Published online Feb 6, 2021. doi: 10.12998/wjcc.v9.i4.764
Peer-review started: October 10, 2020
First decision: November 26, 2020
Revised: December 4, 2020
Accepted: December 26, 2020
Article in press: December 26, 2020
Published online: February 6, 2021
Processing time: 106 Days and 13.9 Hours
Chiari malformations encompass various radiological and clinical entities, sharing the herniation of the rhombencephalic structures through the foramen magnum as a common characteristic. They can be symptomatic or asymptomatic. The therapeutic strategies for these malformations differ on the basis of the diverse pathophysiologic processes that cause them. As Chiari malformations are caused by various pathophysiologic processes, they must be recognized promptly to select the best treatment for each single case.
Core Tip: Chiari malformations include various radiological and clinical entities sharing the herniation of the rhombencephalic structures through the foramen magnum. Depending on the symptomatology, the therapeutic strategies differ on the basis of the underlying pathophysiological processes.
- Citation: Spazzapan P, Bosnjak R, Prestor B, Velnar T. Chiari malformations in children: An overview. World J Clin Cases 2021; 9(4): 764-773
- URL: https://www.wjgnet.com/2307-8960/full/v9/i4/764.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i4.764
Chiari malformations are pathologic conditions in which a part of the rhombencephalic structures (the pons, the medulla oblongata and the cerebellum) that are normally contained within the posterior fossa, herniate through the foramen magnum (FM) into the spinal canal. Chiari malformations are classified as follows[1]:
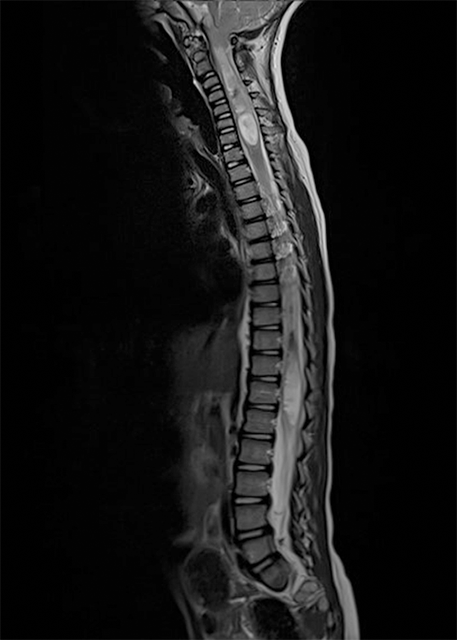
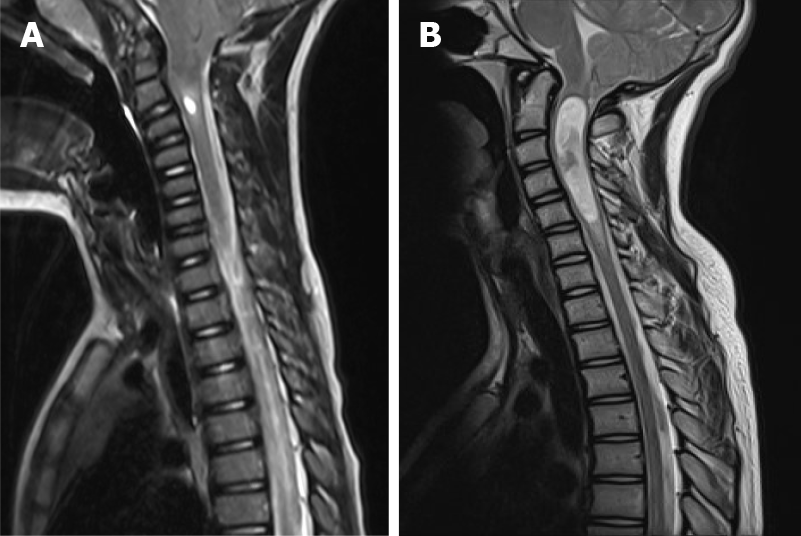
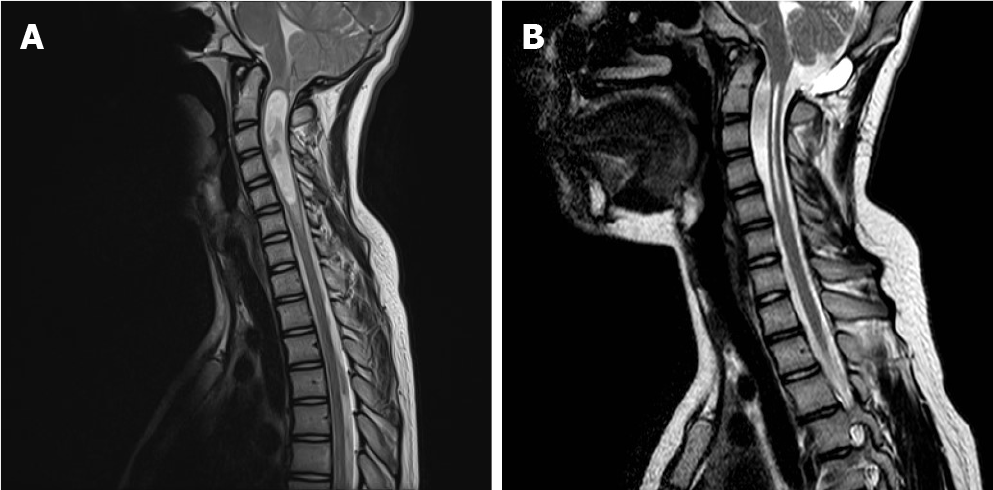
Chiari type 1 (CIM): Most frequent Chiari malformation. The cerebellar tonsils herniate through the FM for at least 5 mm. The brainstem is normally positioned in the posterior fossa. A syringomyelia or hydromyelia can be associated (Figure 1).
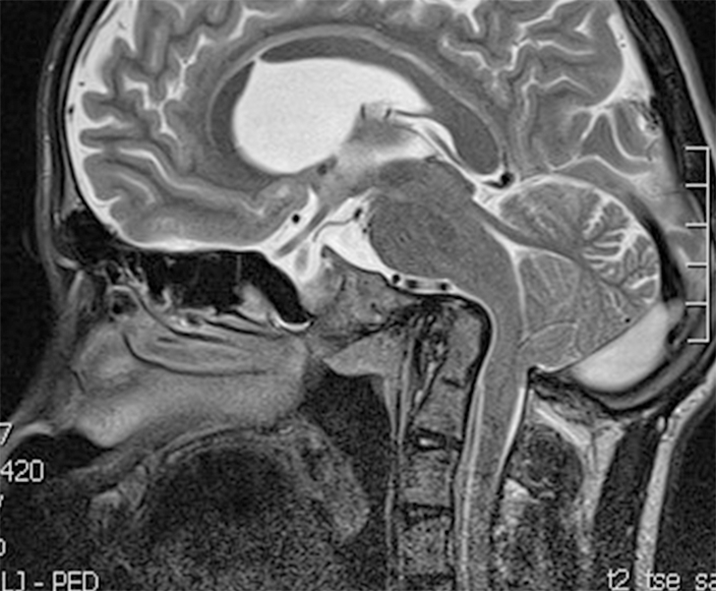
Chiari type 2 (CIIM): It is related the diagnosis of open neural tube defect (myelomeningocele). There is a caudal displacement of the vermis, the brainstem and the fourth ventricle. Syringomyelia is frequent. Additionally, other intracranial anomalies, such as agenesis of the corpus callosum, polymicrogyria and agenesis of the vermis are also present. Together, these malformations form the CIIM complex (Figure 2).
Chiari type 3 (CIIIM): This type is a rare (< 1% of all Chiari malformations) and represents an extreme form of the rhombencephalic herniation. Large portions of the cerebellum and brainstem herniate through the FM and form occipital and cervical subcutaneous cysts. Other anomalies of the central nervous system and severe neurological deficits are present.
Chiari type 4: Consists of aplasia or hypoplasia of the cerebellum and it is not a true herniation of the cerebellum. For this reason, the inclusion of these malformations within the Chiari group is questionable.
Children with CIM malformation are often asymptomatic and this malformation usually represents an incidental finding. It can be, however, associated with headaches and, in case of an associated syringomyelia, peripheral neurological deficits. In CIIM, the neurological deficits are mainly related to the associated malformations that form the CIIM complex and to the open neural tube defect. In CIIIM, the neurological deficits are severe already at birth. This malformation is very rare and nowadays almost never encountered in clinical practice, at least in the western countries. For this reason, this present review is focused especially on CIM and CIIM, as these are most frequently seen in patents seeking neurosurgical and neurological management. Treatment of Chiari malformations can be conservative or surgical. Surgical treatment is indicated in symptomatic children, particularly when the Chiari malformation causes an obstruction to the cerebrospinal fluid (CSF) flow through the FM or compression of the brainstem[1-3].
Several different theories have been developed to explain the origin of the Chiari malformations. Hans Chiari, who first described these conditions, thought that they were intimately related to the hydrocephalus[1]. Later, McLone and Knepper developed the embryological theory of the CSF leakage into the amniotic sac, which may be responsible for the hypotension in the developing neural tube[2]. Because of a small posterior fossa, this may cause the herniation of the rhomboencephalic structures, and other malformations in the central nervous system. A third pathophysiological theory is the hydrodynamic theory, based on an early disbalance of the CSF pulsations in the supra- and infratentorial compartment. This disbalances of the CSF pulsations between the two compartments may initiate a caudal migration of the tentorium, a smaller posterior fossa and consequently a Chiari malformation[3]. All these theories are only partially valid and all of them are incomplete from a pathophysiological point of view, showing that Chiari malformations are complex and versatile conditions with a diverse pathophysiologic origin[2,3].
The CIM is not a congenital malformation, it is rather an acquired one. One could also call it “deformation”, or more precisely an ectopy of the cerebellar tonsils[4]. The CIM has never been described in neonates and histological examinations of the herniated tonsils excluded the presence of neuronal dysplasia, which are typical for other malformations. On the contrary, the CIIM and CIIIM are true malformations of the central nervous system, related to the embryological and developmental anomalies[5].
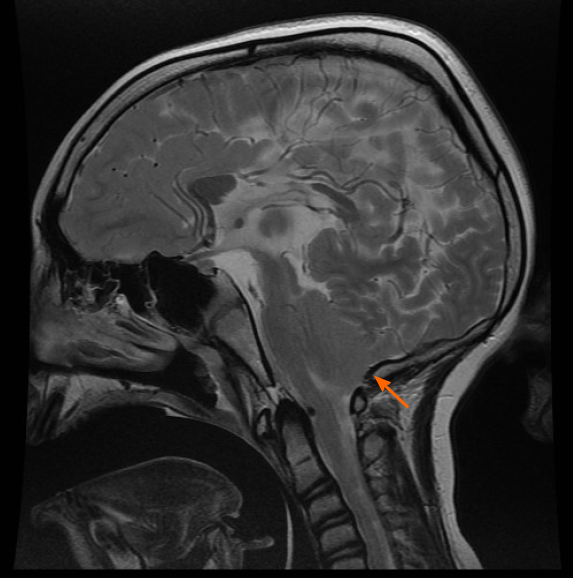
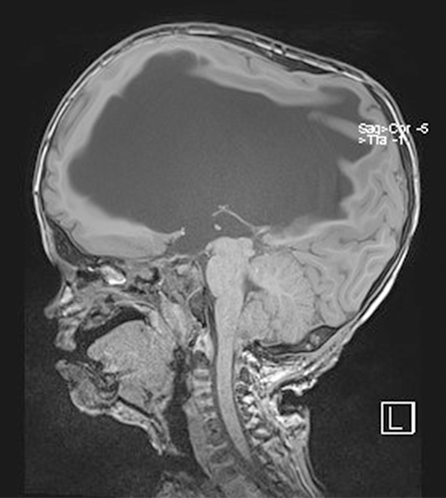
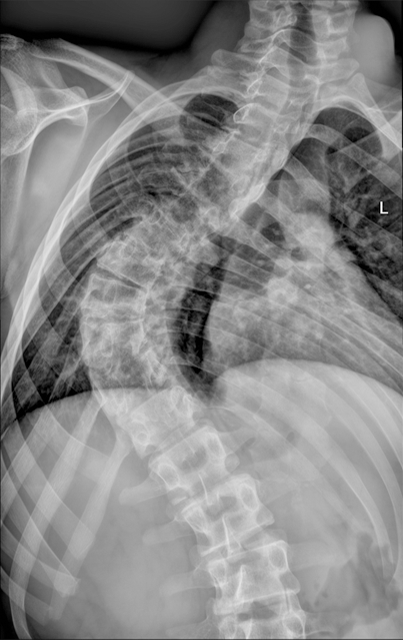
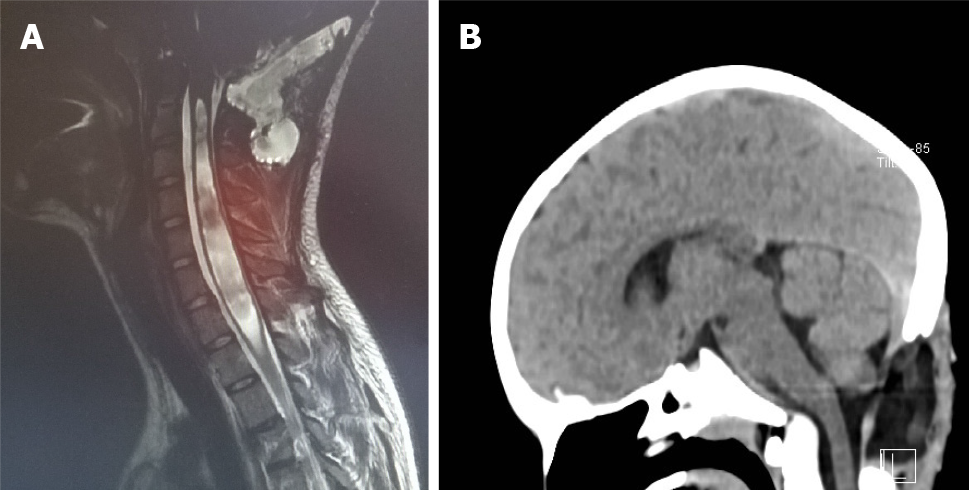
The definition of the CIM is based on the general rule of a 5 mm herniation through the FM. This is a potentially confusing statement, since it is not based on precise measurements and, above all, it has no direct clinical importance. There is no relation between the extent of the cerebellar herniation in millimetres and the presence of syringomyelia or neurological deficits. The CIM should therefore be defined as a functional obstruction of the FM, caused by the inferior part of the cerebellum, which may impede the CSF flow or may lead to the compression of other neural structures[4]. This functional obstruction can be caused by several different pathophysiological processes and the correct recognition of them is the basis of a correct therapeutic decision: (1) The CIM may be related to a disorder of the CSF flow. Hans Chiari first postulated that this malformation, later called Chiari, could be caused by hydrocephalus[1]. He actually observed such malformations in cadaveric specimen, who presented extreme pathologic conditions that are not encountered today in daily clinical practice anymore. Beyond this, several studies showed that hydrocephalus is associated to 10% of CIM and that a correct treatment of hydrocephalus, often with an endoscopic third ventriculostomy, leads to the resolution of both pathologies, hydrocephalus and CIM[6-9]; (2) The CIM may be related to bone anomalies. The theory of a small posterior fossa as a cause of the CIM is not evidence based, but on the contrary, the CIM is often associated with bone anomalies of the craniocervical junction, such as basilar invagination (Figure 3), craniocervical instability and atlanto-occipital fusion[10]; (3) The CIM related to craniosynostosis and obstruction to vein outflow. The CIM can be related to raised intracranial venous pressure, which is typically caused by Vein of Galen aneurysmal malformation and by syndromic craniosynostoses, such as Pfeiffer, Crouzon and Apert syndrome, for example. These entities are all associated with the stenosis of the jugular foramen, intracranial venous congestion, brain swelling and tonsillar herniation[11,12]. Venous hypertension also obstructs normal CSF resorption and consequently leads to hydrocephalus (Figure 4). In case of vein of Galen aneurysmal malformation, both venous hypertension related to CIM and hydrocephalus usually resolve after an effective embolization of the venous malformation[13]. In selected cases of syndromic craniosynostoses, the CIM can be successfully treated by cranial vault expansion, which lowers the intracranial pressure and enables a normal venous circulation; and (4) The CIM may be related to metabolic disorders. Due to several metabolic disorders, the bone structures of the posterior fossa can be underdeveloped and this may result in CIM. This can be associated to a deficit of growth hormone, which is related to CIM in 9% to 20% of cases[14]. Additionally, the CIM may also be related to rickets, hypophosphatemia and hypovitaminosis D.
The CIM is often asymptomatic and the diagnosis is frequently accidental, made during radiologic exams due to other conditions. In symptomatic cases, the CIM most frequently causes headaches, located mainly in the occipital and cervical region. These headaches exacerbate typically during Valsalva manoeuvres, like coughing, laughing and sneezing. If these characteristics are not present, the chance that the headache is not related to the CIM is higher[6].
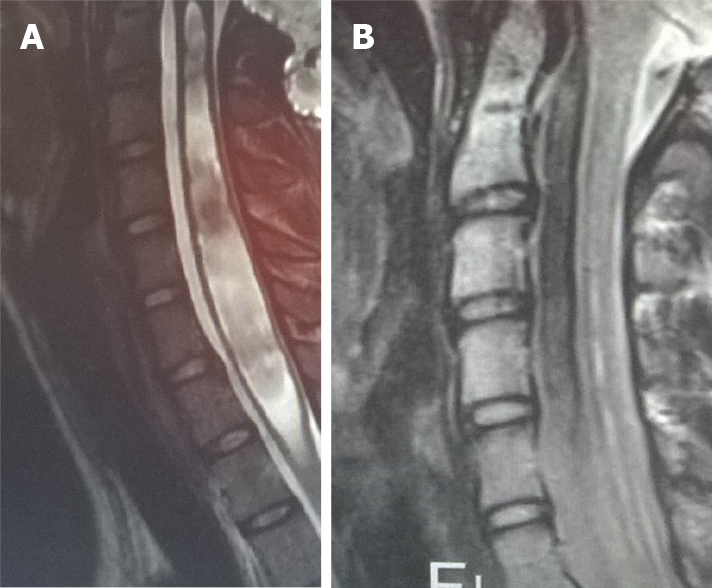
In case of an associated syringomyelia, neurological deficits may occur in upper and lower limbs, as well as in the trunk[6]. The syringomyelia may also cause sensory dissociation: the loss of pain and temperature sensibility with the preservation of the proprioception and the light touch. An important symptom related to the syringomyelia is also scoliosis, which may be encountered in 8% to 10% of patents[14]. Other, occasionally encountered symptoms are caused by the brain stem compression (nystagmus, ophthalmoplegia, tongue atrophy) and the cranial nerves dysfunction (dysphagia, central apnoea, vocal cords palsy, respiratory stridor). Cerebellar signs and symptoms are not present in CIM, since no eloquent neurological functions are located in the tonsils (Figure 5).
The symptoms of the CIIM may vary on the basis of the age of the child. In the neonatal period, it is possible to find a central apnoea, inspiratory stridor, vocal cord palsy, dysphagia and bradycardia. This clinical picture is rare (1% to 10% of children born with an open neural tube defect) and is potentially harmful[4-6]. These children need to be surgically treated as soon as possible. Symptoms that are present early after birth usually regress only moderately and determinate a poor prognosis. In older children, the symptoms of the CIIM are often related to dysfunction of the lower motor neuron of the upper limbs (hypotonia, loss of strength) and of the first motor neuron directed to the lower limbs (hypertonia, orthopaedic deformations, sphincter function deterioration).
The general rule to set the diagnosis of the CIM is a herniation of both tonsils beyond the FM for at least 5 mm. The hydrosyringomyelia, which may be also present, is caused by an obstruction to the CSF flow through the foramina of Luschka and Magendie from the fourth ventricle to the subarachnoid spaces. The magnetic resonance imaging (MRI) can detect also other abnormalities, which can cause the CIM or can be just associated to the CIM. The most important are the following: Atlanto-occipital fusion, basilar invagination (Figure 3), cervical vertebral fusion and scoliosis. The children with a basilar invagination and scoliosis must be treated with particular attention and a craniocervical decompression must often be associated to a craniocervical fixation[15].
The radiological imaging can also show hydrocephalus (Figure 6). Chiari and hydrocephalus represent a rare, but important association, which must be promptly recognized, since a correct hydrocephalus treatment can lead to the resolution of the CIM. Another important diagnostic tool in the setting of children with CIM is polysomnography, which can recognize frequent central apnoea in 4% of children (five or more apnoea events in every hour). Apnoea is caused by compression of the respiratory centres in the brainstem[16].
Beyond the tonsillar herniation, children with the CIIM have also other malformations, such as caudal elongation of the vermis and brainstem. The CIIM is related to the presence of an open neural tube defect and is often associated to hydrocephalus. There is often also a syringomyelia (40% to 95%)[17]. Other abnormalities found in CIIM are cranial and spinal malformations (corpus callosum agenesis, polymicrogyria, vermian agenesis) (Figure 2).
The indications for surgical treatment of the CIM are not uniform and vary among different centres. In an international survey related to the indications for CIM surgery, the majority of surgeons agreed that surgery is necessary in children with syringomyelia, progressive scoliosis and/or neurological deficits[18,19] (Figure 7). The indications for asymptomatic children without the syringomyelia were much less uniform. Furthermore, the differences were noted also regarding the surgical approaches and techniques. A general agreement is that a herniation of at least 5 mm is pathological, but does not need any treatment when it does not cause clinical symptoms or syringomyelia[19]. The indication for surgery must be based on clinical and radiological criteria. It is well known that large herniations can remain asymptomatic for many years and can also regress within a long term follow-up[20]. On the other hand, many patients with a herniation of less than 5 mm may present with large syringes in the medulla. In these cases (often described as Chiari 0 malformation), a craniocervical decompression is indicated[21] (Figures 8 and 9). Between these two extremes, many different conditions can exist. If both the herniation and the syringomyelia are asymptomatic, we unlikely propose a surgical treatment and rather decide a close follow-up. The same is valid also for children, who present just with a hydromyelia. Here, at our centre we can propose surgery just in the case of hydromyelia progression or if neurologic deficits occur. Among children with headaches and without syringomyelia, the most important decision-making factor is worsening of the headaches during the Valsalva manoeuvres. Without this exacerbation of headaches, the operation is most likely unsuccessful[8,9].
A rare and extreme category of patients are neonates with difficulties in swallowing, with nystagmus, inspiratory stridor and frequent apnoea. These signs indicate a brainstem dysfunction. If the MRI confirms a CIM, an early craniocervical decompression may bring some relief[9,19].
Many studies have shown the efficacy of craniocervical decompression in the treatment of children with CIM. In the literature, the rate of symptoms regression in well selected children is around 80% to 85%. The overall possibility of having the syringomyelia regression is around 90%[22,23]. On the other hand, in 5% to 10% of cases, the syringomyelia can recur after surgery. In this subgroup, as well as in the group of patients that did not benefit from the initial surgical treatment, a second decompression can be successfully performed, with the resection of one or both tonsils. On the basis of these data, some surgeons perform the tonsillar resection already during the first surgical procedure[23].
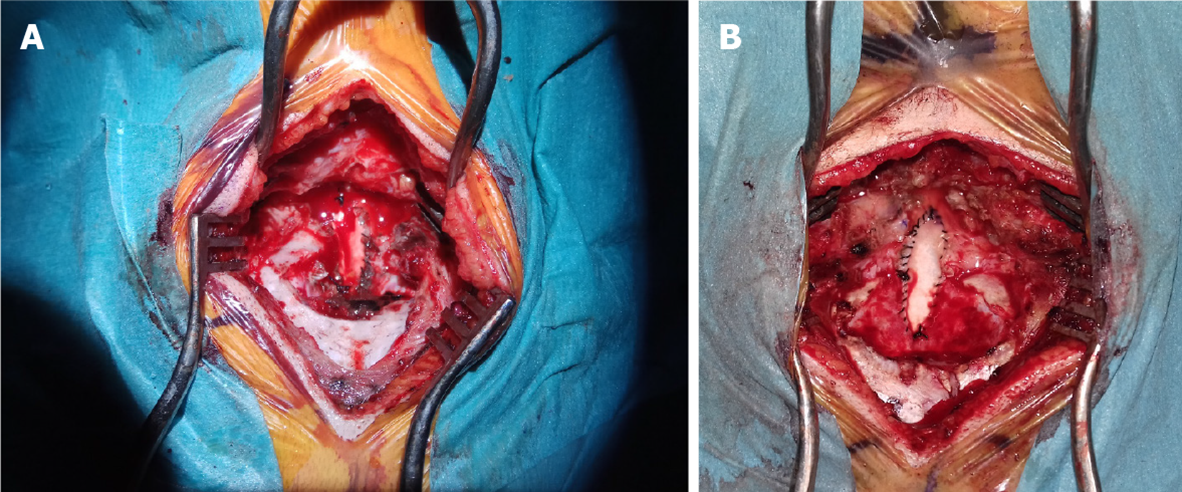
The craniocervical decompression can be done in a sitting or in a prone position. The former allows a better visualization of the anatomy of the craniocervical junction, but is potentially related to air embolism, which represents a harmful complication. During surgery, a suboccipital craniotomy is done, together with the C1 Laminectomy. If the tonsil herniate to the C2 Level, the laminectomy may be extended toward this level. The dura is opened in a Y-shape and a dural graft is sutured in the dural opening (Figure 10). This makes the dural sac larger and the CSF may flow free from the obstacles. Many variations of this surgical technique have been described[24]. Some authors perform just a bony decompression, without any dural incision, other incise only the dura, leaving the arachnoid membranes intact, in order to prevent the possibility of postoperative scarring, some others incise only the outer dural layer[6,23,25]. In some patients, the arachnoid membranes are resected, while in others, the exploration of the fourth ventricle may be performed. Some surgeons plug the obex to facilitate the syrinx resolution[18]. Finally, as already mentioned, some surgeons coagulate and excise the tonsils. This is possible since there is no eloquent function located in this structures, which, beyond this, become gliotic with time due their compression within the narrow craniocervical canal[5]. A useful tool for the decision whether to open the dura or not is the intraoperative ultrasound. If a good tonsillar pulsation is seen, the durotomy can be avoided, since this indicates a sufficient CSF flow. If the pulsations are not visible, the dural incision is advised, with the eventual arachnoid resection and exploration of the fourth ventricle[23,25].
The success of surgical treatment of the CIIM is largely based of the preoperative clinical conditions. Older children usually have a good outcome and neurologic deficits may resolve to some extent. In the neonatal period, the results are poor, since the symptoms are more severe and related to the brainstem dysfunction[23]. If a child with a CIIM presents with the symptoms, the progressive hydrocephalus must first be ruled out. This means that a ventriculoperitoneal shunt must be inserted or revised if pre-existent, even though the endoscopic third ventriculostomy also shows promising results in the treatment of the myelomeningocele-related hydrocephalus. In the rare cases where the hydrocephalus is excluded, a craniocervical decompression is justified. The surgical technique is basically the same as in the CIM. Since the tonsils often herniate to the low cervical levels, the skin incision usually ends at the point where the vermal herniation ends. Of a great importance is to exactly detect the location of the torcular, because the dural sinuses, together with the tentorium, are often displaced caudally and can be located very close to the FM (Figure 2). The extent of the suboccipital craniectomy is therefore usually small[23,24].
Craniocervical decompression is a relatively safe procedure, however, it is not without potential complication[25,26]. The most common complications include injury to the vascular and neural structures, pseudomeningocele (Figure 11), bleeding from the dural lacunae of the posterior fossa, the CSF fistula and meningitis. The best way to avoid the CSF leak is to perform a watertight suture of the dura, fascia and skin and to perform regular compressive bandages after surgery. Less frequent complications comprise a craniocervical instability and acute hydrocephalus. A rare complication is also the prolapse or ptosis of the cerebellum through the craniectomy site[27]. A particular situation must be kept in mind in small children, who have a large osteogenic potential that can cause a craniocervical reossification and a recurrence of their symptoms.
While the CIIM is a true malformation of the central nervous system, the CIM is a consequence of different pathophysiologic processes that cause the herniation of the tonsils through the FM. Differentiating and recognizing these various conditions is of paramount importance in the selection of the right treatment. Overall, the craniocervical decompression is a safe and effective treatment, when taking into account all the possible pitfalls and potential complications. It can prevent the progression of clinical and radiological deterioration in children with symptoms or syringomyelia related to the Chiari malformation. If the indication for surgical treatment is correct, the outcome will be reasonably successful.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Slovenia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Naswhan AJ S-Editor: Fan JR L-Editor: A P-Editor: Wu XYJ
| 1. | Chiari H. Concerning alterations in the cerebellum resulting from cerebral hydrocephalus. 1891. Pediatr Neurosci. 1987;13:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | McLone DG, Knepper PA. The cause of Chiari II malformation: a unified theory. Pediatr Neurosci. 1989;15:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 295] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 3. | GARDNER WJ. Hydrodynamic mechanism of syringomyelia: its relationship to myelocele. J Neurol Neurosurg Psychiatry. 1965;28:247-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 409] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Thompson DNP. Chiari I-a 'not so' congenital malformation? Childs Nerv Syst. 2019;35:1653-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Pueyrredon F, Spaho N, Arroyave I, Vinters H, Lazareff J. Histological findings in cerebellar tonsils of patients with Chiari type I malformation. Childs Nerv Syst. 2007;23:427-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Tubbs RS, Beckman J, Naftel RP, Chern JJ, Wellons JC 3rd, Rozzelle CJ, Blount JP, Oakes WJ. Institutional experience with 500 cases of surgically treated pediatric Chiari malformation Type I. J Neurosurg Pediatr. 2011;7:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 254] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 7. | Massimi L, Pravatà E, Tamburrini G, Gaudino S, Pettorini B, Novegno F, Colosimo C Jr, Di Rocco C. Endoscopic third ventriculostomy for the management of Chiari I and related hydrocephalus: outcome and pathogenetic implications. Neurosurgery. 2011;68:950-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Kandasamy J, Kneen R, Gladstone M, Newman W, Mohamed T, Mallucci C. Chiari I malformation without hydrocephalus: acute intracranial hypertension managed with endoscopic third ventriculostomy (ETV). Childs Nerv Syst. 2008;24:1493-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Di Rocco C, Frassanito P, Massimi L, Peraio S. Hydrocephalus and Chiari type I malformation. Childs Nerv Syst. 2011;27:1653-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Bagci AM, Lee SH, Nagornaya N, Green BA, Alperin N. Automated posterior cranial fossa volumetry by MRI: applications to Chiari malformation type I. AJNR Am J Neuroradiol. 2013;34:1758-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 11. | Cinalli G, Spennato P, Sainte-Rose C, Arnaud E, Aliberti F, Brunelle F, Cianciulli E, Renier D. Chiari malformation in craniosynostosis. Childs Nerv Syst. 2005;21:889-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 185] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Rijken BF, Lequin MH, Van Veelen ML, de Rooi J, Mathijssen IM. The formation of the foramen magnum and its role in developing ventriculomegaly and Chiari I malformation in children with craniosynostosis syndromes. J Craniomaxillofac Surg. 2015;43:1042-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Girard N, Lasjaunias P, Taylor W. Reversible tonsillar prolapse in vein of Galen aneurysmal malformations: report of eight cases and pathophysiological hypothesis. Childs Nerv Syst. 1994;10:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Hamilton J, Chitayat D, Blaser S, Cohen LE, Phillips JA 3rd, Daneman D. Familial growth hormone deficiency associated with MRI abnormalities. Am J Med Genet. 1998;80:128-132. [PubMed] |
| 15. | Bollo RJ, Riva-Cambrin J, Brockmeyer MM, Brockmeyer DL. Complex Chiari malformations in children: an analysis of preoperative risk factors for occipitocervical fusion. J Neurosurg Pediatr. 2012;10:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Felix O, Amaddeo A, Olmo Arroyo J, Zerah M, Puget S, Cormier-Daire V, Baujat G, Pinto G, Fernandez-Bolanos M, Fauroux B. Central sleep apnea in children: experience at a single center. Sleep Med. 2016;25:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Spazzapan P, Velnar T. Myelomeningocele in slovenia: results of a 10 years follow-up. J Neurosurg Sci. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Schijman E, Steinbok P. International survey on the management of Chiari I malformation and syringomyelia. Childs Nerv Syst. 2004;20:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Singhal A, Cheong A, Steinbok P. International survey on the management of Chiari 1 malformation and syringomyelia: evolving worldwide opinions. Childs Nerv Syst. 2018;34:1177-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Novegno F, Caldarelli M, Massa A, Chieffo D, Massimi L, Pettorini B, Tamburrini G, Di Rocco C. The natural history of the Chiari Type I anomaly. J Neurosurg Pediatr. 2008;2:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Iskandar BJ, Hedlund GL, Grabb PA, Oakes WJ. The resolution of syringohydromyelia without hindbrain herniation after posterior fossa decompression. J Neurosurg. 1998;89:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 107] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Leon TJ, Kuhn EN, Arynchyna AA, Smith BP, Tubbs RS, Johnston JM, Blount JP, Rozzelle CJ, Oakes WJ, Rocque BG. Patients with "benign" Chiari I malformations require surgical decompression at a low rate. J Neurosurg Pediatr. 2019;23:498-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Caldarelli M, Novegno F, Vassimi L, Romani R, Tamburrini G, Di Rocco C. The role of limited posterior fossa craniectomy in the surgical treatment of Chiari malformation Type I: experience with a pediatric series. J Neurosurg. 2007;106:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Knerlich-Lukoschus F, Jünger S, Messing-Jünger M. "Management: opinions from different centers"-the Sankt Augustin experience. Childs Nerv Syst. 2019;35:1885-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Messing-Jünger M, Röhrig A. Primary and secondary management of the Chiari II malformation in children with myelomeningocele. Childs Nerv Syst. 2013;29:1553-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Arnautovic A, Splavski B, Boop FA, Arnautovic KI. Pediatric and adult Chiari malformation Type I surgical series 1965-2013: a review of demographics, operative treatment, and outcomes. J Neurosurg Pediatr. 2015;15:161-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 27. | Zakaria R, Kandasamy J, Khan Y, Jenkinson MD, Hall SR, Brodbelt A, Pigott T, Mallucci CL. Raised intracranial pressure and hydrocephalus following hindbrain decompression for Chiari I malformation: a case series and review of the literature. Br J Neurosurg. 2012;26:476-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |