Published online Feb 6, 2021. doi: 10.12998/wjcc.v9.i4.871
Peer-review started: September 20, 2020
First decision: November 25, 2020
Revised: December 10, 2020
Accepted: December 28, 2020
Article in press: December 28, 2020
Published online: February 6, 2021
Processing time: 120 Days and 1.3 Hours
Hepatocellular adenomas are rare tumors that can occur in patients with glycogen storage disease type I.
We herein report two cases of histologically proven hepatocellular adenomas in patients with glycogen storage disease type I. Magnetic resonance imaging (MRI) was performed after bolus injection of gadoxetate disodium, a liver-specific gadolinium-based MRI contrast agent. In the present cases, some of the hepatocellular adenomas showed unexpectedly a “bull’s eye” appearance on T2-weighted and post-contrast images, which was not previously described as imaging findings of hepatocellular adenomas in glycogen storage disease. A bull’s eye appearance on T2-weighted images can be encountered in both benign (i.e., abscess) or malignant (i.e., epithelioid hemangioendothelioma, cholangio-carcinoma, and metastases) hepatic lesions.
We present two cases of hepatocellular adenomas in patients with glycogen storage disease type 1, in which gadoxetate disodium-MRI showed atypical imaging findings for hepatocellular adenomas. At present there is no systematic study evaluating MRI findings of hepatocellular adenomas in patients with glycogen storage disease, further studies are needed to specifically investigate this issue.
Core Tip: Magnetic resonance imaging findings of hepatocellular adenomas in glycogen storage disease may differ from the typical ones observed in the common population.
- Citation: Vernuccio F, Austin S, Meyer M, Guy CD, Kishnani PS, Marin D. "Bull’s eye” appearance of hepatocellular adenomas in patients with glycogen storage disease type I — atypical magnetic resonance imaging findings: Two case reports. World J Clin Cases 2021; 9(4): 871-877
- URL: https://www.wjgnet.com/2307-8960/full/v9/i4/871.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i4.871
Hepatocellular adenomas (HCAs) occur in about 29% of patient with glycogen storage disease (GSD) type I (GSD I)[1]. HCAs in GSD I are usually multiple and smaller compared to the general population[2], but it is a general belief that computed tomography (CT) and magnetic resonance imaging (MRI) characteristics of individual lesions in patients with GSD are similar to those reported for sporadic or solitary HCAs[3]. Just one previous case report suggested the presence of various signal intensities during the hepatocyte-specific phase of multiple HCAs in a patient with GSD type I[4]. However, no systematic study describing MRI features on the different sequences in HCAs associated with GSD type I has been published so far and it is not clear yet whether the radiological presentation of individual HCAs in GSD is truly different from that in the general population.
At Duke University — which is a tertiary referral center for GSD — 24 patients with GSD I have been imaged with MRI for focal liver lesions. We herein present two cases of histologically proven HCAs in GSD I, in which MRI was performed after administration of gadoxetate disodium, a liver-specific paramagnetic gadolinium-based MRI contrast agent that is used for imaging of focal liver disease. To the best of our knowledge, this is the first report describing the “bull’s eye” appearance as imaging findings of HCA in GSD I.
A 26-year-old female (Case 1) and a 17-year-old male (Case 2) were referred to our hospital because of history of GSD I.
A liver MRI exam was performed on a 3-T MRI scanner in both patients (Magnetom Skyra, Siemens Healthineers, United States). The following MRI sequences were acquired in the axial plane for both patient: T2 half-Fourier acquisition single-shot turbo spin-echo [repetition time (TR)/echo time (TE) = 800/89], T2-weighted respiratory-triggered turbo spin-echo (TR/TE = 4102/81 ms), T1-weighted in-phase (TR/TE = 4.36/2.63 ms), and out-of-phase (TR/TE = 4.36/1.34 ms), diffusion weighted images (TR/TE = 6400/62 for Case 1; TR/TE = 5.945/58 for Case 2), and T1-weighted fat saturated three-dimensional spoiled gradient-echo images (TR/TE = 4.06/2.37 for Case 1; TR/TE = 4.36/1.34 ms for Case 2) acquired after intravenous injection at the recommended dose of 0.1 mL/kg body weight (gadoxetate disodium, Eovist, Bayer HealthCare Pharmaceuticals) during the arterial phase (25 s, after contrast medium injection), portal venous phase (60 s), equilibrium phase (180 s), and the hepatobiliary phase (at 20 min in Case 1 and at 30 min in Case 2 from the start of contrast medium injection). Moreover, a coronal T2- half-Fourier acquisition single-shot turbo spin-echo (TR/TE = 1000/87), and a coronal T1-weighted fat saturated three-dimensional spoiled gradient-echo images in the hepatobiliary phase were acquired.
Case 1 had a partial hepatectomy for an HCA in the left lobe 10 years prior.
Case 1: Good metabolic control on cornstarch since the age of 1 year.
Case 2: Good metabolic control on cornstarch and later protein supplement started at the age of 19 mo and gastrostomy tube at the age of 4 years before high school.
Case 1: Body mass index equal to 29 kg/m2.
Case 2: Body mass index equal to 20 kg/m2.
Case 1: Alpha Fetoprotein and carcinoembrionic were 2.9 ng/mL and 0.7 ng/mL, respectively.
Case 2: Alpha fetoprotein was 0.8 ng/mL.
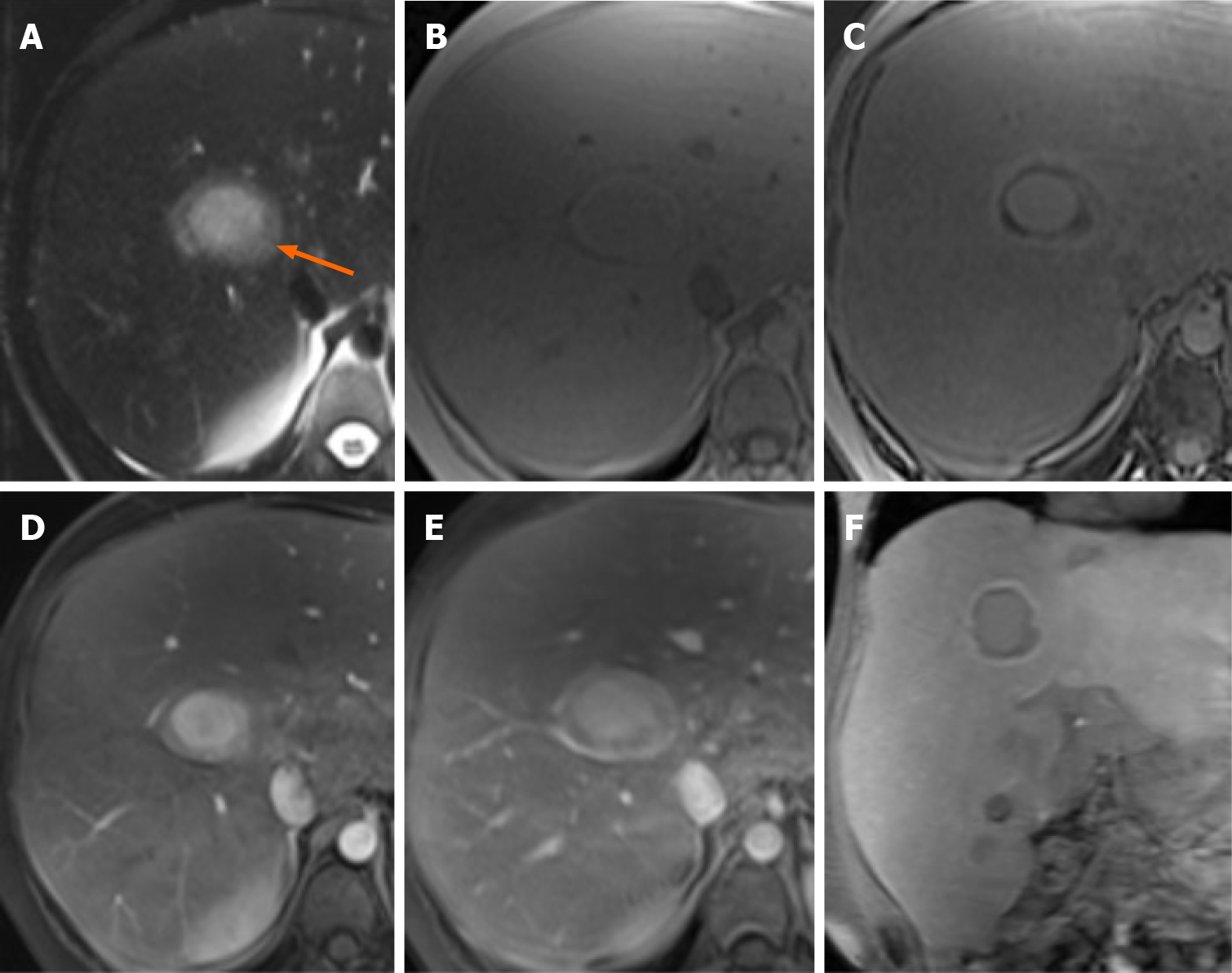
Case 1: MRI images showed an enlarged fatty liver with multiple focal lesions (> 10). On the T2-weighted images, the largest lesion showed a two-layered “bull’s eye” appearance with a central markedly hyperintense core surrounded by a peripheral slightly hyperintense halo (Figure 1A). This “bull’s eye” appearance corresponded to a peripheral marked signal drop in the out-of-phase compared to the in-phase images (Figure 1B and C), suggesting a fatty component in the periphery of the lesion. On contrast-enhanced MRI images, the core vividly enhanced in the arterial phase, while the periphery enhanced to a lesser degree relative to the core (Figure 1D). On the portal venous phase, the core showed persisting enhancement, while the periphery became isointense compared to the liver parenchyma (Figure 1E). In the hepatobiliary phase images, the core of the lesion was still recognizable as isointense to the liver parenchyma, the periphery showed a marked hypointensity, with a peripheral slightly hyperintense rim (Figure 1F). Other two smaller lesions showed similar appearance in the same patient. Patient was transplanted 2 years later: pathology confirmed the diagnosis of HCAs.
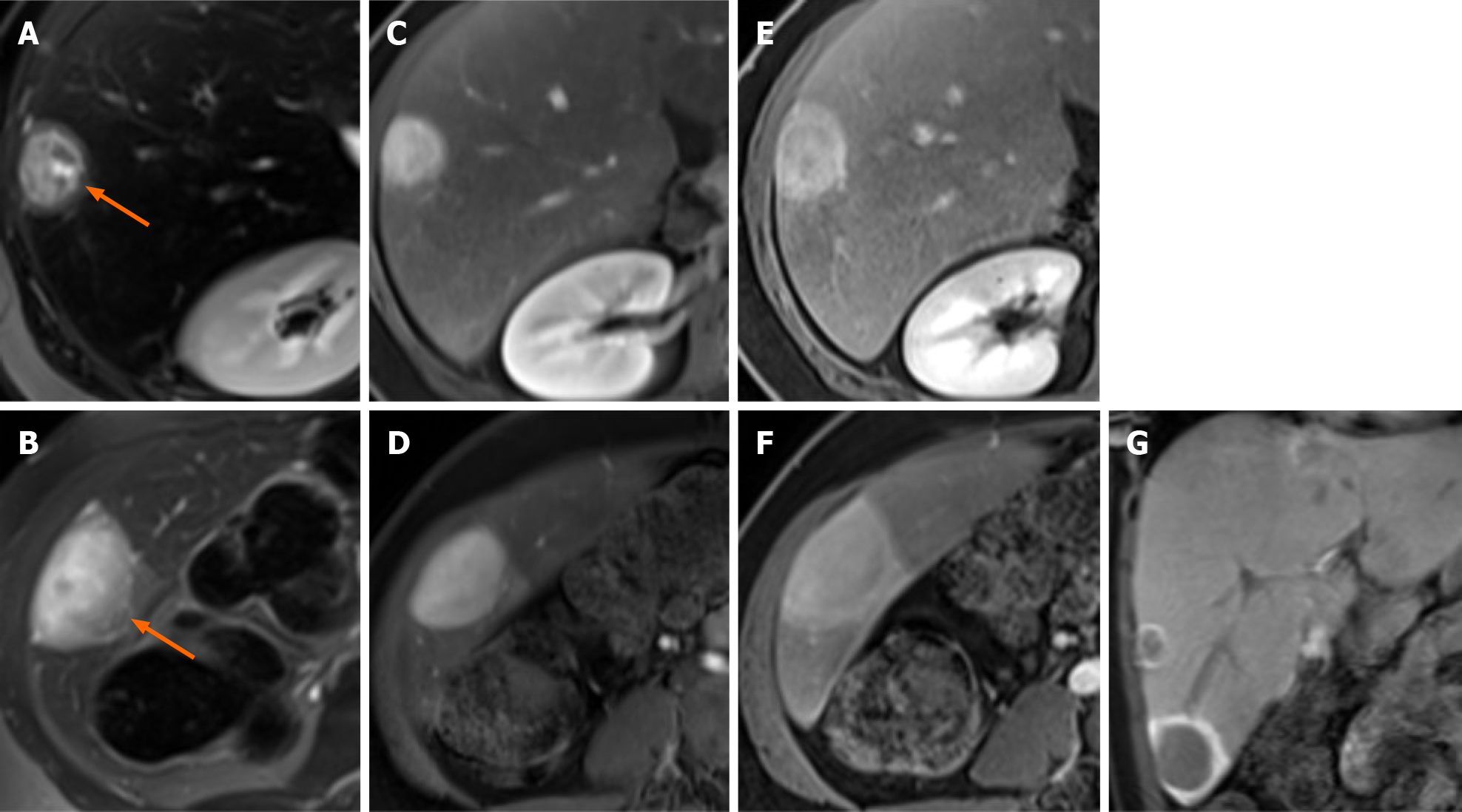
Case 2: MRI images showed an enlarged steatotic liver with multiple focal lesions (> 10) consistent with HCAs. On the T2-weighted images, the two largest lesions showed a two-layered “bull’s eye” appearance, similar to the one described in patient one, but with a thinner peripheral halo (Figure 2A and B). On dual phase images, no fat-content within the lesions was detected. On contrast-enhanced MRI images, the core of the lesions enhanced in the arterial phase, and showed contrast retention in the portal venous phase; moreover, the peripheral slightly T2-hyperintense halo showed enhancement in the portal venous phase (Figure 2D and E). During the hepatobiliary phase images, the lesions were markedly hypointense compared to the adjacent liver parenchyma, with a peripheral markedly hyperintense rim (Figure 2F), which is typical in HCAs. The patient underwent surgical resection: Histology confirmed the diagnosis of HCAs and the peripheral zone was diagnosed as a thin, less than 1 cm, capsule without any dilated bile ducts. No other lesions showed the same appearance in this patient (Figure 2).
Synthesizing MRI findings and pathology (data not shown), we diagnosed both GSD I patients with HCAs.
Case 1 was transplanted, while Case 2 underwent lobectomy.
Case 1 is well at last 6-year-follow-up. Case 2 was followed-up elsewhere and the last clinical follow-up at our center was performed at 2 wk after surgery.
HCAs in GSD type I have a different epidemiologic presentation as well as a different outcome, compared to the general population[1,2]. Indeed, the M:F ratio of HCAs in GSD type I is 1:1[2], the only reported subtypes are inflammatory or beta catenin activated HCAs and adenomatosis (i.e., presence of more than 10 hepatic adenomas) is the most frequently documented presentation[5]. Despite usually considered to have benign prognosis, progressive disease and complications of HCAs (i.e., hemorrhage and malignant transformation) have been reported[6-9]. In a series of 32 GSD patients with HCAs, evolution of HCAs into hepatocellular carcinoma has been reported in about 12.5% of cases in a median interval time from the diagnosis of HCA to the diagnosis of hepatocellular carcinoma of 6.7 years[10]. In this series recently published by Jang et al[10], the radiological changes of HCAs in the 4 patients that developed hepatocellular carcinoma included an increase either in the number or size of HCA in two patients and an increase in both size and number in the remaining two patients. The underlying etiology of HCAs in GSD is unknown; the most agreed hypothesis is that a chronic hepatic inflammation and underlying genetic may predispose these patients to develop HCAs[11,12].
Although CT generally provides some imaging clues, such as the hypervascularity in the arterial phase and contrast retention in the portal venous phase, MRI currently represents the most sensitive (sensitivity: 77%-91%) and specific (specificity: 88%-100%) imaging modality for the diagnosis and follow-up of HCAs[7,13-15]. It also has the advantage of no radiation. MRI findings of HCAs correlate with pathology[13,14]. Specifically, the high intensity on T2-weighted sequence, as well as the enhancement in the arterial phase and its persistence in the portal venous phase correlate with the heterogeneous sinusoidal dilatation.
We present two cases of HCAs in patients with GSD type I, in which gadoxetate disodium-MRI showed atypical imaging findings for HCAs. In both patients, the HCAs showed a “bull’s eye” appearance on T2-weighted images with a markedly hyperintense core surrounded by a peripheral slightly hyperintense halo. This appearance on T2-weighted images was evident, but not described, in the only MRI case report of HCAs in GSD, and at pathology, it probably corresponded to the incomplete capsule surrounding the lesions[4]. This “bull’s eye” sign differs from the “atoll sign”, characteristic of inflammatory HCAs and seen in about one third of HCAs in the general population[13]. The “atoll sign” is characterized by a peripheral rim of high signal intensity with the center of the lesion appearing isointense to the background liver on T2 mimicking an atoll.
In Case 1, the “bull’s eye” appearance on T2-weighted images was related to a thick rim of intralesional fat on dual phase sequences. Such appearance on dual-phase images has been described in insulinomas[16] and has never been described before for HCAs. In Case 2, the “bull’s eye” appearance on T2-weighted images was related at histology with a thin peripheral capsule. In this patient, both the lesions were peripheral and the bull’s eye appearance on T2-weighted images could lead to misdiagnosis with both benign (i.e., abscess) or malignant (i.e., epithelioid hemangioendothelioma, cholangiocarcinoma, and metastases) hepatic lesions[17-20]. However, in our cases the other MRI sequences, patient history and pathology were consistent with the imaging diagnosis of HCAs.
Due to paucity of reports availability, it is not clear whether the radiological presentation of HCAs in GSD I is different from that in the general population and if the evolution of HCAs is similar to that of the general population[7]. On the basis of our observation, we can speculate that MRI findings of HCAs in GSD may differ from the typical ones observed in the general population. Further studies are needed to confirm this hypothesis, investigate the possible underlying pathological mechanisms and assess whether the different radiological features may predict evolution into hepatocellular carcinoma; therefore, we plan to further analyze our GSD I cohort with this purpose.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Battelino T S-Editor: Gao CC L-Editor: A P-Editor: Liu JH
| 1. | Wang DQ, Fiske LM, Carreras CT, Weinstein DA. Natural history of hepatocellular adenoma formation in glycogen storage disease type I. J Pediatr. 2011;159:442-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Reddy SK, Austin SL, Spencer-Manzon M, Koeberl DD, Clary BM, Desai DM, Smith AD, Kishnani PS. Liver transplantation for glycogen storage disease type Ia. J Hepatol. 2009;51:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Grazioli L, Federle MP, Brancatelli G, Ichikawa T, Olivetti L, Blachar A. Hepatic adenomas: imaging and pathologic findings. Radiographics. 2001;21:877-92; discussion 892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 193] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Sakamoto A, Hayashi H, Sakamoto I, Isomoto I, Eguchi S, Takatsuki M, Kanematsu T, Abe K, Hayashi T, Uetani M. Multiple hepatocellular adenomas in a patient with glycogen storage disease type I: various enhancement patterns in MRI with Gd-EOB-DTPA. Abdom Imaging. 2012;37:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Nault JC, Couchy G, Balabaud C, Morcrette G, Caruso S, Blanc JF, Bacq Y, Calderaro J, Paradis V, Ramos J, Scoazec JY, Gnemmi V, Sturm N, Guettier C, Fabre M, Savier E, Chiche L, Labrune P, Selves J, Wendum D, Pilati C, Laurent A, De Muret A, Le Bail B, Rebouissou S, Imbeaud S; GENTHEP Investigators; Bioulac-Sage P; Letouzé E; Zucman-Rossi J. Molecular Classification of Hepatocellular Adenoma Associates With Risk Factors, Bleeding, and Malignant Transformation. Gastroenterology 2017; 152: 880-894. e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 269] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 6. | Reddy SK, Kishnani PS, Sullivan JA, Koeberl DD, Desai DM, Skinner MA, Rice HE, Clary BM. Resection of hepatocellular adenoma in patients with glycogen storage disease type Ia. J Hepatol. 2007;47:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Vernuccio F, Ronot M, Dioguardi Burgio M, Cauchy F, Choudhury KR, Dokmak S, Soubrane O, Valla D, Zucman-Rossi J, Paradis V, Vilgrain V. Long-term Evolution of Hepatocellular Adenomas at MRI Follow-up. Radiology. 2020;295:361-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Franco LM, Krishnamurthy V, Bali D, Weinstein DA, Arn P, Clary B, Boney A, Sullivan J, Frush DP, Chen YT, Kishnani PS. Hepatocellular carcinoma in glycogen storage disease type Ia: a case series. J Inherit Metab Dis. 2005;28:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 9. | Kishnani PS, Austin SL, Abdenur JE, Arn P, Bali DS, Boney A, Chung WK, Dagli AI, Dale D, Koeberl D, Somers MJ, Wechsler SB, Weinstein DA, Wolfsdorf JI, Watson MS; American College of Medical Genetics and Genomics. Diagnosis and management of glycogen storage disease type I: a practice guideline of the American College of Medical Genetics and Genomics. Genet Med. 2014;16:e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 298] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 10. | Jang HJ, Yang HR, Ko JS, Moon JS, Chang JY, Seo JK. Development of Hepatocellular Carcinoma in Patients with Glycogen Storage Disease: a Single Center Retrospective Study. J Korean Med Sci. 2020;35:e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Kim SY, Weinstein DA, Starost MF, Mansfield BC, Chou JY. Necrotic foci, elevated chemokines and infiltrating neutrophils in the liver of glycogen storage disease type Ia. J Hepatol. 2008;48:479-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Kishnani PS, Chuang TP, Bali D, Koeberl D, Austin S, Weinstein DA, Murphy E, Chen YT, Boyette K, Liu CH, Chen YT, Li LH. Chromosomal and genetic alterations in human hepatocellular adenomas associated with type Ia glycogen storage disease. Hum Mol Genet. 2009;18:4781-4790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Ronot M, Bahrami S, Calderaro J, Valla DC, Bedossa P, Belghiti J, Vilgrain V, Paradis V. Hepatocellular adenomas: accuracy of magnetic resonance imaging and liver biopsy in subtype classification. Hepatology. 2011;53:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 14. | van Aalten SM, Thomeer MG, Terkivatan T, Dwarkasing RS, Verheij J, de Man RA, Ijzermans JN. Hepatocellular adenomas: correlation of MR imaging findings with pathologic subtype classification. Radiology. 2011;261:172-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Ba-Ssalamah A, Antunes C, Feier D, Bastati N, Hodge JC, Stift J, Cipriano MA, Wrba F, Trauner M, Herold CJ, Caseiro-Alves F. Morphologic and Molecular Features of Hepatocellular Adenoma with Gadoxetic Acid-enhanced MR Imaging. Radiology. 2015;277:104-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 16. | Dioguardi Burgio M, Bruno O, Agnello F, Torrisi C, Vernuccio F, Cabibbo G, Soresi M, Petta S, Calamia M, Papia G, Gambino A, Ricceri V, Midiri M, Lagalla R, Brancatelli G. The cheating liver: imaging of focal steatosis and fatty sparing. Expert Rev Gastroenterol Hepatol. 2016;10:671-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 17. | Paolantonio P, Laghi A, Vanzulli A, Grazioli L, Morana G, Ragozzino A, Colagrande S. MRI of hepatic epithelioid hemangioendothelioma (HEH). J Magn Reson Imaging. 2014;40:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Bächler P, Baladron MJ, Menias C, Beddings I, Loch R, Zalaquett E, Vargas M, Connolly S, Bhalla S, Huete Á. Multimodality Imaging of Liver Infections: Differential Diagnosis and Potential Pitfalls. Radiographics. 2016;36:1001-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Park HJ, Kim SH, Jang KM, Lee SJ, Park MJ, Choi D. Differentiating hepatic abscess from malignant mimickers: value of diffusion-weighted imaging with an emphasis on the periphery of the lesion. J Magn Reson Imaging. 2013;38:1333-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Karaosmanoglu AD, Onur MR, Ozmen MN, Akata D, Karcaaltincaba M. Magnetic Resonance Imaging of Liver Metastasis. Semin Ultrasound CT MR. 2016;37:533-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |