Published online Feb 6, 2021. doi: 10.12998/wjcc.v9.i4.864
Peer-review started: August 14, 2020
First decision: November 26, 2020
Revised: November 29, 2020
Accepted: December 10, 2020
Article in press: December 10, 2020
Published online: February 6, 2021
Processing time: 160 Days and 20.1 Hours
Treatment for neck lymph node metastases after adequate initial surgery in medullary thyroid carcinoma (MTC) has been controversial. Ultrasound (US)-guided radiofrequency ablation (RFA) has been widely used in recurrent well-differentiated thyroid carcinoma. Here, we report for the first time the use of RFA in a patient with recurrent MTC.
We report the case of a 56-year-old woman with cervical lymph node metastases of MTC. Four years previously, she had undergone a total thyroidectomy and neck lymph node dissection. A neck US revealed many enlarged nodes during the follow-up period. Moreover, the serum calcitonin jumped to 198.17 pg/mL, which strongly indicated the recurrence of MTC. Subsequently, two metastatic lymph nodes were confirmed by US-guided fine-needle aspiration-cytology and fine-needle aspiration-calcitonin, and then the patient was treated with RFA. Four months later, the neck US and a contrast-enhanced US showed obvious shrinkage in the ablation zones, and the serum calcitonin dropped to 11.80 pg/mL.
This case suggests that RFA may be an effective and safe treatment for local recurrent MTC.
Core Tip: Adequate surgery is the mainstay of treatment in medullary thyroid carcinoma. However, neck lymph node metastases after adequate initial treatment are present in a majority of cases. So far, treatment for neck lymph node metastases after adequate initial surgery in medullary thyroid carcinoma has been controversial. Radiofrequency ablation, which is considered a moderate approach between secondary surgery and surveillance, may be attempted for these recurrent patients. This case suggests that radiofrequency ablation may be an effective and safe treatment for local recurrent medullary thyroid carcinoma.
- Citation: Tong MY, Li HS, Che Y. Recurrent medullary thyroid carcinoma treated with percutaneous ultrasound-guided radiofrequency ablation: A case report. World J Clin Cases 2021; 9(4): 864-870
- URL: https://www.wjgnet.com/2307-8960/full/v9/i4/864.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i4.864
The prevalence of medullary thyroid carcinoma (MTC) accounts for approximately 3% to 5% of all thyroid malignancies[1] in sporadic (85% of cases) or hereditary (15% of cases) form[2]. MTC is more aggressive and progressive than well-differentiated thyroid carcinoma and is characterized by early lymph nodal metastases[1,3,4]. Adequate surgery is the mainstay of treatment in MTC. However, neck lymph node metastases after adequate initial treatment are present in the majority of cases[5,6]. In these cases, a second surgical treatment can be considered, but its real therapeutic role is controversial because of the absence of randomized trials comparing reoperation to observation alone. In patients without infiltration of vital neck structures or symptomatic lesions, reoperation may lead to overtreatment and consequential complications[7,8]. Active surveillance management, as an alternative, is not acceptable for many anxious patients. Therefore, radiofrequency ablation (RFA), which is considered a moderate approach between surgery and surveillance, may be attempted for these recurrent patients.
Ultrasound (US)-guided tumor ablation has become a mature treatment for recurrent differentiated thyroid carcinoma[9]. However, to the best of our knowledge, no case has been published that elucidates the efficacy and safety of RFA in local recurrent MTC. We herein describe the outcome of recurrent MTC with neck lymph node metastases treated by RFA in a patient who refused to undergo a second surgery.
A 56-year-old female presented to the department of ultrasound with recurrent MTC.
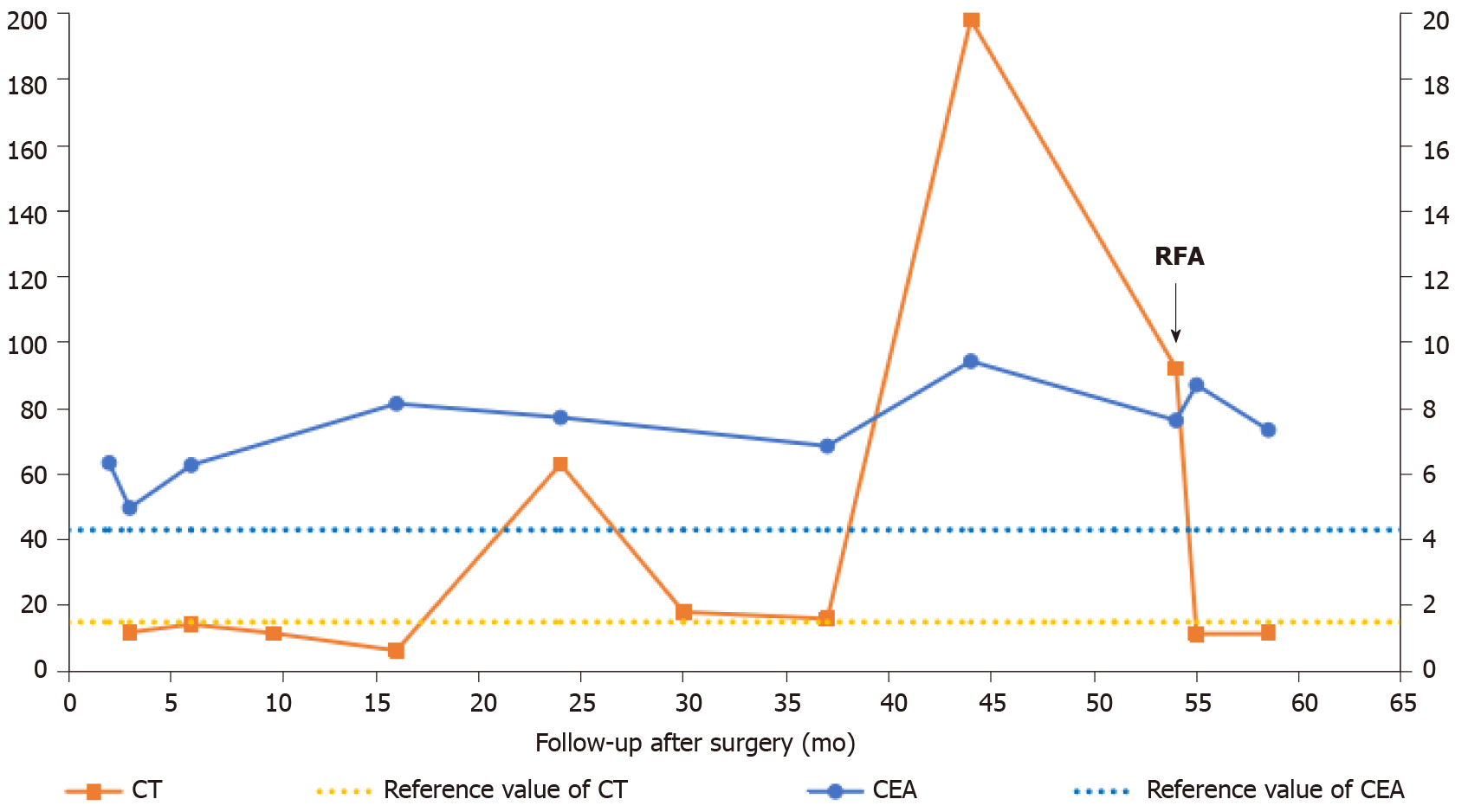
The patient was diagnosed with sporadic MTC at the age of 51. She presented a hypoechoic solid nodule that was approximately 0.5 cm × 0.4 cm in the left thyroid gland with no pain, diarrhea, flushing or any other specific symptom. The preoperative serum calcitonin was 1165.97 pg/mL. The patient was then treated with total thyroidectomy with central neck dissection and modified left lateral neck dissection. The nodule was pathologically confirmed to be MTC. Nine of fourteen metastatic lymph nodes were found in the excised lymphoid adipose tissue at the left cervical level II-IV, VB and VI (Table 1). Three months after surgery, the serum calcitonin dropped to 11.86 pg/mL (Figure 1).
| Level | Number of metastasis | Total number |
| Left II | 1 | 3 |
| Left III | 1 | 1 |
| Left IV | 1 | 3 |
| Left VB | 0 | 0 |
| Left VI | 6 | 7 |
| Total | 9 | 14 |
After the initial treatment, the patient was followed up with neck US, serum calcitonin and carcinoembryonic antigen every 3-6 mo. During the follow-up, an enlarged lymph node (0.4 cm × 0.3 cm) with no visible hilum at left level VI was detected by the neck US in the 9th mo. A similar lymph node (0.5 cm × 0.4 cm) appeared in the left supraclavicular fossa in the 44th mo. In addition, many lymph nodes at the right cervical level II-III and left level V became enlarged. Serum calcitonin jumped to 198.17 pg/mL in the 44th mo. Afterwards, serum calcitonin remained at a high level, which was significantly higher than the reference (Figure 1). The serum carcinoembryonic antigen level had been relatively stable since the initial surgery. The abdominal and lung computed tomography scan revealed no suspicious metastasis.
She presented with no lump or tenderness in the neck. Trachea was in the middle of the neck.
After the recurrent signs occurred, the patient visited our department for further diagnosis and treatment in the 54th mo. Serum calcitonin was increased at 92.18 pg/mL (normal range: < 15 pg/mL).
We performed the neck US on the patient, finding six morphologically suspicious lymph nodes. To identify the lymph nodes that harbored metastatic lesions, we conducted US-guided fine-needle aspiration (FNA) on these six nodules. The results of FNA-cytology and FNA-calcitonin are displayed in Table 2. Four of the nodes turned out to be reactive hyperplasia nodes, whereas the other two revealed suspicious malignancy. Moreover, the FNA-calcitonin values of these two suspicious lymph nodes were significantly higher (2000 pg/mL) than the others indicating metastatic lesions.
| Location/level (size) | Cytological pathology | FNA-calcitonin, pg/mL |
| Right/II | Reactive hyperplasia | < 2.00 |
| Right/III | Reactive hyperplasia | < 2.00 |
| Left/V (5.0 mm × 3.0 mm) | Reactive hyperplasia | < 2.00 |
| Left/V (7.9 mm × 4.1 mm) | Reactive hyperplasia | < 2.00 |
| Left/VI1 | No tumor cell found | > 2000 |
| Left/supraclavicular fossa1 | A few epithelioid cells | > 2000 |
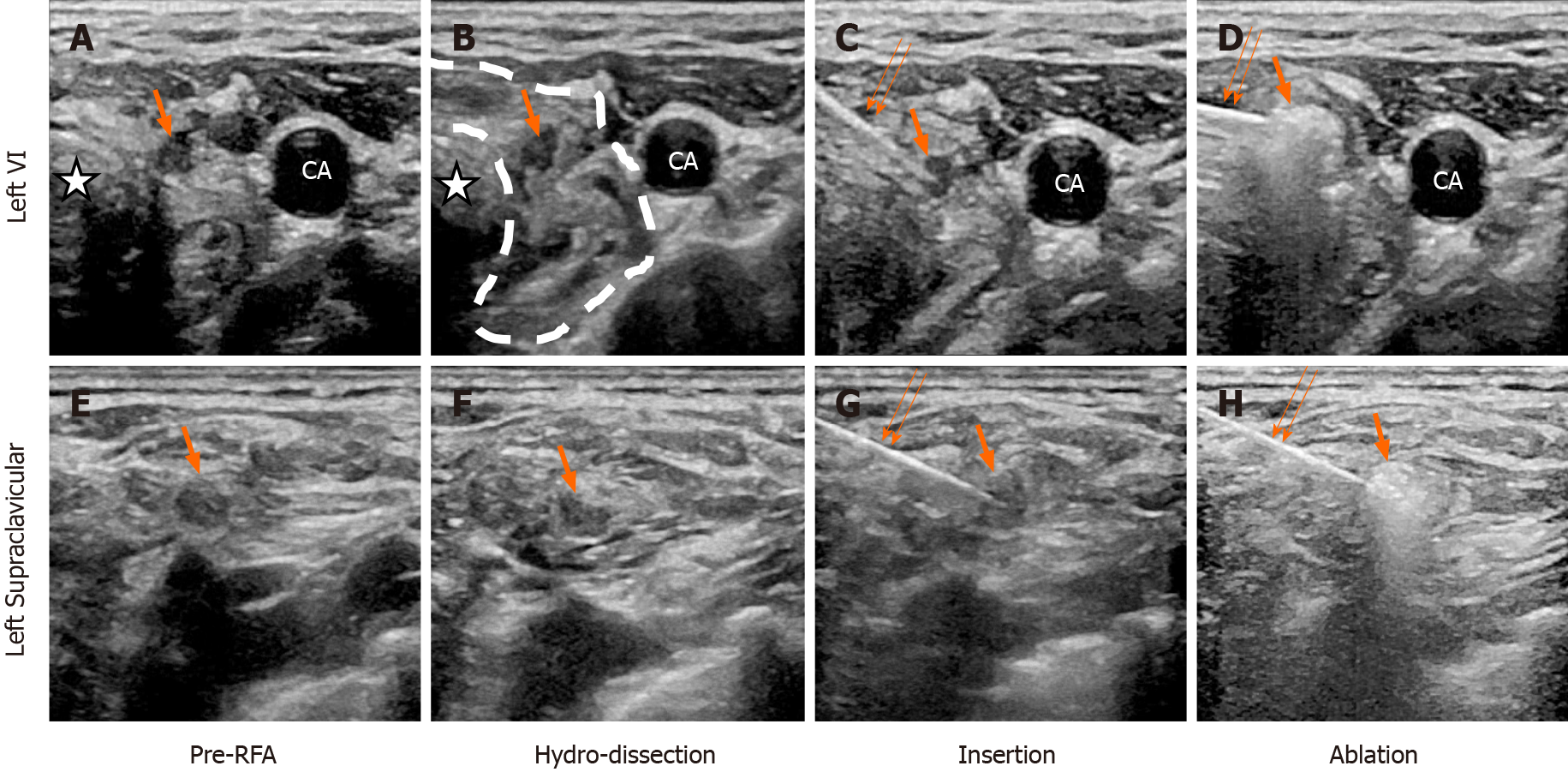
Postoperative recurrent MTC with metastatic lymph nodes of left VI and left supraclavicular fossa.
After identification of the metastatic lymph nodes (Figure 2A and 2E) by US-guided FNA, we performed RFA on them. The RFA and postoperative contrast-enhanced ultrasound (CEUS) was performed using the LOGIQ E9 Ultrasound System (GE Healthcare, Chicago, IL, United States) with a 9L linear array transducer (6-15 MHz). The ultrasound was conducted using a unipolar RF generator (VIVA RF generator, VRS01; STAR Med Co., Ltd., Korea) and an 18-gauge internally cooled electrode with a 7-mm active tip (VIVA RF Electrode; STAR Med Co., Ltd.). The procedure was performed by a US interventional physician with 15 years of experience in RFA treatment. Briefly, 1% lidocaine was injected for local anesthesia on the puncture point. To prevent thermal injury, saline was injected to isolate the target ablation zone with surrounding tissue, including the trachea, recurrent laryngeal nerves, and supraclavicular nerves (Figure 2B and 2F). Then, the electrode tip was inserted into the lesions (Figure 2C and 2G), fixed at their centers and kept motionless during the ablation procedure. After a short period, transient hyperechoic zones consisting of vapor were observed at the electrode tip (Figure 2D and 2H). During the procedure, the RF generator monitored the temperature at the electrode tip. The power was automatically reduced once the temperature reached 60 ºC.
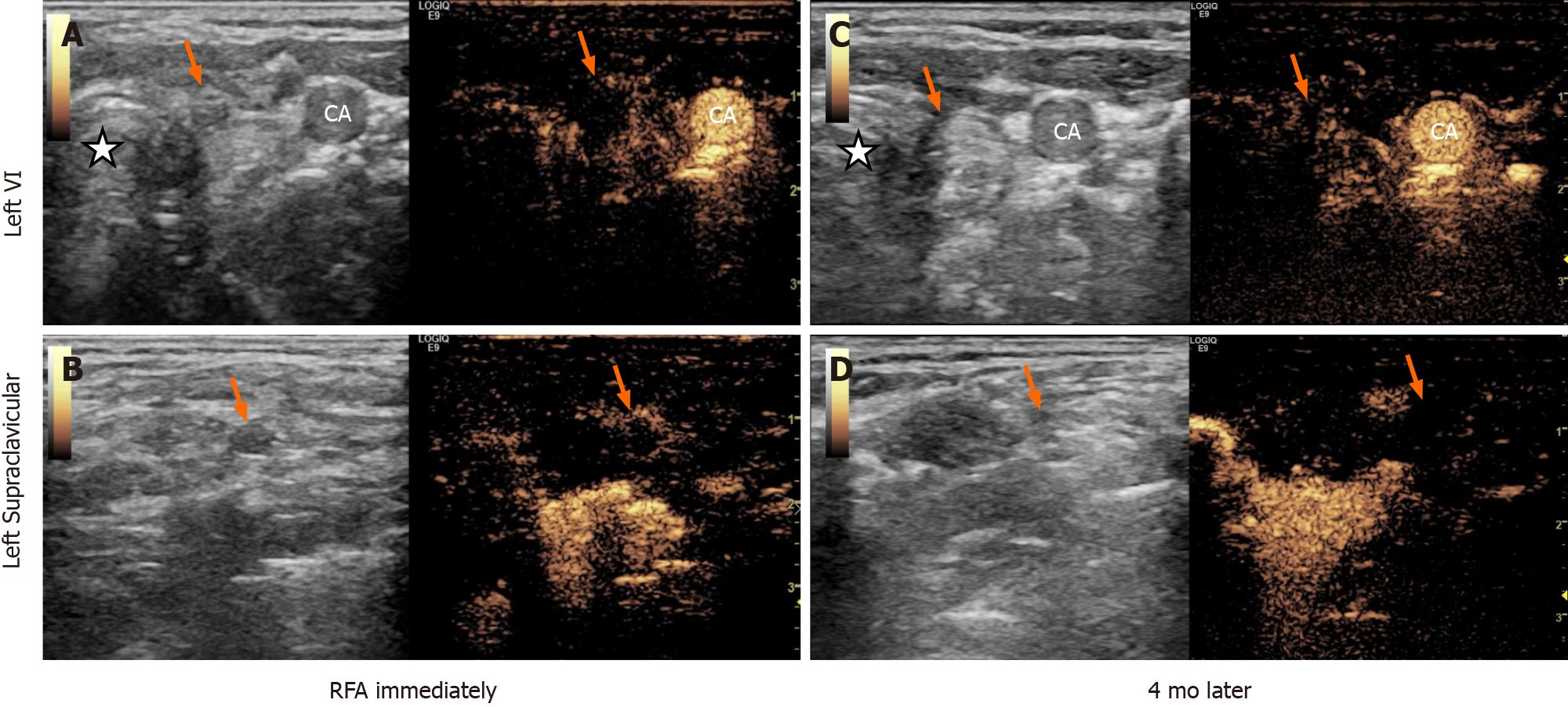
Following the RFA procedure, we immediately performed US and CEUS on the patient. The US revealed two hyperechoic ablated zones that covered the metastatic lesions completely, and CEUS confirmed no enhancement in them (Figure 3A and 3B). No major complications such as intolerable pain, hematoma or nerve injury occurred during or after the treatment. One month after RFA, the patient’s serum calcitonin level dropped to 11.40 pg/mL. Four months later, the neck US and CEUS showed obvious shrinkage in the ablation zones (Figure 3C and 3D), and the serum calcitonin remained at 11.80 pg/mL.
In this case, we described for the first time the effective use of RFA in the treatment of recurrent MTC with cervical lymph node metastases. The action of RFA, performed with hydrodissection and spot-ablation techniques, performs the main role in coagulating the nodes. After absorption of necrotic tissue, significant shrinkage of metastatic lymph nodes coupled with marked clinical improvement in metastasis-related symptoms was observed. Remarkably, no nerve injuries or inflammatory changes induced by RFA were encountered, which is a critical advantage as compared to a secondary surgery.
RFA has been recently used to treat patients with metastatic lymph nodes from recurrent papillary thyroid carcinoma[10,11] and primary papillary thyroid microcarcinoma without cervical lymph node metastasis[12-15]. Complete disappearance of the whole lesion was reported in the majority of cases during the follow-up periods. In terms of safety, few major complications have been encountered, demonstrating the safety of RFA[11]. Therefore, RFA is an acceptable treatment to manage both primary papillary thyroid microcarcinoma and metastatic lymph nodes from papillary thyroid carcinoma in terms of efficacy and safety for nonsurgical candidates.
RFA for recurrent MTCs has not been reported in the literature. However, for the present case, in which the patient had undergone an initial complete surgery and then postoperative lymph nodes metastasized, we decided to perform RFA on the patient for the following reasons. First, because the recurrent MTC did not appear to infiltrate vital neck structures or symptomatic lesions, reoperation may have led to overtreatment and produced negative complications[7,8]. Second, adhesions or inflammatory changes induced by the initial surgery may produce difficulties for the secondary surgery. Third, the patient was very anxious, and active surveillance management was deemed unacceptable. Fourth, the patient refused to undergo a second surgery.
To perform RFA, a critical issue is to accurately identify the metastatic lymph nodes. Calcitonin measurement in FNA washout has been suggested as a useful supplement to conventional FNA-cytology in patients with metastatic MTC or increased serum calcitonin[16-19]. The cut-off level for FNA-calcitonin has been reported to range from 10.4 to 67 pg/mL[19-22]. Specifically, Kihara et al[21] reported that when the cut-off value was 21.0 pg/mL, FNA-calcitonin had the highest sensitivity and specificity (nearly 100%), noting the contrast with approximately 45%-63% sensitivity of routine FNA-cytology[23,24]. In this case, FNA-calcitonin of the lymph nodes at left level VI and left supraclavicular fossa were significantly higher (> 2000 pg/mL) than the other lymph nodes, despite the negative FNA-cytology result, indicating that these two lymph nodes were responsible for the persistent increase in serum calcitonin.
After identifying the metastatic lymph nodes, we conducted RFA on these two lesions. In our patient, after two insertions of an RF electrode using a spot ablation technique, the lesions were completely ablated as indicated by the postoperative CEUS and follow-up sonography evaluations. These imaging changes in the lesions were accompanied by a clinically significant decline in serum calcitonin values. Overall, this case provides evidence to support the mainstay role of RFA in the treatment of local recurrent MTC. As a local minimally invasive therapy, RFA is an acceptable treatment for managing metastatic lymph nodes in MTC in terms of efficacy and safety, which is necessary in prolonging disease-free survival time and delaying systemic therapy. Moreover, a greater number of patients and a long follow-up period should be employed to elucidate the long-term efficacy of RFA.
To the best of our knowledge, this is the first case study of RFA in the treatment of local recurrent MTC. The effective and safe response suggests the possibility that RFA may be a novel option to control local recurrent MTC, warranting clinical verifications with larger sample sizes.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hansen AW S-Editor: Fan JR L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Viola D, Elisei R. Management of Medullary Thyroid Cancer. Endocrinol Metab Clin North Am. 2019;48:285-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Wells SA Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, Raue F, Frank-Raue K, Robinson B, Rosenthal MS, Santoro M, Schlumberger M, Shah M, Waguespack SG; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25:567-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 1470] [Article Influence: 147.0] [Reference Citation Analysis (0)] |
| 3. | Elisei R, Pinchera A. Advances in the follow-up of differentiated or medullary thyroid cancer. Nat Rev Endocrinol. 2012;8:466-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Meng K, Luo H, Chen H, Guo H, Xia W. Prognostic value of numbers of metastatic lymph node in medullary thyroid carcinoma: A population-based study using the SEER 18 database. Medicine (Baltimore). 2019;98:e13884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Fialkowski E, DeBenedetti M, Moley J. Long-term outcome of reoperations for medullary thyroid carcinoma. World J Surg. 2008;32:754-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Tisell LE, Hansson G, Jansson S, Salander H. Reoperation in the treatment of asymptomatic metastasizing medullary thyroid carcinoma. Surgery. 1986;99:60-66. [PubMed] |
| 7. | Miccoli P, Minuto MN, Ugolini C, Molinaro E, Basolo F, Berti P, Pinchera A, Elisei R. Clinically unpredictable prognostic factors in the outcome of medullary thyroid cancer. Endocr Relat Cancer. 2007;14:1099-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Scollo C, Baudin E, Travagli JP, Caillou B, Bellon N, Leboulleux S, Schlumberger M. Rationale for central and bilateral lymph node dissection in sporadic and hereditary medullary thyroid cancer. J Clin Endocrinol Metab. 2003;88:2070-2075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 225] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 9. | Mauri G, Cova L, Ierace T, Baroli A, Di Mauro E, Pacella CM, Goldberg SN, Solbiati L. Treatment of Metastatic Lymph Nodes in the Neck from Papillary Thyroid Carcinoma with Percutaneous Laser Ablation. Cardiovasc Intervent Radiol. 2016;39:1023-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Baek JH, Kim YS, Sung JY, Choi H, Lee JH. Locoregional control of metastatic well-differentiated thyroid cancer by ultrasound-guided radiofrequency ablation. AJR Am J Roentgenol. 2011;197:W331-W336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Chung SR, Suh CH, Baek JH, Park HS, Choi YJ, Lee JH. Safety of radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: a systematic review and meta-analysis. Int J Hyperthermia. 2017;33:920-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Zhang Y, Zhang MB, Luo YK, Li J, Zhang Y, Tang J. Effect of chronic lymphocytic thyroiditis on the efficacy and safety of ultrasound-guided radiofrequency ablation for papillary thyroid microcarcinoma. Cancer Med. 2019;8:5450-5458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Kim JH, Baek JH, Sung JY, Min HS, Kim KW, Hah JH, Park DJ, Kim KH, Cho BY, Na DG. Radiofrequency ablation of low-risk small papillary thyroidcarcinoma: preliminary results for patients ineligible for surgery. Int J Hyperthermia. 2017;33:212-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Jeong SY, Baek JH, Choi YJ, Chung SR, Sung TY, Kim WG, Kim TY, Lee JH. Radiofrequency ablation of primary thyroid carcinoma: efficacy according to the types of thyroid carcinoma. Int J Hyperthermia. 2018;34:611-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Zhang M, Luo Y, Zhang Y, Tang J. Efficacy and Safety of Ultrasound-Guided Radiofrequency Ablation for Treating Low-Risk Papillary Thyroid Microcarcinoma: A Prospective Study. Thyroid. 2016;26:1581-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 16. | Boi F, Maurelli I, Pinna G, Atzeni F, Piga M, Lai ML, Mariotti S. Calcitonin measurement in wash-out fluid from fine needle aspiration of neck masses in patients with primary and metastatic medullary thyroid carcinoma. J Clin Endocrinol Metab. 2007;92:2115-2118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Kudo T, Miyauchi A, Ito Y, Takamura Y, Amino N, Hirokawa M. Diagnosis of medullary thyroid carcinoma by calcitonin measurement in fine-needle aspiration biopsy specimens. Thyroid. 2007;17:635-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Abraham D, Gault PM, Hunt J, Bentz J. Calcitonin estimation in neck lymph node fine-needle aspirate fluid prevents misinterpretation of cytology in patients with metastatic medullary thyroid cancer. Thyroid. 2009;19:1015-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Trimboli P, Cremonini N, Ceriani L, Saggiorato E, Guidobaldi L, Romanelli F, Ventura C, Laurenti O, Messuti I, Solaroli E, Madaio R, Bongiovanni M, Orlandi F, Crescenzi A, Valabrega S, Giovanella L. Calcitonin measurement in aspiration needle washout fluids has higher sensitivity than cytology in detecting medullary thyroid cancer: a retrospective multicentre study. Clin Endocrinol (Oxf). 2014;80:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | de Crea C, Raffaelli M, Maccora D, Carrozza C, Canu G, Fadda G, Bellantone R, Lombardi CP. Calcitonin measurement in fine-needle aspirate washouts vs. cytologic examination for diagnosis of primary or metastatic medullary thyroid carcinoma. Acta Otorhinolaryngol Ital. 2014;34:399-405. [PubMed] |
| 21. | Kihara M, Hirokawa M, Kudo T, Hayashi T, Yamamoto M, Masuoka H, Higashiyama T, Fukushima M, Ito Y, Miya A, Miyauchi A. Calcitonin measurement in fine-needle aspirate washout fluid by electrochemiluminescence immunoassay for thyroid tumors. Thyroid Res. 2018;11:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Trimboli P, Crescenzi A, Saggiorato E, Treglia G, Giovanella L. Novel acquisitions in the diagnosis of medullary thyroid carcinoma. Minerva Endocrinol. 2017;42:238-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Elisei R, Bottici V, Luchetti F, Di Coscio G, Romei C, Grasso L, Miccoli P, Iacconi P, Basolo F, Pinchera A, Pacini F. Impact of routine measurement of serum calcitonin on the diagnosis and outcome of medullary thyroid cancer: experience in 10,864 patients with nodular thyroid disorders. J Clin Endocrinol Metab. 2004;89:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 276] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 24. | Bugalho MJ, Santos JR, Sobrinho L. Preoperative diagnosis of medullary thyroid carcinoma: fine needle aspiration cytology as compared with serum calcitonin measurement. J Surg Oncol. 2005;91:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |