] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 10. | Boughey JC, McCall LM, Ballman KV, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, Leitch AM, Flippo-Morton T, Hunt KK. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. 2014;260:608-614; discussion 614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 325] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 11. | Xie C, Wan X, Quan H, Zheng M, Fu L, Li Y, Lou L. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci. 2018;109:1207-1219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 256] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 12. | Taurin S, Yang C-H, Reyes M, Cho S, Jarboe EA, Werner TL, Coombs DM, Chen P, Janát-Amsbury MM. Treatment of endometrial cancer cells with a new small tyrosine kinase inhibitor targeting mutated fibroblast growth factor receptor-2. 2017;77:3244-3244. [DOI] [Full Text] |
| 13. | Horak ER, Leek R, Klenk N, LeJeune S, Smith K, Stuart N, Greenall M, Stepniewska K, Harris AL. Angiogenesis, assessed by platelet/endothelial cell adhesion molecule antibodies, as indicator of node metastases and survival in breast cancer. Lancet. 1992;340:1120-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 590] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 14. | Makhoul I, Klimberg VS, Korourian S, Henry-Tillman RS, Siegel ER, Westbrook KC, Hutchins LF. Combined neoadjuvant chemotherapy with bevacizumab improves pathologic complete response in patients with hormone receptor negative operable or locally advanced breast cancer. Am J Clin Oncol. 2015;38:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Cao L, Yao GY, Liu MF, Chen LJ, Hu XL, Ye CS. Neoadjuvant Bevacizumab plus Chemotherapy versus Chemotherapy Alone to Treat Non-Metastatic Breast Cancer: A Meta-Analysis of Randomised Controlled Trials. PLoS One. 2015;10:e0145442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Bear HD, Tang G, Rastogi P, Geyer CE Jr, Robidoux A, Atkins JN, Baez-Diaz L, Brufsky AM, Mehta RS, Fehrenbacher L, Young JA, Senecal FM, Gaur R, Margolese RG, Adams PT, Gross HM, Costantino JP, Swain SM, Mamounas EP, Wolmark N. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N Engl J Med. 2012;366:310-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 362] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 17. | Han B, Chu T, Zhang X, Zhong H, Zhang B, Wang H, Gu A, Zhang W, Shi C, Zhong R. Efficacy and Safety of Anlotinib in Combination with Chemotherapy as First-Line Therapy in Advanced Non-Small Cell Lung Cancer (NSCLC) Patients. J Thorac Oncol. 2019;14:S398. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Wang HY, Chu JF, Zhang P, Wang JQ, Yan Z, Yao SN, Yao ZH, Liu YY. Safety and Efficacy of Chemotherapy Combined with Anlotinib Plus Anlotinib Maintenance in Chinese Patients with Advanced/Metastatic Soft Tissue Sarcoma. Onco Targets Ther. 2020;13:1561-1568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Sun Y, Niu W, Du F, Du C, Li S, Wang J, Li L, Wang F, Hao Y, Li C, Chi Y. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol. 2016;9:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 322] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 20. | Vlastos G, Jean ME, Mirza AN, Mirza NQ, Kuerer HM, Ames FC, Hunt KK, Ross MI, Buchholz TA, Buzdar AU, Singletary SE. Feasibility of breast preservation in the treatment of occult primary carcinoma presenting with axillary metastases. Ann Surg Oncol. 2001;8:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | He M, Tang LC, Yu KD, Cao AY, Shen ZZ, Shao ZM, Di GH. Treatment outcomes and unfavorable prognostic factors in patients with occult breast cancer. Eur J Surg Oncol. 2012;38:1022-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Tsai C, Zhao B, Chan T, Blair SL. Treatment for occult breast cancer: A propensity score analysis of the National Cancer Database. Am J Surg. 2020;220:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Ge LP, Liu XY, Xiao Y, Gou ZC, Zhao S, Jiang YZ, Di GH. Clinicopathological characteristics and treatment outcomes of occult breast cancer: a SEER population-based study. Cancer Manag Res. 2018;10:4381-4391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Johnson HM, Irish W, Vohra NA, Wong JH. The effect of local therapy on breast cancer-specific mortality of women with occult breast cancer and advanced nodal disease (N2/N3): a population analysis. Breast Cancer Res Treat. 2019;177:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Barton SR, Smith IE, Kirby AM, Ashley S, Walsh G, Parton M. The role of ipsilateral breast radiotherapy in management of occult primary breast cancer presenting as axillary lymphadenopathy. Eur J Cancer. 2011;47:2099-2106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Ping S, Ming WH, Bin SH, Wen WD, Cheng Q, Jun CC, Bin HY, Jian CZ. Comparison of clinical characteristics between occult and non-occult breast cancer. J BUON. 2014;19:662-666. [PubMed] |
| 27. | Rosen PP, Kimmel M. Occult breast carcinoma presenting with axillary lymph node metastases: a follow-up study of 48 patients. Hum Pathol. 1990;21:518-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 60] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Merson M, Andreola S, Galimberti V, Bufalino R, Marchini S, Veronesi U. Breast carcinoma presenting as axillary metastases without evidence of a primary tumor. Cancer. 1992;70:504-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Sohn G, Son BH, Lee SJ, Kang EY, Jung SH, Cho SH, Baek S, Lee YR, Kim HJ, Ko BS, Yu, Lee JW, Ahn SH. Treatment and survival of patients with occult breast cancer with axillary lymph node metastasis: a nationwide retrospective study. J Surg Oncol. 2014;110:270-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Montagna E, Bagnardi V, Rotmensz N, Viale G, Cancello G, Mazza M, Cardillo A, Ghisini R, Galimberti V, Veronesi P, Monti S, Luini A, Raviele PR, Mastropasqua MG, Goldhirsch A, Colleoni M. Immunohistochemically defined subtypes and outcome in occult breast carcinoma with axillary presentation. Breast Cancer Res Treat. 2011;129:867-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |