Published online Feb 6, 2021. doi: 10.12998/wjcc.v9.i4.912
Peer-review started: September 18, 2020
First decision: November 23, 2020
Revised: December 6, 2020
Accepted: December 16, 2020
Article in press: December 16, 2020
Published online: February 6, 2021
Processing time: 121 Days and 4.6 Hours
Most cases of Apert syndrome (AS) are found after birth. Cases of AS diagnosed by ultrasound combined with magnetic resonance imaging (MRI) and whole exome sequencing (WES) during pregnancy are rare.
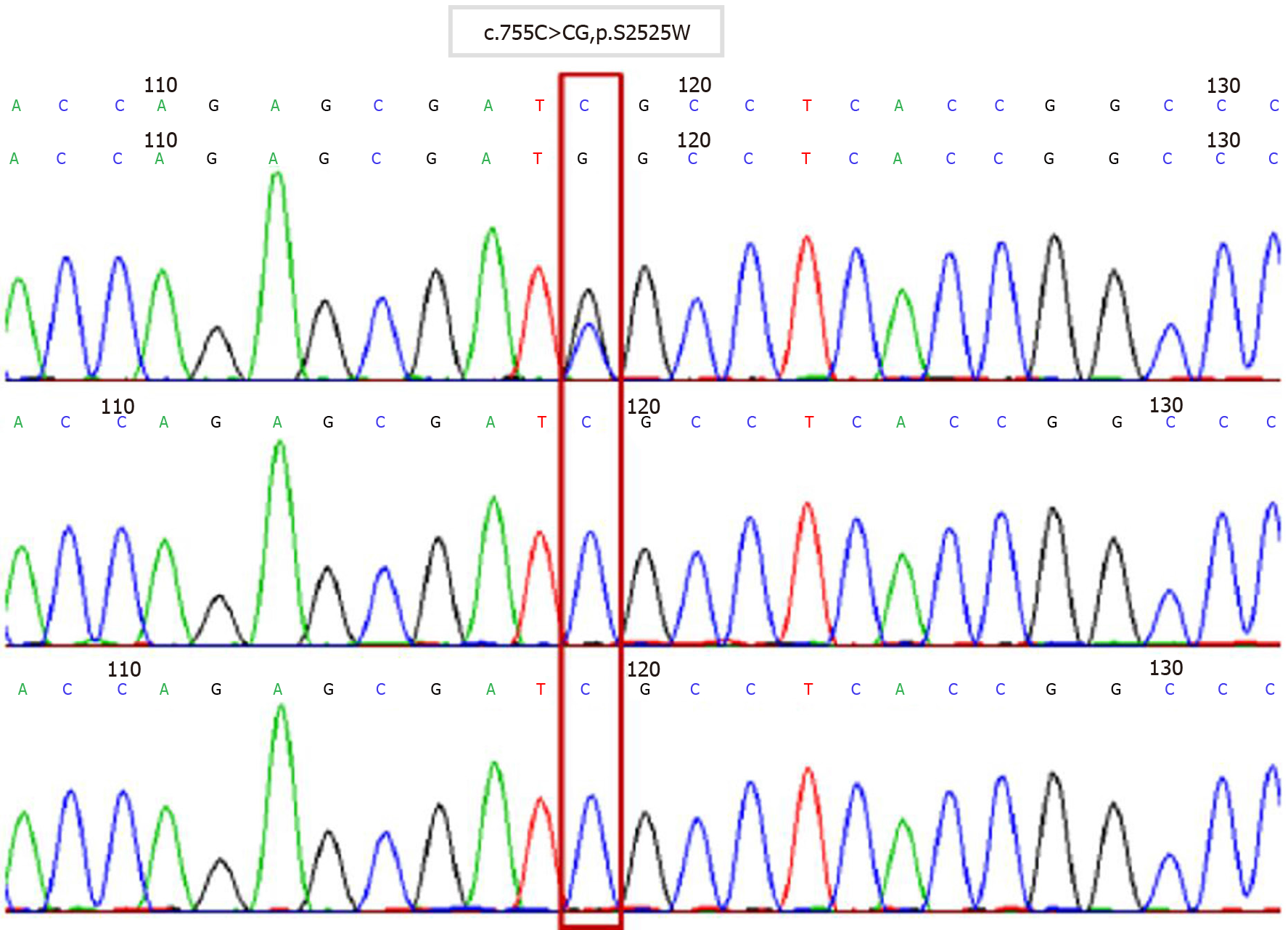
We present the case of a 34-year old female patient (gravida 2, para 1) whose fetus was diagnosed with AS during pregnancy. Fetal ultrasound performed at 30, 2/7 wk of pregnancy showed abnormalities. MRI and three-dimensional ultrasound performed at 31, 1/7 wk of pregnancy showed the possibility of AS. Chromosome examination and core family WES were conducted at 31, 5/7 wk of pregnancy. The results showed that FGFR2 in the fetus had a c.755C>G missense mutation in its nucleotide, and AS was confirmed.
This case highlights the importance of imaging examinations. Prenatal ultrasound combined with MRI can identify fetal morphological abnormalities accurately, which can be confirmed by WES.
Core Tip: Apert syndrome (AS) during pregnancy is rare. We present a case of AS diagnosed by prenatal ultrasound combined with magnetic resonance imaging and whole exome sequencing in late pregnancy. Our case also shows that S252W mutation of AS is related to syndactyly and craniofacial dysplasia, and suggests that young paternal age is also related to AS. We reviewed the three-dimensional fetal ultrasound at 23, 5/7 wk of gestation and found some suspicious images such as craniosynostosis and raised forehead. This shows that improved awareness of AS and prenatal imaging are important.
- Citation: Chen L, Huang FX. Apert syndrome diagnosed by prenatal ultrasound combined with magnetic resonance imaging and whole exome sequencing: A case report. World J Clin Cases 2021; 9(4): 912-918
- URL: https://www.wjgnet.com/2307-8960/full/v9/i4/912.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i4.912
Apert syndrome (AS) is characterized by craniosynostosis, hypoplasia of the middle of the face, and syndactyly[1-3]. Since AS is difficult to detect during pregnancy, it is usually diagnosed after birth. The incidence of AS is 1 in 65000 births[4]. At present, few cases of AS during pregnancy have been reported, and the reported cases are usually based on prenatal ultrasound diagnosis. AS diagnosed by prenatal ultrasound combined with magnetic resonance imaging (MRI) and whole exome sequencing (WES) during pregnancy has not been reported previously. Here, we report a case of AS diagnosed by prenatal ultrasound combined with MRI and WES in late pregnancy and showed that young paternal age is also related to AS.
On April 13, 2020, a 34-year old female patient at 31, 5/7 wk of pregnancy was admitted to our hospital due to the abnormalities on fetal imaging examination 10 d previously.
There was no history of present disease.
There was no history of past disease.
The family history was unremarkable.
Physical examination on admission showed no abnormalities.
Laboratory examinations showed no abnormalities.
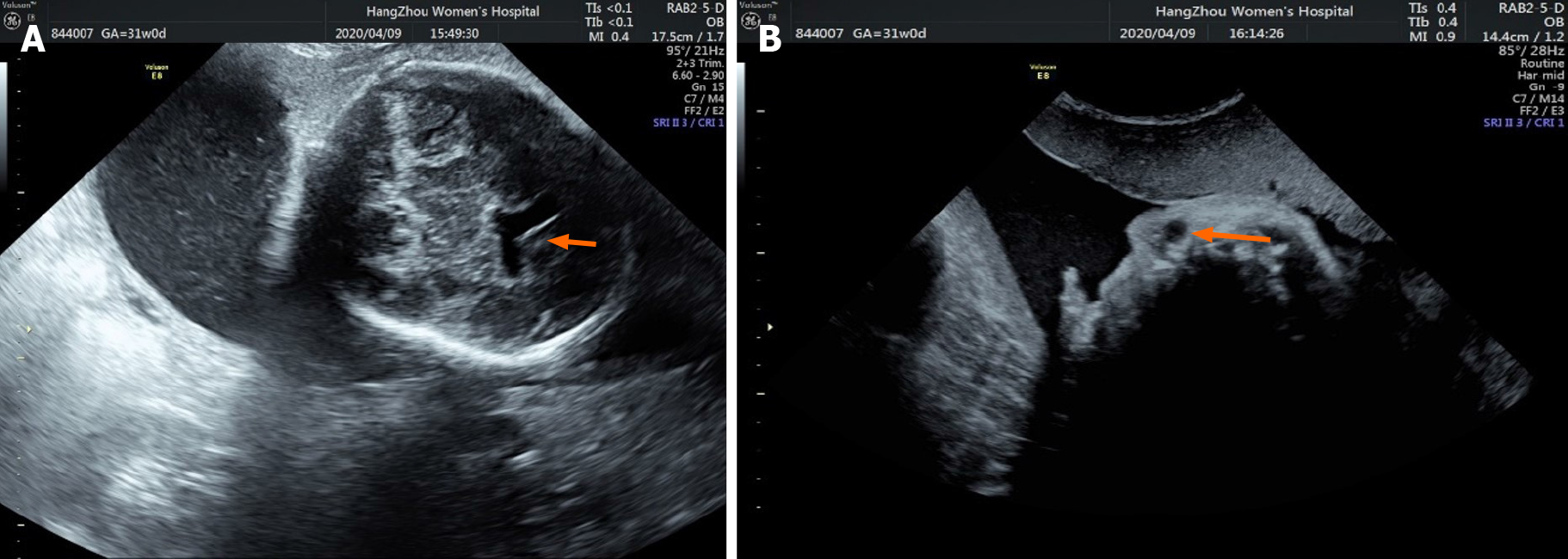
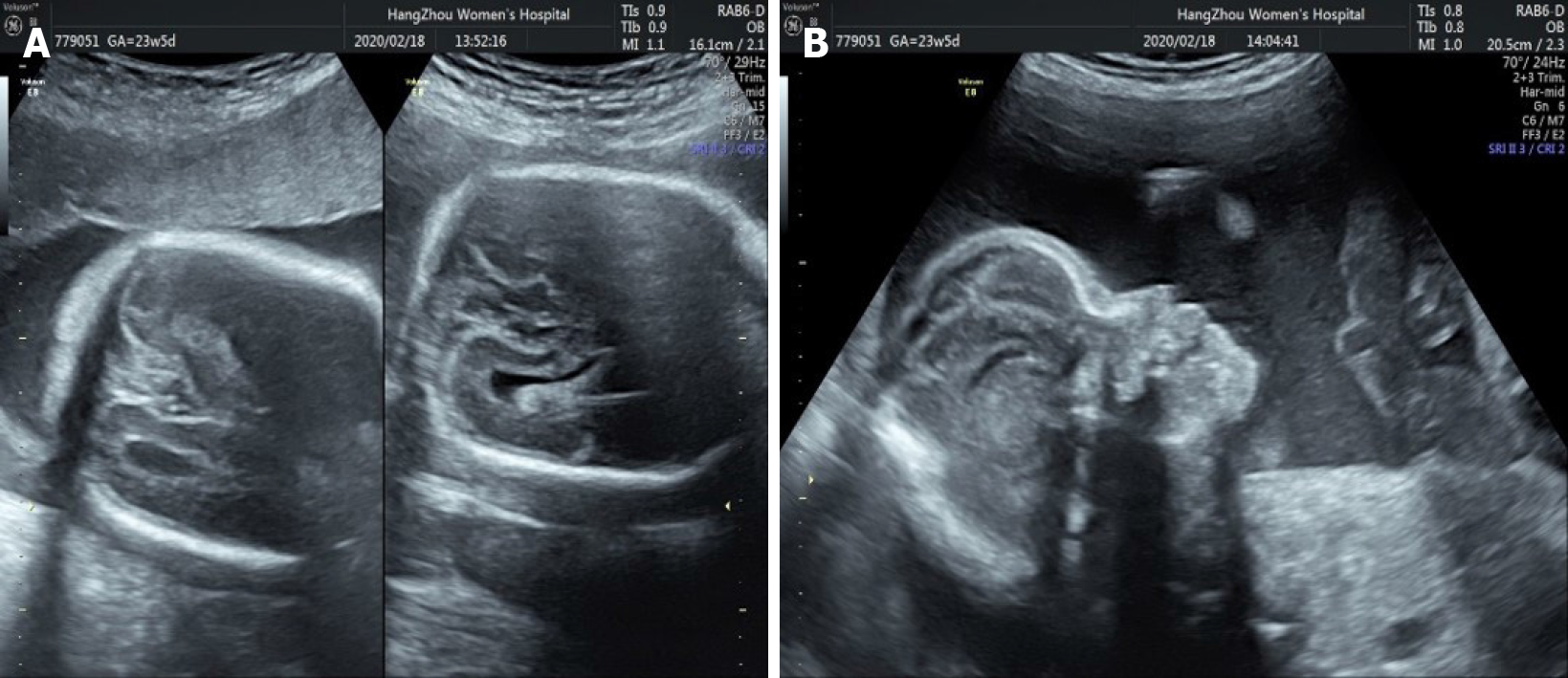
Fetal ultrasound performed at 30, 2/7 wk of pregnancy showed that the anterior horn of the lateral ventricle of the fetus was widened by 10 mm and 5 mm on the right and left, respectively (Figure 1A). A cystic dark area 9 mm × 9 mm × 8 mm in size was seen near the right eye, suggesting the possibility of a nasolacrimal duct cyst (Figure 1B).
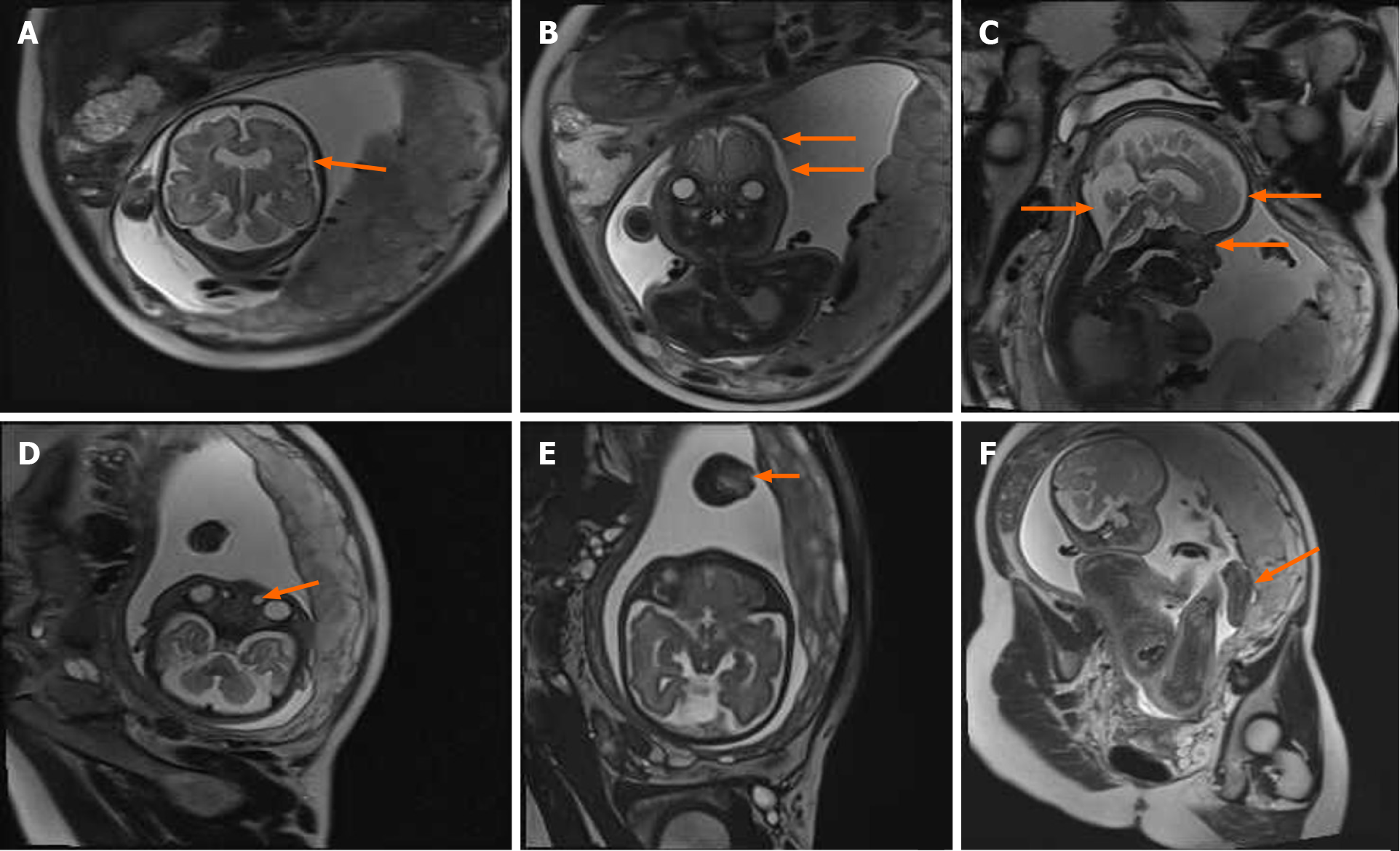
MRI performed at 31, 1/7 wk of pregnancy showed craniosynostosis (Figure 2A) in the coronal position, and the fetal skull was narrow and pointed (Figure 2B). In the sagittal position, the forehead was uplifted and raised, the nasal root was slightly concave (Figure 2C), eye distance was widened (the ratio of inner to outer diameter was 0.4) (Figure 2B). Cystic shadows were seen at the inner edges of both eyes, the diameter was 3.8 mm on the left and 5.7 mm on the right, indicating a nasolacrimal duct cyst on both sides (Figure 2D). Both sides of the anterior horn of the lateral ventricle were widened, about 7.4 mm on the right side and 5.7 mm on the left. The posterior cranial fossa was widened and the width was 10.4 mm (Figure 2C). The midline above the cerebellum showed a cystoid shadow of about 12 mm × 10 mm × 22 mm, suggesting an arachnoid cyst (Figure 2C). MRI also showed syndactyly of the hands and feet (Figure 2E and F).
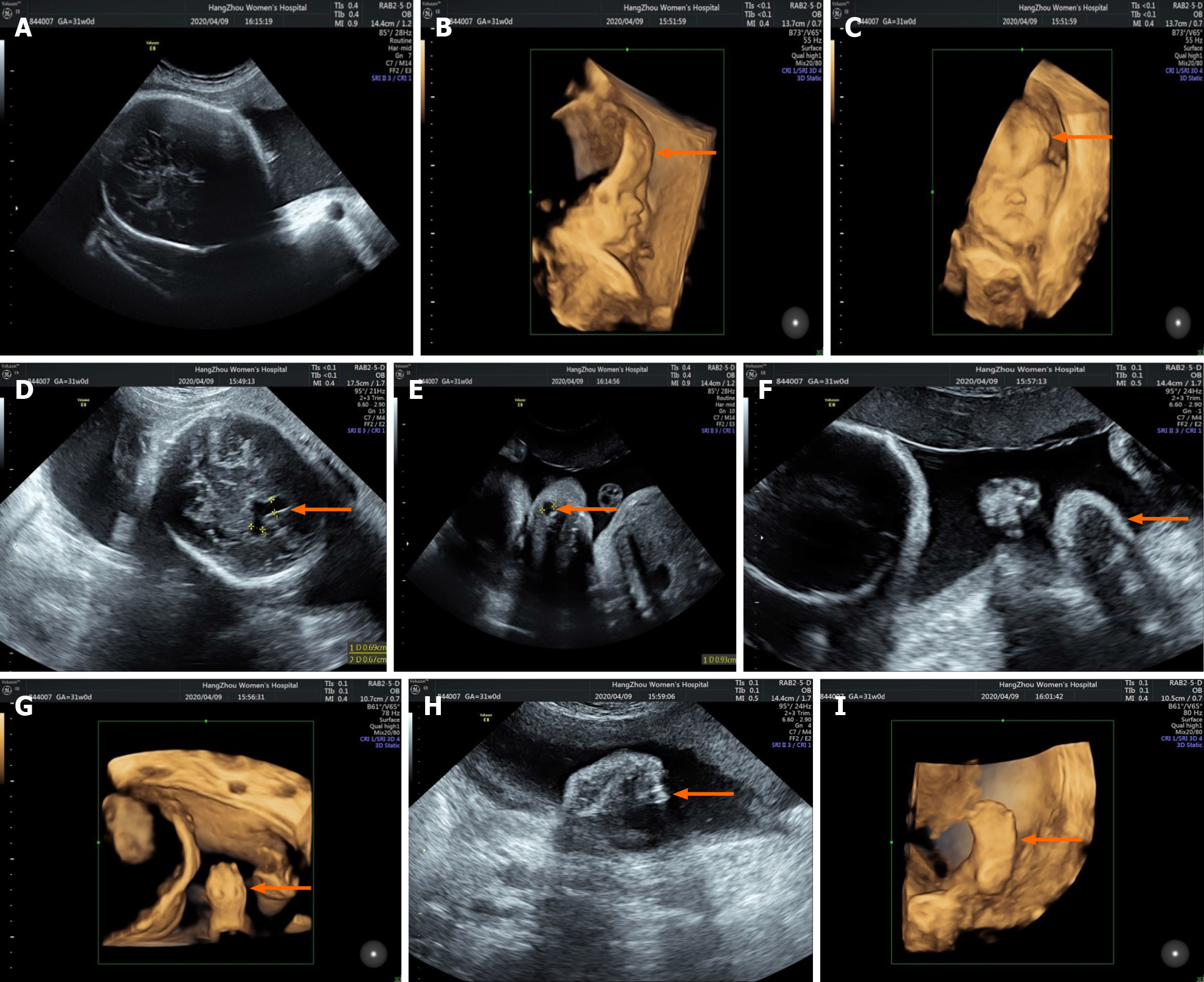
Three-dimensional (3D) prenatal ultrasound performed on the same day as MRI showed craniosynostosis (Figure 3A), forehead protrusion (Figure 3B and C), widened anterior horns of the lateral ventricle (Figure 3D), nasolacrimal duct cyst (Figure 3E) and syndactyly (Figure 3F–I).
Apert syndrome.
According to the observed abnormalities such as acrocephalia, craniosynostosis, midfacial dysplasia and syndactyly, AS was considered. After antenatal consultation, the patient selected WES for further diagnosis and requested termination of the pregnancy.
At 31 5/7 wk of gestation, the patient received ultrasound-guided umbilical vein puncture plus amniotic cavity 6, 9-diamino2-ethoxylacridine lactate hydrate (Rivanol, Guangxi Hefeng Pharmaceutical Co., Ltd., China) injection for labor induction. Umbilical cord blood (3.5 mL) was successfully extracted during the operation. The peripheral blood of the patient and her husband was simultaneously extracted for chromosome examination and core family WES analysis.
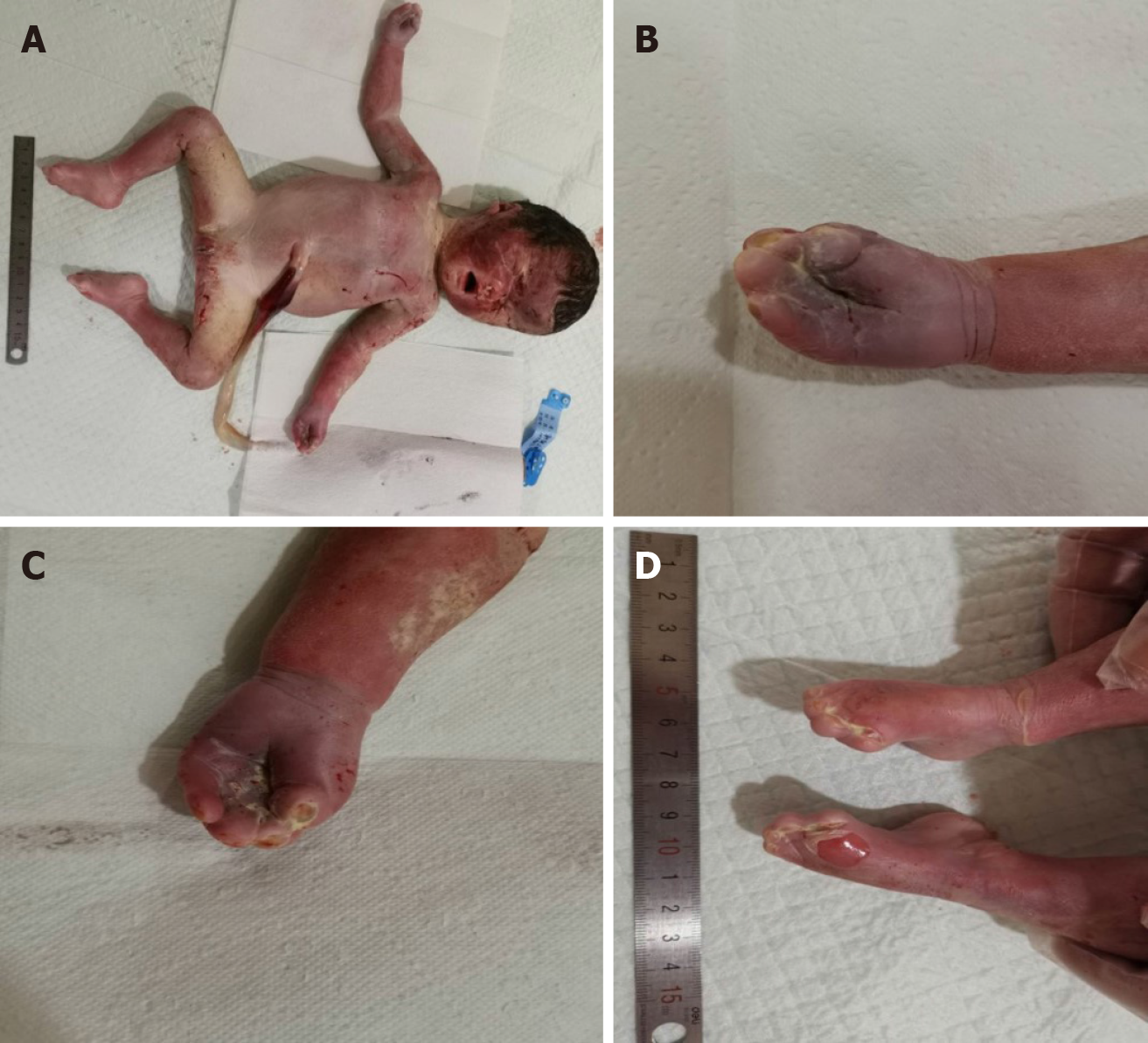
The fetus was stillborn 2 d after Rivanol injection with a weight of 2.3 kg, a pointed head, bulging forehead, collapsed nose root, protruding eyes, and complete syndactyly of the hands and feet (Figure 4A–D).
Twenty-eight days after the operation, chromosome examination and WES showed that FGFR2 of the fetus had a c.755C>G missense mutation in its nucleotides (Figure 5). According to the relevant guidelines of the American College of Medical Genetics and Genomics, the disease gene variation database and literature reports, we confirmed that c.755C>G was the pathogenic variation and AS was diagnosed. Neither parent had the same genetic mutation.
AS is one of the most common severe craniomaxillofacial deformity syndromes. It is characterized by craniofacial deformity and syndactyly, and is often accompanied by varying degrees of mental retardation and other abnormalities[5,6]. Premature coronal suture closure, acrocephaly, prominent forehead, abnormal development in the middle of the face, collapse of the nasal root, and syndactyly were found in our case, which were consistent with previous reports[7,8].
AS is an autosomal disorder and 98% of cases are caused by functional mutations in the FGFR2 gene located on chromosome 10[4]. The sudden mutation of S252W and P253R of the FGFR2 gene is the main cause of AS. Patients with the S252W mutation mainly present with craniofacial dysplasia, while those with the 253R mutation mainly present with syndactyly[9-11]. In the present case, WES indicated that the nucleotides of FGFR2 had a c.755C>G missense mutation, which changed the amino acid at position 252 from serine to tryptophan. Although the genetic mutation was S252W, craniofacial dysplasia and syndactyly were obvious. That means that the mutation of S252W is also related to syndactyly.
Older paternal age is considered to be a high risk factor for AS, and with increased paternal age, the frequency of gene FGFR2 mutations is also increased, and the corresponding incidence of AS is increased[12]. Advanced paternal age is not well defined at present, which is usually defined as ≥ 40 years of age at conception[13]. The 34-year-old father of this fetus does not belong to the high-risk category, but AS still occurred. This suggests that the older age of the father had little relationship with the occurrence of AS in this case.
It is difficult to diagnose AS with prenatal ultrasound. Most cases are found after birth. With the improvement of ultrasound detection technology, reports on the prenatal diagnosis of AS have increased, and most of them were found in the middle and late stages of pregnancy. In this case, 3D ultrasonography of the fetus in the second trimester showed no abnormalities, and the diagnosis was not made until the third trimester. We reviewed the 3D fetal ultrasound at 23, 5/7 wk of gestation and found suspicious images such as craniosynostosis (Figure 6A) and raised forehead (Figure 6B). It is speculated that missed diagnosis may be related to the low incidence and insufficient diagnostic experience and cognition of the disease. In addition, due to the close proximity between the fingers and toes of the fetus in utero and low mobility, ultrasound observation is not easy, which may also cause missed diagnosis. Ultrasound is the preferred method of prenatal screening, but it is affected by fetal position and resolution of the instrument[14]. Fetal MRI can be used to scan various sections with high soft tissue resolution[15]. The technical level of operators has little influence on resolution. In this case, fetal MRI was performed after abnormal ultrasound examination to confirm skull morphology and intracranial tissue, providing the morphological basis for the diagnosis of AS. Due to the poor quality of life of children with AS, ultrasound combined with MRI should be used to detect fetal malformations as early as possible, and further genetic testing can be performed to terminate the pregnancy in a timely manner, or children can receive rehabilitation treatment as soon as possible.
To improve the diagnosis of AS, prenatal ultrasound combined with MRI should be used to identify fetal morphological abnormalities as soon as possible. For AS diagnosed by imaging, WES can be used to finally confirm AS. These methods are important to help pregnant women choose whether to continue the pregnancy or to develop an early treatment plan for the newborn infant.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Prestes-Carneiro L S-Editor: Zhang L L-Editor: Webster JR P-Editor: Liu JH
| 1. | Cohen MM Jr, Kreiborg S. A clinical study of the craniofacial features in Apert syndrome. Int J Oral Maxillofac Surg. 1996;25:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 75] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | GH. Anatomy and management of the foot in Apert syndrome: A review of the literature. The Foot. 2006;16:98-102. [DOI] [Full Text] |
| 3. | Pettitt DA, Arshad Z, Mishra A, McArthur P. Apert syndrome: A consensus on the management of Apert hands. J Craniomaxillofac Surg. 2017;45:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Breik O, Mahindu A, Moore MH, Molloy CJ, Santoreneos S, David DJ. Apert syndrome: Surgical outcomes and perspectives. J Craniomaxillofac Surg. 2016;44:1238-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Green SM. Pathological anatomy of the hands in Apert's syndrome. J Hand Surg Am. 1982;7:450-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Journeau P, Lajeunie E, Rénier D, Salon A, Guéro S, Pouliquen JC. Syndactyly in Apert syndrome. Utility of a prognostic classification. Ann Chir Main Memb Super. 1999;18:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Ferreira JC, Carter SM, Bernstein PS, Jabs EW, Glickstein JS, Marion RW, Baergen RN, Gross SJ. Second-trimester molecular prenatal diagnosis of sporadic Apert syndrome following suspicious ultrasound findings. Ultrasound Obstet Gynecol. 1999;14:426-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Athanasiadis AP, Zafrakas M, Polychronou P, Florentin-Arar L, Papasozomenou P, Norbury G, Bontis JN. Apert syndrome: the current role of prenatal ultrasound and genetic analysis in diagnosis and counselling. Fetal Diagn Ther. 2008;24:495-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Slaney SF, Oldridge M, Hurst JA, Moriss-Kay GM, Hall CM, Poole MD, Wilkie AO. Differential effects of FGFR2 mutations on syndactyly and cleft palate in Apert syndrome. Am J Hum Genet. 1996;58:923-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Kodaka T, Kanamori Y, Sugiyama M, Hashizume K. A case of acrocephalosyndactyly with low imperforate anus. J Pediatr Surg. 2004;39:E32-E34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 11. | Das S, Munshi A. Research advances in Apert syndrome. J Oral Biol Craniofac Res. 2018;8:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Munshi A, Khetarpal P, Das S, Rao V, Valecha M, Bansal M, Kumar R. Apert's syndrome: Study by whole exome sequencing. Genes Dis. 2018;5:119-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Toriello HV, Meck JM; Professional Practice and Guidelines Committee. Statement on guidance for genetic counseling in advanced paternal age. Genet Med. 2008;10:457-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Griffiths PD, Porteous M, Mason G, Russell S, Reeves MJ. The use of in utero MRI to supplement ultrasound in the foetus at high risk of developmental brain or spine abnormality. Brit J Radiol. 2012;85:e1038. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Hosny IA, Elghawabi HS. Ultrafast MRI of the fetus: an increasingly important tool in prenatal diagnosis of congenital anomalies. Magn Reson Imaging. 2010;28:1431-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |