Published online Feb 6, 2021. doi: 10.12998/wjcc.v9.i4.822
Peer-review started: October 9, 2020
First decision: November 29, 2020
Revised: December 4, 2020
Accepted: December 11, 2020
Article in press: December 11, 2020
Published online: February 6, 2021
Processing time: 108 Days and 2.5 Hours
Congenital cystic adenomatoid malformation (CCAM) and bronchopulmonary sequestration (BPS) are the most common lung diseases in fetuses. There are differences in the prognosis and treatment of CCAM and BPS, and the clinical diagnosis and treatment plan is usually prepared prior to birth. Therefore, it is quite necessary to make a clear diagnosis before delivery. CCAM and BPS have similar imaging features, and the differentiation mainly relies on the difference in supply vessels. However, it is hard to distinguish them due to invisible supplying vessels on some images.
To explore the application value of magnetic resonance imaging (MRI) in the differential diagnosis of fetal CCAM and BPS.
Data analysis for 32 fetuses with CCAM and 14 with BPS diagnosed by prenatal MRI at Huzhou Maternal and Child Health Care Hospital and Anhui Provincial Children’s Hospital from January 2017 to January 2020 was performed to observe the source blood vessels of lesions and their direction. Pathological confirmation was completed through CT examination and/or operations after birth.
After birth, 31 cases after birth were confirmed to be CCAM, and 15 were confirmed to be BPS. The CCAM group consisted of 21 macrocystic cases and 10 microcystic cases. In 18 cases, blood vessels were visible in lesions. Blood supply of the pulmonary artery could be traced in eight cases, and in 10 cases, only vessels running from the midline to the lateral down direction were observed. No lesions were found in four macrocystic cases and one microcystic case with CCAM through CT after birth; two were misdiagnosed by MRI, and three were misdiagnosed by prenatal ultrasonography. The BPS group consisted of 12 intralobar cases and three extralobar cases. Blood vessels were visible in lesions of nine cases, in four of which, the systemic circulation blood supply could be traced, and in five of which, only vessels running from the midline to the lateral up direction were observed. Three were misdiagnosed by MRI, and four were misdiagnosed by prenatal ultrasonography.
CCAM and BPS can be clearly diagnosed based on the origin of blood vessels, and correct diagnosis can be made according to the difference in the direction of the blood vessels, but it is hard distinguish microcystic CCAM and BPS without supplying vessels. In some CCAM cases, mainly the macrocystic ones, the lesions may disappear after birth.
Core Tip: Congenital cystic adenomatoid malformation (CCAM) and broncho-pulmonary sequestration (BPS) can be clearly diagnosed based on the origin of blood vessels, and correct diagnosis can be made according to the difference in the direction of the blood vessels, but it is hard to distinguish microcystic CCAM and BPS without supplying vessels. In some CCAM cases, mainly the macrocystic ones, the lesions may disappear after birth.
- Citation: Li Z, Lv YD, Fang R, Li X, Luo ZQ, Xie LH, Zhu L. Usefulness of prenatal magnetic resonance imaging in differential diagnosis of fetal congenital cystic adenomatoid malformation and bronchopulmonary sequestration. World J Clin Cases 2021; 9(4): 822-829
- URL: https://www.wjgnet.com/2307-8960/full/v9/i4/822.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i4.822
Congenital cystic adenomatoid malformation (CCAM) and bronchopulmonary sequestration (BPS), especially CCAM[1], are the most common lung diseases in fetuses[2]. There are differences in the prognosis and treatment of CCAM and BPS, and the clinical diagnosis and treatment plan is usually prepared prior to birth[3]. Therefore, it is quite necessary to make a clear diagnosis before delivery. CCAM and BPS have similar imaging features, and the differentiation mainly relies on the difference in supply vessels[4]. However, it is hard to distinguish them due to invisible supplying vessels on some images[5,6], especially microcystic CCAM and BPS[7]. At present, prenatal ultrasonography is widely used for screening[8,9], but it may lead to missed diagnosis or misdiagnosis due to fetal position and ribs. Magnetic resonance imaging (MRI) belongs to a nonradioactive examination with the advantages of large scanning field, satisfactory soft tissue contrast, and high tissue resolution. It is not affected by mother’s body size and amniotic fluid volume, as well as fetal position and fetal bones. Therefore, MRI can better display the details of normal anatomy and abnormal pathological structure of fetuses, which means that it has advantages over ultrasonography, and can provide additional information[10]. In this study, a retrospective study was performed to analyze CCAM and BPS cases diagnosed by prenatal MRI, and explore the application value of prenatal MRI in the differential diagnosis of fetal CCAM and BPS.
The follow-up data of 32 singleton fetuses with CCAM and 14 with BPS diagnosed by prenatal MRI at Huzhou Maternity & Child Health Care Hospital and Anhui Provincial Children’s Hospital from January 2017 to January 2020 were collected. All fetuses were born smoothly and accepted prenatal MRI examination within 24-48 h after prenatal ultrasonography. This study was approved by the Ethics Committee of Huzhou Maternity & Child Health Care Hospital and Anhui Provincial Children’s Hospital, and all pregnant women voluntarily signed an informed consent form prior to examination.
MRI examination was carried out using a 1.5-T Avanto superconducting imaging system (Siemens, Munich, Germany). This system has a gradient field strength of 45 mT∙m-1∙s-1, a 32-channel phased array heart coil, and 1-2 excitations. We employed three MRI sequences: (1) True fast imaging with steady-state precession (True FISP) sequence with a minimum repeat time (TR) of 3.6-4.2 ms, echo time (TE) of 1.0-2.0 ms, reversal angle of 90°, matrix of 256 × 192, and scan time of 0.5-2.0 s per layer (total, 10.0-20.0 s); (2) Half-Fourier acquisition single-shot turbo spin-echo (HASTE) sequence with TR of 1150-1500 ms, TE of 42-145 ms, reversal angle of 160°, and matrix of 256 × 198; and (3) Two-dimensional turbo FLASH T1WI (TFL) sequence with TR of 1680-2000 ms, TE of 2.9-4.5 ms, inversion angle of 15°, and matrix of 256 × 154. Another MRI examination was performed using a 1.5 T MRI scanner (Philips Medical Systems, Netherlands) with a 4-channel abdominal surface coil and 1-2 excitations, and single-shot fast spin-echo (SSFSE) sequence and balanced fast field echo (B-FFE) sequence were performed using the following parameters: (1) SSFSE sequence: TR of 12000.0 ms, TE of 120.0 ms, thickness of 5.0-7.0 mm, reversal angle of 80°, and matrix of 216 × 218; and (2) B-FFE sequence: TR and TE of minimum values set by the system, thickness of 5.0-7.0 mm, reversal angle of 90°, and matrix 216 × 218.
Each pregnant woman was introduced in the device with feet first, and examined in supine position or left lateral position with quiet respiration. Localization scan of the lower abdomen in the coronal plane was first carried out, followed by routine brain, chest, and abdomen scanning in the cross-sectional, sagittal, and coronal planes. Finally, the chest and abdomen scans were performed.
Ultrasonography was completed with Voluson experd730 (GE) and × 300 Color Doppler Ultrasound Diagnostic System (Siemens), with a convex array probe and frequency of 4.0-8.0 MHz.
CCAM cases were classified as macrocystic and microcystic ones based on the size of cyst. Macrocystic CCAM refers to the disease with a cyst diameter ≥ 5 mm, and microcystic CCAM refers to the disease with a cyst diameter < 5 mm[11,12].
BPS cases were classified as extralobar and intralobar ones[13,14]. Extralobar BPS was wrapped by separate visceral pleura and separated from normal lung tissues. The lesion tissues of intralobar BPS were located in normal lung tissues, and wrapped by visceral pleura with normal lung tissues.
MRI images were analyzed by two experienced associate chief radiologists based on the double-blind method, involving lesion signal, location, blood supply vessels, vessel direction, and heart position. In case of disagreement, they should determine through mutual consultation.
Postnatal CT examination and/or pathological confirmation were completed.
Thirty-one cases were confirmed to be CCAM, including five cases with lesions disappearing after CT review. Fifteen cases were confirmed to be BPS, including 12 intralobar and three extralobar cases.
In the CCAM group, pregnant women were aged 20-37 years, with an average age of 27.5 ± 3.8 years. At the time of MRI examination, their gestational age was 20-37 wk, with an average of 29.2 ± 4.6 wk. There were 17 male infants and 14 female infants, including 23 of term labor and eight of premature labor; the earliest was born at 35+1 wk. In the BPS group, pregnant women were aged 20-28 years, with an average age of 28.1 ± 4.3 years. At the time of MRI examination, their gestational age was 21-31 wk, with an average of 28.5 ± 4.6 wk. There were nine male infants and six female infants, including 11 of term labor and four of premature labor; the earliest was born at 35+4 wk.
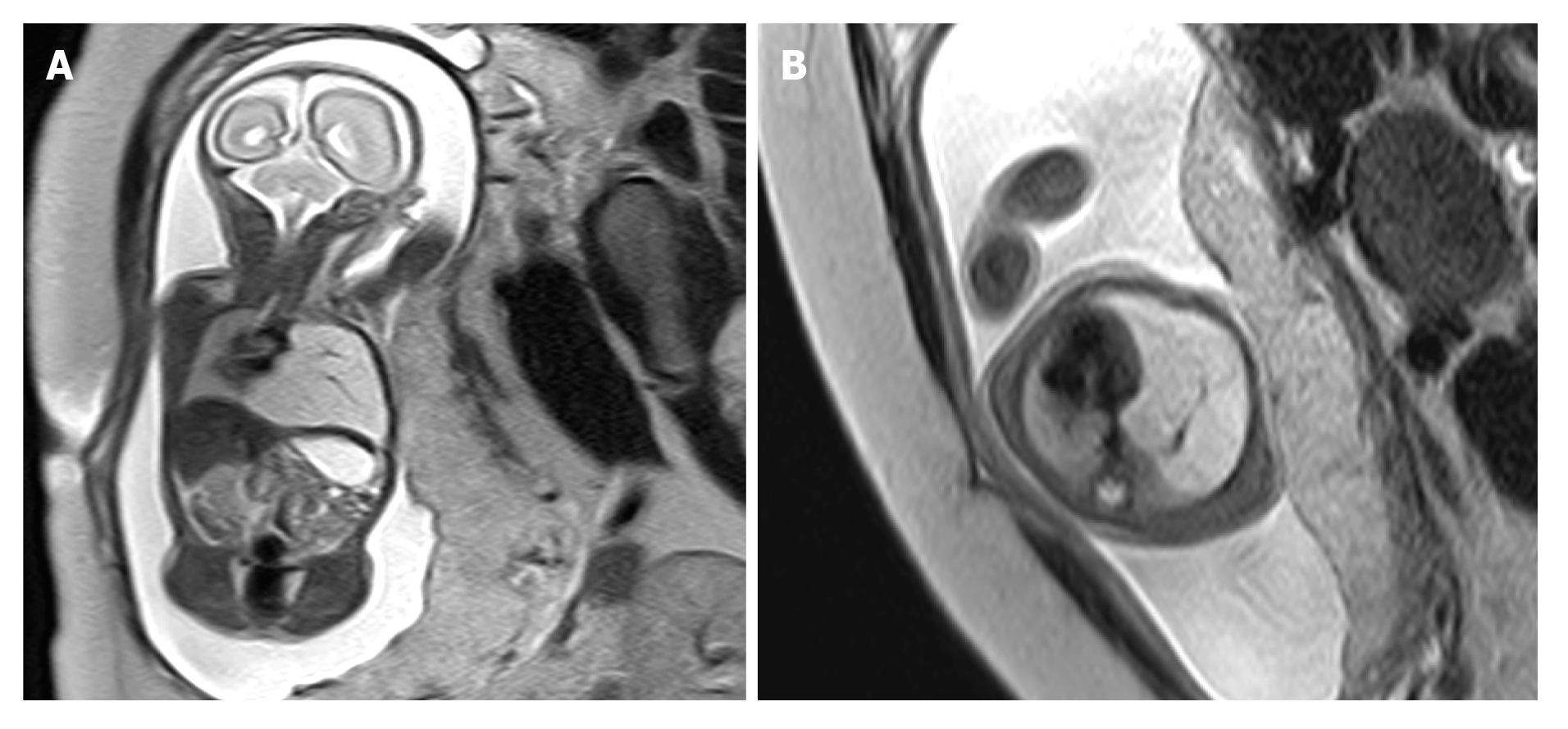
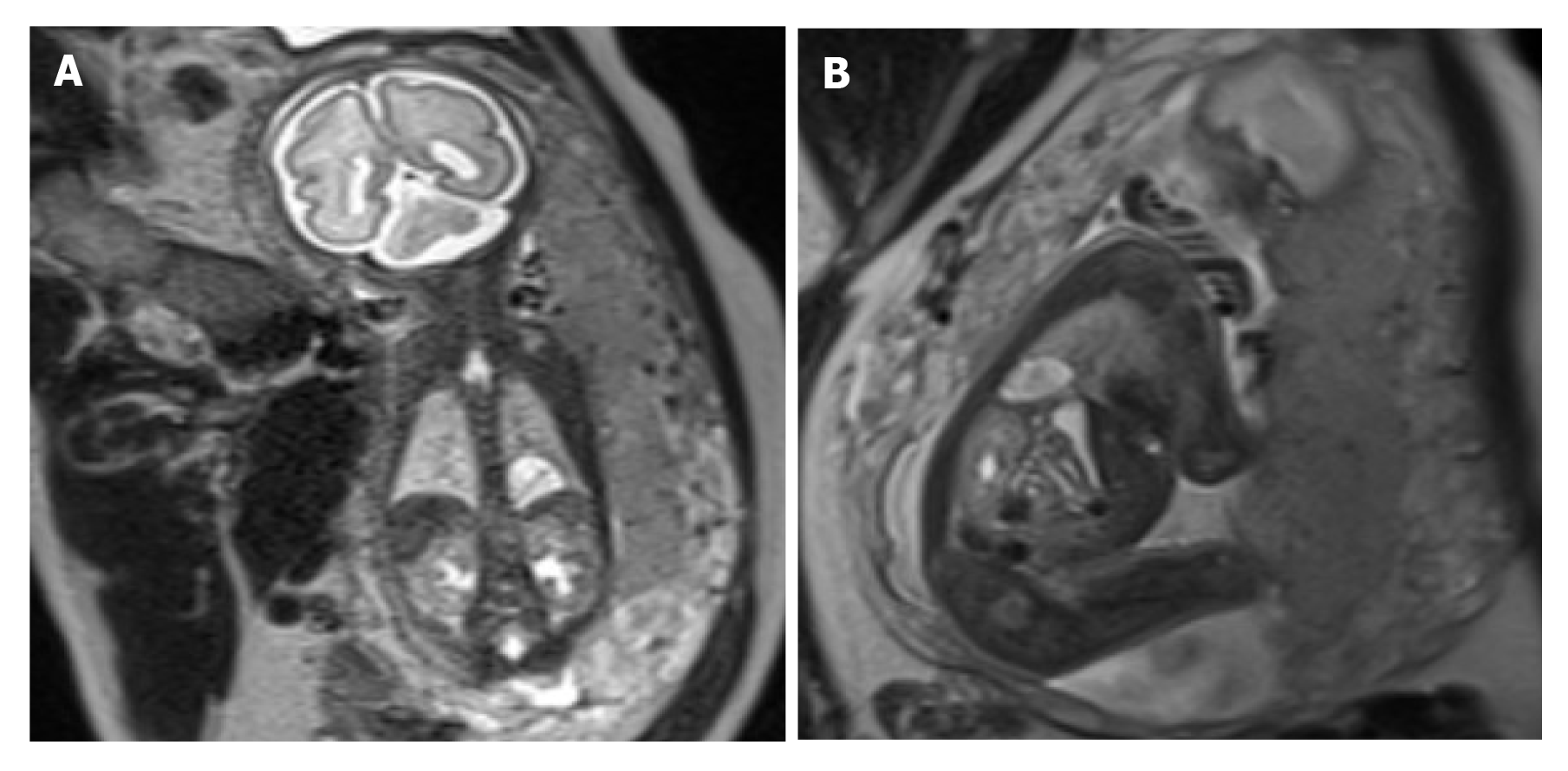
There were 21 macrocystic and 10 microcystic CCAM cases, 20 of which had lesions in the left lung, and 11 had the lesions in the right lung. Two cases had hearts shifting left and four had hearts shifting right. In eight of the 18 cases with visible blood vessels, pulmonary artery supply could be traced in eight cases, and vessels running from the midline to the lateral down direction were only observed in ten (Figure 1). Postnatal CT examination showed that no lesions were found in five infants, and prenatal MRI showed that four were macrocystic and one was microcystic; pulmonary artery supply could be traced in two cases, and visible blood vessels in lesions running from the midline to the lateral down direction were observed in three. Two cases were misdiagnosed by prenatal MRI as microcystic CCAM (without blood vessels in the lesion), with a misdiagnosis rate of 6.5%; three cases were misdiagnosed by prenatal ultrasonography as microcystic CCAM, with a misdiagnosis rate of 9.7%. In the BPS group, 12 cases had lesions in the left lung and three had lesions in the right lung; two cases had hearts shifting right; and in four of the nine cases with visible blood vessels in lesions, systemic circulation supply could be traced, and vessels running from the midline to the lateral up direction were only observed in five cases (Figure 2). Three cases were misdiagnosed as intralobar BPS by prenatal MRI, with a misdiagnosis rate of 20.0%; four cases were misdiagnosed as intralobar BPS by ultrasonography, with a misdiagnosis rate of 26.7%.
The etiology of CCAM is still unclear, and it is believed to be abnormal proliferative hamartoma differing from tissue origin of the lung[1]. CCAM, accompanied by abnormal development of local lung tissue, is associated with substantial dysplasia of bronchial atresia[15,16]. Moerman et al[17] described the autopsy results of four cases with CCAM, each of which had segmental bronchial absence or atresia, and the results can provide further evidence for the hypothesis of primary defect due to CCAM. In addition, they also proposed that primary defect was bronchial atresia due to limited stop or defect in the process of bronchopulmonary germination and branching. The complete atresia would result in bronchial absence.
The etiology of BPS is still unknown. Pryce et al[18] proposed a theory of traction, which has become a relatively recognized theory. In the theory, it is described that blood capillaries are connected with the dorsal aorta when lung bud tissues are not separated from archenteron at the early stage of embryonic development; with the development of embryo, when lung bud tissues are separated from archenteron, the blood capillaries connected with dorsal aorta that should have been degraded and absorbed would be partially retained due to unsafe degradation for some reasons. In the future, embryonic lung tissues with blood supplied by arterial branches will be formed with the development of lung buds, and finally form an isolated lung without communication with the normal bronchus after birth.
Compared with ultrasonography, MRI has the advantages of large field of view, multi-parameter, high soft tissue resolution, no limitation of fetal position and maternal size, and clearer display of anatomical structure of the fetus and placenta than ultra-sonography, all of which have made it an important supplement to obstetric ultrasonography[19]. True FISP/B-FFE and HASTE/SSFSE sequences are commonly used in fetal MRI examinations, and they are fast in scanning speed, which can shorten the imaging time and greatly reduce artifacts of the fetus and pregnant women; in addition, the images of fetal organs with high resolution can be obtained, pregnant women do not need to take sedatives, and high-quality images can be obtained by scanning after holding breath. True FISP/B-FFE and HASTE/SSFSE can show high-uniformity signals of fetal lungs. True FISP/B-FFE can make fetal heart and large blood vessels show high signals, which can clearly indicate the structure of the four cardiac chambers and large blood vessels, as well as oppressive changes made by lesions on heart and large vessels. HASTE/SSFSE can show “black-blood” signal of the heart, which can display the location and size of fetal heart, but not internal structure. However, due to the bright “water” effect, it can display the morphology, boundary, and internal structure of fetal lung more clearly, thus better distinguishing fetal lung diseases from surrounding normal lung tissues. In HASTE/SSFSE sequence, CCAM and BPS can show high signals, and blood supply vessels show low signals. Therefore, this sequence can be used to better discover the source blood vessels of lesions. In this study, in the 31 CCAM cases, 18 had visible blood vessels, and as for eight of which, pulmonary artery supply can be traced. In 14 BPS cases, nine had visible blood vessels, and as for four of which, systemic circulation supply can be traced.
CCAM can take place at any part of the two lungs, and different types may indicate different prenatal MRI presentations. Prenatal MRI of macrocystic CCAM may show blocks with T2WI signal higher than the signal of normal lung tissues; the lesions can be clearly distinguished from surrounding normal lung tissues, with an irregular morphology and different size in cysts, and the larger vesica would make MRI lesion area show higher signals[10]. Macrocystic signal can be close to amniotic fluid signal, and the cyst wall and its spacing with low T2WI signal are partially visible. The volume of the affected lung or lobe may increase, and the heart and large blood vessels may be changed under stress. Microcystic prenatal MRI may show high T2WI signal, but lower than the amniotic fluid signal, indicating substantial lesions. Larger CCAM may oppress large blood vessels and the heart, accompanied by poor reflux of blood and changes in pleural effusion. In some cases, CCAM lesions gradually became smaller or even disappeared at the later stage of pregnancy[20]. More than 15% of CCAM lesions can disappear after birth[21]. In this study, postnatal CT found no lesions in five cases, macrocystic CCAM was confirmed in four cases, and microcystic CCAM was confirmed in one case, with a lesion disappearance rate of 16.1% (5/31). BPS usually took place at the lower lobe of the left lung, and prenatal MRI showed that the lung lobe with lesions would enlarge. The lesions showed uniform high signal on T2WI, with clear boundaries, and the signal was between the amniotic fluid and normal lung tissue. In the case of a large lesion, the heart would shift to the normal side under stress to varying degrees, which can result in fetal edema and lung dysplasia[22]. Some extralobar BPS lesions may take place in the lower diaphragmatic region of the upper abdomen, showing cystic signals.
CCAM blood supply vessels mainly originate from the pulmonary artery[23,24], and BPS blood supply vessels mainly originate from the aorta[25]. Based on different origins of blood vessels, CCAM and BPS can be distinguished. Empty blood vessels may be found in some prenatal MRI examinations, but the sourcing blood vessels cannot be traced, which would make it hard to distinguish microcystic CCAM and BPS. In this study, it was found that due to the higher position of the pulmonary artery as compared with the aorta, the pulmonary artery supplying blood for CCAM ran from the midline to the lateral down direction, and the aorta supplying blood for BPS ran from the midline to the lateral up direction. The difference in running direction of supplying vessels can indicate the types of lesions. In this study, only empty blood vessels were observed by prenatal MRI in 15 cases, but no origin of blood vessel was traced. In ten cases, blood vessels in the lesion ran from the midline to the lateral down direction, which were diagnosed as CCAM. The postnatal CT showed that the lesions disappeared in three cases, and CCAM was confirmed in seven cases through CT and/or surgical pathology. In five cases, blood vessels in the lesion ran from the midline to the lateral up direction, which were diagnosed as BPS and further confirmed through postnatal CT and/or surgical pathology. It was not hard to distinguish macrocystic CCAM and BPS, but hard to distinguish microcystic CCAM and BPS without visible supplying vessels. In this study, three cases of BPS were misdiagnosed as microcystic CCAM, and two cases of microcystic CCAM were misdiagnosed as BPS.
In conclusion, CCAM is the most common fetal lung malformation, with an incidence higher than that of BPS. It can be clearly diagnosed according to the origin of blood vessels, and correctly diagnosed according to the direction of the blood vessels, but it would be hard to distinguish microcystic CCAM and BPS without visible supplying vessels. In some CCAM cases, mainly the macrocystic ones, the lesions may disappear after birth.
Congenital cystic adenomatoid malformation (CCAM) and bronchopulmonary sequestration (BPS) have similar imaging features, and the differentiation mainly relies on the difference in supply vessels.
To make a better diagnosis and differential diagnosis of CCAM and BPS through prenatal magnetic resonance imaging (MRI).
To improve the accuracy of prenatal MRI in CCAM and BPS.
The MRI images of CCAM and BPS were retrospectively analyzed to find the blood supply vessels and the direction of travel.
In this study, it was found that due to the higher position of the pulmonary artery as compared with the aorta, the pulmonary artery supplying blood for CCAM ran from the midline to the lateral down direction, and the aorta supplying blood for BPS ran from the midline to the lateral up direction.
CCAM and BPS can be correctly diagnosed according to the direction of the blood vessels.
More cases are needed to confirm our findings.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Park SB S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Wang LYT
| 1. | Hellmund A, Berg C, Geipel A, Bludau M, Heydweiller A, Bachour H, Müller A, Müller A, Gembruch U. Prenatal Diagnosis and Evaluation of Sonographic Predictors for Intervention and Adverse Outcome in Congenital Pulmonary Airway Malformation. PLoS One. 2016;11:e0150474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Shanti CM, Klein MD. Cystic lung disease. Semin Pediatr Surg. 2008;17:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Schwartz DS, Reyes-Mugica M, Keller MS. Imaging of surgical diseases of the newborn chest. Intrapleural mass lesions. Radiol Clin North Am. 1999;37:1067-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Hou HM, Dong M, Li MJ, Qiao YN, Sun J. Clinical value of prenatal ultrasound in diagnosis of congenital cystic adenomatoid malformation of the lung. J Med Imaging 2018; 28: 452-458. Available from: http://en.cnki.com.cn/Article_en/CJFDTotal-XYXZ201803028. htm. |
| 5. | Daltro P, Werner H, Gasparetto TD, Domingues RC, Rodrigues L, Marchiori E, Gasparetto EL. Congenital chest malformations: a multimodality approach with emphasis on fetal MR imaging. Radiographics. 2010;30:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Li Z, Zhu M, Dong S, Luo Z, Fei Z, Fang X, Qi L. [Clinical value of prenatal MRI in the diagnosis and differential diagnosis of fetal bronchopulmonary sequestration]. Zhonghua Fuchanke Zazhi. 2016;51:23-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Dong SZ, Zhu M, Zhong YM, Zhu H, Pan HH. Diagnosis of fetal congenital cystic adenomatoid malformation of the lung by MRI. Radiol Practice. 2011;26:172-175. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Paladini D, Quarantelli M, Sglavo G, Pastore G, Cavallaro A, D'Armiento MR, Salvatore M, Nappi C. Accuracy of neurosonography and MRI in clinical management of fetuses referred with central nervous system abnormalities. Ultrasound Obstet Gynecol. 2014;44:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | von Scheidt F, Eicken A, Wowra T, Brunner H, Apitz C. Bilateral Pulmonary Sequestration in a Preterm Infant. J Pediatr 2018; 194: 260-260. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Li Z, Lv Y, He P, Luo Z, Pan L, Du Y, Fang R, Liu Y, Li L, Zhu L. Clinical value of prenatal MRI for diagnosis of isolated ventriculomegaly and prediction of early postnatal developmental outcomes. Prenat Diagn. 2019;39:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Laje P, Liechty KW. Postnatal management and outcome of prenatally diagnosed lung lesions. Prenat Diagn. 2008;28:612-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Sun ZY, Xia LM, Chen XL, Wang CY, Yang XH, Yang WZ. Congenital cystic adenomatoid malformation of fetus: manifestations and diagnosis of MRI. Chin J Radiol. 2007;41:490-492. |
| 13. | Colon N, Schlegel C, Pietsch J, Chung DH, Jackson GP. Congenital lung anomalies: can we postpone resection. J Pediatr Surg. 2014;47:87-92. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Liu HS, Li SQ, Huang C, Qin YZ, Li L. Diagnosis and Treatment of Pulmonary Sequestration: Report of 53 Cases. Xiehe Yixue Zazhi. 2011;02:61-64. [DOI] [Full Text] |
| 15. | Bolde S, Pudale S, Pandit G, Ruikar K, Ingle SB. Congenital pulmonary airway malformation: A report of two cases. World J Clin Cases. 2015;3:470-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 16. | Riedlinger WF, Vargas SO, Jennings RW, Estroff JA, Barnewolt CE, Lillehei CW, Wilson JM, Colin AA, Reid LM, Kozakewich HP. Bronchial atresia is common to extralobar sequestration, intralobar sequestration, congenital cystic adenomatoid malformation, and lobar emphysema. Pediatr Dev Pathol. 2006;9:361-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Moerman P, Fryns JP, Vandenberghe K, Devlieger H, Lauweryns JM. Pathogenesis of congenital cystic adenomatoid malformation of the lung. Histopathology. 1992;21:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | PRYCE DM. Lower accessory pulmonary artery with intralobar sequestration of lung; a report of seven cases. J Pathol Bacteriol. 1946;58:457-467. [PubMed] |
| 19. | Williams HJ, Johnson KJ. Imaging of congenital cystic lung lesions. Paediatr Respir Rev. 2002;3:120-127. [PubMed] |
| 20. | Liu YP, Chen CP, Shih SL, Chen YF, Yang FS, Chen SC. Fetal cystic lung lesions: evaluation with magnetic resonance imaging. Pediatr Pulmonol. 2010;45:592-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Alshamiri KM, Abbod HB. Congenital cystic adenomatoid malformation. Int J Pediatr Adolesc Med. 2017;4:159-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Witlox RSGM, Lopriore E, Rijken M, Klumper FJCM, Oepkes D, van Klink JMM. Long-Term Neurodevelopmental and Respiratory Outcome after Intrauterine Therapy for Fetal Thoracic Abnormalities. Fetal Diagn Ther. 2019;45:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Cheney LB, Patel B, Lam A, Arbuckle S, Morris J, Holland AJ. Extralobar pulmonary sequestration in association with congenital cystic adenomatoid malformation: an unusual abdominal mass. ANZ J Surg. 2011;81:556-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Yu G, Hong C, Ma XY, Wang LM, Zhong YF. Analysis of perinatal outcome of fetus with congenital cystic adenomatoid malformation. Zhonghua Fuchanke Zazhi. 48:683-685. [DOI] [Full Text] |
| 25. | Khalek N, Johnson MP. Management of prenatally diagnosed lung lesions. Semin Pediatr Surg. 2013;22:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |