Published online Dec 26, 2021. doi: 10.12998/wjcc.v9.i36.11487
Peer-review started: August 14, 2021
First decision: September 29, 2021
Revised: October 10, 2021
Accepted: November 18, 2021
Article in press: November 18, 2021
Published online: December 26, 2021
Processing time: 131 Days and 11.4 Hours
Sodium taurocholate cotransport polypeptide (NTCP) deficiency disease is a genetic metabolic disorder due to mutations in the SLC10A1 gene and impaired bile acid salt uptake by the basolateral membrane transport protein NTCP in hepatocytes. A variety of clinical manifestations and genetic mutation loci have been reported for this disease. However, specific therapeutic measures are lacking, and the long-term effects are unknown.
An infant with elevated bile acids and behavioral neurodevelopmental delay failed to respond to bile acid-lowering therapy. Genetic testing for metabolic liver disease revealed that the child had NTCP deficiency due to the SLC10A1 mutation: c.422dupA (p.Y141X), which is a novel mutation site. The current follow-up revealed a gradual decrease in bile acid levels after 1 year of age, but the child still had behavioral neurodevelopmental delays.
The clinical manifestations, genetic characteristics, treatment and long-term prognosis due to NTCP deficiency remain poorly defined and need to be further confirmed by more studies and reports.
Core Tip: The mutation loci and clinical manifestations of sodium taurocholate cotransport polypeptide deficiency disease are currently under further investigation. Our case emphasizes the need to re-examine the clinical manifestations, prognosis and interventions associated with hypercholesterolemia due to this disease and suggests that behavioral neurodevelopmental delay may also be a clinical manifestation of this disease.
- Citation: Liu HY, Li M, Li Q. De novo mutation loci and clinical analysis in a child with sodium taurocholate cotransport polypeptide deficiency: A case report. World J Clin Cases 2021; 9(36): 11487-11494
- URL: https://www.wjgnet.com/2307-8960/full/v9/i36/11487.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i36.11487
Sodium taurocholate cotransport polypeptide (NTCP) is a carrier protein encoded by solute carrier family 10 member 1 (SLC10A1), 23 kbp in length, located on chromosome 14q24.2 and containing five exons. The protein product, NTCP, consists of 349 amino acid residues and has a molecular weight of 38 kDa. NTCP is located in the basolateral membrane of hepatocytes and is a sodium-dependent transporter protein involved in the transport of bile acids from the blood to the hepatocytes to maintain the uninterrupted hepatic-intestinal circulation of bile salts, and its deficiency leads to elevated levels of bile acids in the blood (hypercholesterolemia)[1-3]. Several genetic mutation loci have been found to contribute to the disease, with hypercholestasis as the main manifestation and some cases accompanied by disorders of lipid metabolism and cholestatic liver disease. Here, we report a case of a newly identified mutated locus and suggest that behavioral neurodevelopmental delay may also be a clinical manifestation of the disease.
Elevated bile acids in an 11-mo-old patient.
The child was hospitalized in a local hospital after birth due to a “neonatal infection”. After improvement of liver function, she was found to have elevated bile acids and was discharged from the hospital after treatment with “anti-infective drugs and ursodeoxycholic acid” to promote bile acid excretion and continued to take “ursodeoxycholic acid (15 mg/kg per time, bid)” for more than 9 mo after discharge. The bile acid level did not improve significantly. There was no history of vomiting and diarrhea during the disease, no yellow staining of skin and sclera, no voluntary scratching and no white clay-like stools. Mental, diet and sleep condition were sound, and urine and stool were normal.
The child was hospitalized in a local hospital after birth due to a “neonatal infection”.
The child was a gestation 3 production 3, gestational age 39 wk, born at full term, birth weight 2970 g, head held up at 3 mo after birth, could sit up on her own and recognize people at 7 mo, can crawl at present, cannot stand unassisted, can babble and combine vowels and consonants, like ‘ma ma ma ma’ and ‘da da da da,’ can wave goodbye and has other gestures. Her parents denied any history of trauma, surgery or blood transfusion, and there were no special circumstances with her family history. Both of her parents and two elder brothers had no elevated bile acids, and her motor-intellectual development was normal.
Body temperature 36.4 ˚C , respiration 32 times/min, heart rate 112 times/min, weight 9 kg (percentiles (P) 50-75), height 75 cm (P75) and head circumference 43 m (P25-50). General condition: no yellowish staining of the skin; no pigmentation; no petechiae and hemorrhages; no enlargement of superficial lymph nodes; no deformity of skull and facial features; fontane 0.3 cm × 0.3 cm; the sclera was not yellowish; the lips and mouth were pink; the heart and lungs functioned properly; the abdomen was in proper condition; the liver and spleen were not enlarged; and the nervous system functioned properly.
Psychomotor development assessment: the development screen test, an intelligence development screening test for children aged 0-6 years in China, showed a developmental quotient of 73, which is equivalent to 8 mo of age for intellectual development, 8 mo of age for motor development and 10 mo of age for social adaptation, with suspected psychomotor developmental delay (test results of developmental quotient 70-84 are considered suspicious).
Blood, urine and stool routine were in proper condition. Liver function was shown in Table 1. No abnormal laboratory results of renal function, cardiac enzymes, thyroid function, serum ion, anemia and blood lipids were found. Immunoglobulin and complement assay were in proper condition. Epstein-Barr virus DNA was negative. Cytomegalovirus DNA was negative. Hepatitis pathogenesis qualitative set were negative, and 25-hydroxy vitamin D level was mildly deficient (57 ng/mL, reference value less than 60 ng/mL is deficiency).
| Age (mo) | Day 5 | 2 mo 20 d | 5 mo 20 d | 9 mo | 9 mo 16 d | 10 mo 17 d | 11 mo 2 d | 11 mo 17 d | 12 mo | 13 mo 15 d | 17 mo | 20 mo |
| Total protein (65-85 g/L) | 49 | 56.6 | 60.1 | 61.4 | 64.4 | 74.2 | 66.1 | 68.5 | 69 | 72 | 65.5 | 67 |
| Glutamic pyruvic transaminase(7-40 μ/L) | 43.2 | 45.4 | 40.2 | 38.4 | 42.6 | 61.8 | 21.9 | 8.7 | 11.2 | 32.2 | 18 | 21 |
| Glutamic oxaloacetic transaminase (13-35 μ/L) | 32.0 | 40.9 | 31.0 | 23.3 | 35.1 | 86.6 | 37.4 | 30.8 | 32.0 | 35.8 | 39.0 | 35.0 |
| Albumin (40-55 g/L) | 30.5 | 41.4 | 42.7 | 40.7 | 42.5 | 43.9 | 40.1 | 44.2 | 43.0 | 47.0 | 40.6 | 42.0 |
| Alkaline phosphatase (< 28 IU/L) | 400.0 | 573.6 | 572.0 | 479.2 | 384.2 | 327.0 | 336.0 | 461.7 | 432.0 | 342.9 | 472.0 | 435.0 |
| Total bilirubin (3.4-17.1 μmol/L) | 131.5 | 7.0 | 3.9 | 3.1 | 5.4 | 3.8 | 2.9 | 3.6 | 3.0 | 4.5 | 5.7 | 5.2 |
| Indirect bilirubin (≤ 3.4 μmol/L) | 126.3 | 3.3 | 2.3 | 2.6 | 3.0 | 1.3 | 2.4 | 3.1 | 2.5 | 3.2 | 2.4 | 2.8 |
| Direct bilirubin (1.7-10.2 μmol/L) | 5.2 | 3.6 | 1.6 | 0.5 | 2.4 | 2.5 | 0.5 | 0.5 | 0.5 | 1.3 | 3.3 | 2.4 |
| Total bile acid (≤ 10 μmol/L) | 231.9 | 193.0 | 239.6 | 235.1 | 210.7 | 543.3 | 576.1 | 533.0 | 220.0 | 180.2 | 145.4 | 137.0 |
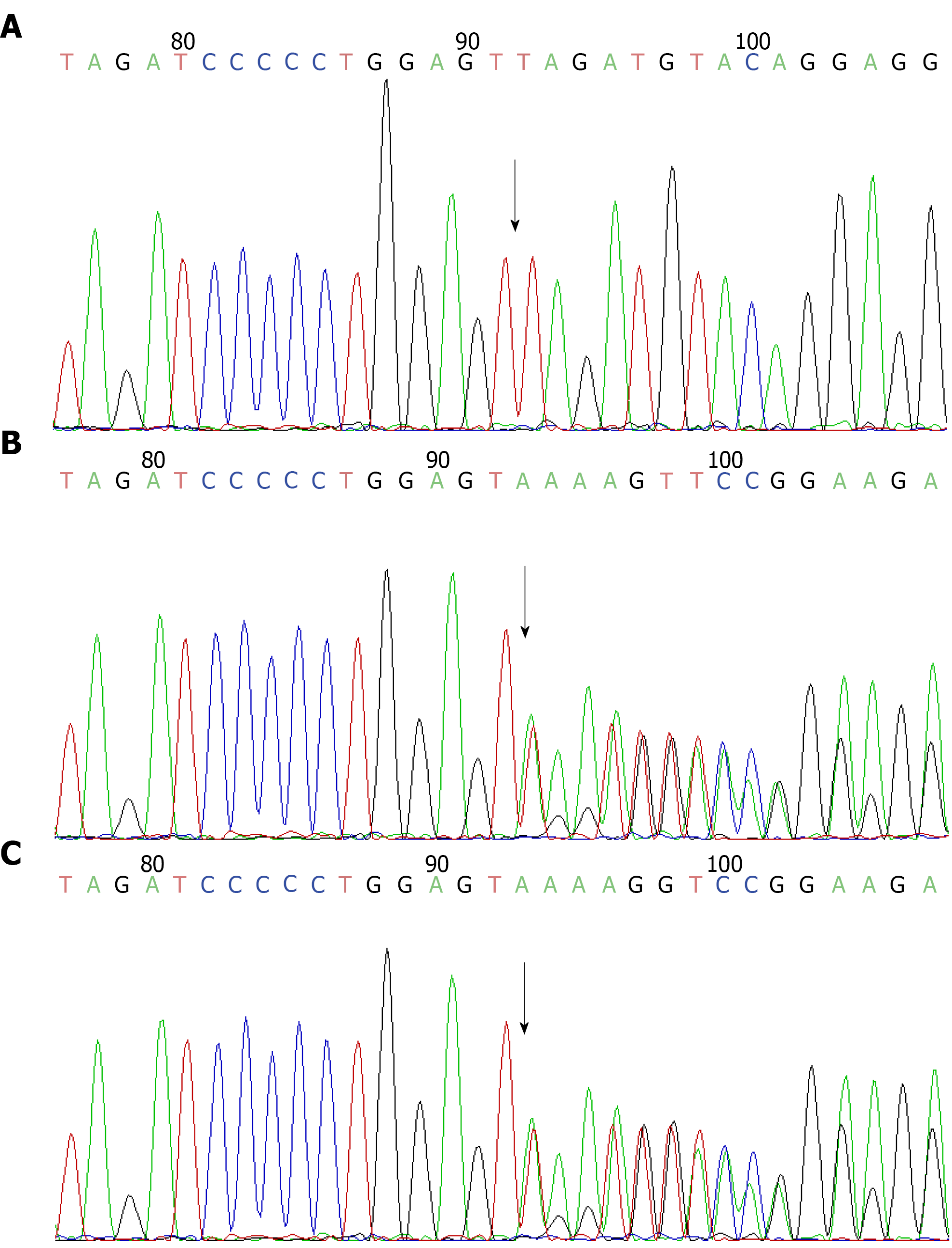
Further examination: in conjunction with the child’s medical history and clinical presentation, genetic testing for metabolic liver disease revealed a pure mutation in the SLC10A1 gene at c.422dupA, resulting in a nonsense mutation in amino acid p.Y141X (see Figure 1). According to the American College of Medical Genetics guidelines, this mutation was judged to be pathogenic, but there were no reports of mutations at this locus in the literature database and no results of pathogenicity analysis. The parents of the child were both heterozygous for this locus by lineage verification analysis. In the present case, the child was female, and both parents were carriers of the heterozygous variant. Both had no clinical manifestations, consistent with autosomal recessive inheritance.
Abdominal ultrasound: no abnormality was found in liver, biliary tract, pancreas and spleen.
NTCP deficiency disease.
She was treated with phenobarbital, rifampicin and difenacoum for 2 wk, vitamin D supplementation, intensive psycho-behavioral developmental interventions and other symptomatic supportive treatments, with no significant decrease in bile acids and normal liver enzymes on dynamic review.
After the age of 1 year, the bile acid level of this child showed a gradual decrease to a level of about 130 μmol/L. There was no liver enzyme damage, no skin pruritus and no white clay-like stools. Current follow-up at 20 mo 4 d, the child’s head circumference was 46 cm (P50), length was 80 cm (P75), weight was 11.5 kg (P50-75). The results of the Gesell Developmental Diagnostic Scale: gross motor score was 89 at the normal state (developmental age 17.8 mo); fine motor score was 77 at the borderline state (developmental age 15.4 mo); adaptive ability score was 78 at borderline state (developmental age 15.6 mo); language ability score was 70 at the delayed state (developmental age 14.0 mo); and personal-social development score was 75 at the delayed state (developmental age 15.0 mo). The child was not developing well except for gross motor development. The family was advised to strengthen the psycho-behavioral developmental intervention and follow up on the clinical and biochemical characteristics of the child.
NTCP deficiency disease is a newly identified genetic metabolic disorder characterized by significant and persistent hypercholesterolemia. As of July 2021, more than 90 cases of NTCP deficiency have been reported in 12 studies, with a common feature of hypercholestasis, an inheritance pattern consistent with autosomal recessive inheritance, and possible involvement in the development of neonatal hyperbilirubinemia, early infantile cholestasis and gestational cholestasis[4-15]. NTCP deficiency disorders currently lack specific treatment. SLC10A1 gene analysis can help to confirm the diagnosis in patients with this disease in time while avoiding unnecessary tests and interventions[7]. The SLC10A1 gene variant c.800C > T (p.Ser267Phe) is the most common among reported patients with NTCP-deficient disease[12], especially in Southeast Asian patients, with an allele frequency of 95.5%[13] and occupies an absolute dominance in the SLC10A1 variant spectrum. In addition, c.263T > C (p. Ile88Thr)[14], c.755G > A (p. Arg252His)[4], c.615 618del (p. Ser206Profs* 12)[15], c.595A > C (p.Ser199Arg)[11] and other SLC10A1 variant types were detected. However, the c. 422dupA (p.Y141X) mutation locus, in this case, has not been reported, and the new nonsense variant c.422dupA (p.Y141X) enriches the SLC10A1 mutation spectrum and clinical manifestations in a child with elevated bile acids as the predominant phenotype with motor mental retardation.
The first international case of an NTCP-deficient child was reported in 2015 by Dutch authors with a mutation at c.755G > A (p. Arg252His) and a clinical phenotype of extremely elevated bile acids (up to 1500 μM, reference value < 16.3), accompanied by mild hypotonia, growth retardation and delayed motor milestones[4]. As reported in the latest follow-up, the child remained developmentally delayed and required special education but had normal height and weight development. Another case reported total bile acid levels exceeding 1000 μmol/L until 4 years of age but gradually decreasing to between 500 and 800 μmol/L after 5 years of age[16]. In other case reports of SLC10A1 mutations, hypercholesterolemia was usually reported as the main manifestation, with some cases accompanied by clinical phenotypes such as disorders of lipid metabolism and cholestatic liver disease.
Multiple clinical studies have reported that c.800C > T (p.Ser267Phe)[6], c.595A > C (p.Ser199Arg)[11], c.263T > C (p.Ile88Thr)[14] and c.615 618del (p. Ser206Profs* 12)[15] loci mutations were found in patients with dyslipidemia and sex hormone disorders, and NTCP deficient individuals were more prone to vitamin D deficiency, sex hormone disorder and dyslipidemia. In a literature report[7], children with c.800C > T (p.Ser267Phe) pure mutation were between 25% and 75% of the same age group in terms of height and weight, 61% had jaundice (yellowing of eyes or skin), 23.1% had hepatomegaly and proceeded with histopathological features including hepatocyte destruction, periportal inflammation and fibrosis, resembling mild chronic viral hepatitis. In addition, Chen et al[10] reported that 2 patients with NTCP deficiency with pure mutations of p. Ser267Phe had intracranial pressure in late gestation. In summary, previous studies have reported that hypercholesterolemia is the main manifestation of the disease, and some cases are associated with lipid metabolism disorders and cholestatic liver disease. Most scholars believe that the manifestation of motor retardation in NTCP-deficient children is still a mystery except the first international report of a child with NTCP deficiency who had mild hypotonia and growth retardation clinical phenotype. However, in the context of this paper, we should re-examine the clinical manifestations, prognosis and interventions related to hypercholestasis caused by this disease, and behavioral neurodevelopmental delay may also be one of the clinical manifestations of this disease.
In addition to NTCP, there are also OATP1B1 and OATP1B3 (encoded by SLCO1B1 and SLCO1B3, respectively), which also function in the uptake of bilirubin into hepatocytes[17,18]. In animal experiments by Roscam Abbing et al[19], it was shown that due to the strong compensatory effect of OATP1B1/1B3, mice were able to maintain normal plasma total bile acid levels even when NTCP uptake function was inhibited. However, normal bile acids do not predict an unimpaired liver function, and it was found that the compensation of OATP1B3 can lead to bile deposition in hepatocytes. Over time the massive deposition of bile pigments can lead to damage of hepatocytes and bile ducts and even increase the risk of gallstones[20,21]. Similarly, Mao et al[22] observed in an animal test model of knockout mice with SLC10A1 gene that SlC10A1 deficiency resulted in various abnormal manifestations of the gallbladder, including gallbladder wall thickening, gallbladder enlargement (wall thickening), gallbladder enlargement with black-green bile and diaphragm cap malformation. Furthermore, in an animal model by Zhang et al[23], it was observed that the metabolism of tyrosine, glycine, taurine, fatty acids and glycerophospholipids as well as the biosynthesis of tryptophan, pantothenic acid and coenzyme A were significantly dysregulated in NTCP knockout mice by metabolic pathway analysis, suggesting that NTCP is closely associated with these metabolic pathways. It is worth suggesting that several amino acids such as tyrosine and tryptophan are closely related to neurological development in humans; thus, the developmental delay associated with NTCP deficiency disease should be taken seriously. Whether it is related to abnormal metabolic pathways should be further investigated.
There is a lack of specific therapeutic agents for NTCP-deficient disease; symptomatic supportive therapy is the main management. The long-term effects of long-term hypercholesterolemia are unknown. According to the current study data, the disease lacks specific therapeutic measures, but the short-term clinical outcome of patients tends to be good, and no serious adverse prognosis such as death or cirrhosis due to this disease has been reported so far[4-16]. Therefore, no other relevant medication has been given in this case for the time being.
The clinical phenotype, genetic features, treatment and long-term prognosis due to NTCP deficiency disease are still poorly defined and need to be further confirmed by more studies and findings. However, to summarize the current literature, hypercholesterolemia is the main manifestation of the disease. Lipid metabolism disorders, cholestatic liver disease and mental-behavioral developmental delay may also be clinical phenotypes. According to current research data, the disease lacks specific therapeutic measures and requires further research findings and follow-up. Thus, in children with persistent hypercholesterolemia, SLC10A1 can be used as the primary screening gene test, and the child’s growth and development, liver function, 25-hydroxy vitamin D, lipids, sex hormones and other biochemical indicators related to lipid metabolism should be closely monitored for a comprehensive assessment to achieve a better outcome for this group of children.
We would like to thank the patients and their families for their participation in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Navarrete Arellano M, Rodrigues AT S-Editor: Wang JL L-Editor: Filipodia P-Editor: Wang JL
| 1. | Hagenbuch B, Meier PJ. Molecular cloning, chromosomal localization, and functional characterization of a human liver Na+/bile acid cotransporter. J Clin Invest. 1994;93:1326-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 337] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Hagenbuch B, Dawson P. The sodium bile salt cotransport family SLC10. Pflugers Arch. 2004;447:566-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 188] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Anwer MS, Stieger B. Sodium-dependent bile salt transporters of the SLC10A transporter family: more than solute transporters. Pflugers Arch. 2014;466:77-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Vaz FM, Paulusma CC, Huidekoper H, de Ru M, Lim C, Koster J, Ho-Mok K, Bootsma AH, Groen AK, Schaap FG, Oude Elferink RP, Waterham HR, Wanders RJ. Sodium taurocholate cotransporting polypeptide (SLC10A1) deficiency: conjugated hypercholanemia without a clear clinical phenotype. Hepatology. 2015;61:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 160] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 5. | Zou TT, Zhu Y, Wan CM, Liao Q. Clinical features of sodium-taurocholate cotransporting polypeptide deficiency in pediatric patients: case series and literature review. Transl Pediatr. 2021;10:1045-1054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Liu R, Chen C, Xia X, Liao Q, Wang Q, Newcombe PJ, Xu S, Chen M, Ding Y, Li X, Liao Z, Li F, Du M, Huang H, Dong R, Deng W, Wang Y, Zeng B, Pan Q, Jiang D, Zeng H, Sham P, Cao Y, Maxwell PH, Gao ZL, Peng L. Homozygous p.Ser267Phe in SLC10A1 is associated with a new type of hypercholanemia and implications for personalized medicine. Sci Rep. 2017;7:9214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Dong C, Zhang BP, Wang H, Xu H, Zhang C, Cai ZS, Wang DW, Shu SN, Huang ZH, Luo XP. Clinical and histopathologic features of sodium taurocholate cotransporting polypeptide deficiency in pediatric patients. Medicine (Baltimore). 2019;98:e17305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Li H, Qiu JW, Lin GZ, Deng M, Lin WX, Cheng Y, Song YZ. Clinical and genetic analysis of a pediatric patient with sodium taurocholate cotransporting polypeptide deficiency. Zhongguo Dang Dai Er Ke Za Zhi. 2018;20:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (1)] |
| 9. | Yan YY, Wang MX, Gong JY, Liu LL, Setchell KDR, Xie XB, Wang NL, Li W, Wang JS. Abnormal Bilirubin Metabolism in Patients With Sodium Taurocholate Cotransporting Polypeptide Deficiency. J Pediatr Gastroenterol Nutr. 2020;71:e138-e141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Chen R, Deng M, Rauf YM, Lin GZ, Qiu JW, Zhu SY, Xiao XM, Song YZ. Intrahepatic Cholestasis of Pregnancy as a Clinical Manifestation of Sodium-Taurocholate Cotransporting Polypeptide Deficiency. Tohoku J Exp Med. 2019;248:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Li H, Deng M, Guo L, Qiu JW, Lin GZ, Long XL, Xiao XM, Song YZ. Clinical and molecular characterization of four patients with NTCP deficiency from two unrelated families harboring the novel SLC10A1 variant c.595A > C (p.Ser199Arg). Mol Med Rep. 2019;20:4915-4924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Deng M, Mao M, Guo L, Chen FP, Wen WR, Song YZ. Clinical and molecular study of a pediatric patient with sodium taurocholate cotransporting polypeptide deficiency. Exp Ther Med. 2016;12:3294-3300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Tan HJ, Deng M, Qiu JW, Wu JF, Song YZ. Monozygotic Twins Suffering From Sodium Taurocholate Cotransporting Polypeptide Deficiency: A Case Report. Front Pediatr. 2018;6:354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Qiu JW, Deng M, Cheng Y, Atif RM, Lin WX, Guo L, Li H, Song YZ. Sodium taurocholate cotransporting polypeptide (NTCP) deficiency: Identification of a novel SLC10A1 mutation in two unrelated infants presenting with neonatal indirect hyperbilirubinemia and remarkable hypercholanemia. Oncotarget. 2017;8:106598-106607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Van Herpe F, Waterham HR, Adams CJ, Mannens M, Bikker H, Vaz FM, Cassiman D. NTCP deficiency and persistently raised bile salts: an adult case. J Inherit Metab Dis. 2017;40:313-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Vaz FM, Huidekoper HH, Paulusma CC. Extended Abstract: Deficiency of Sodium Taurocholate Cotransporting Polypeptide (SLC10A1): A New Inborn Error of Metabolism with an Attenuated Phenotype. Dig Dis. 2017;35:259-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Van Dyke RW, Stephens JE, Scharschmidt BF. Bile acid transport in cultured rat hepatocytes. Am J Physiol. 1982;243:G484-G492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta. 2003;1609:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 596] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 19. | Roscam Abbing RLP, Slijepcevic D, Donkers JM, Havinga R, Duijst S, Paulusma CC, Kuiper J, Kuipers F, Groen AK, Oude Elferink RPJ, van de Graaf SFJ. Blocking Sodium-Taurocholate Cotransporting Polypeptide Stimulates Biliary Cholesterol and Phospholipid Secretion in Mice. Hepatology. 2020;71:247-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Appleby RN, Nolan JD, Johnston IM, Pattni SS, Fox J, Walters JR. Novel associations of bile acid diarrhoea with fatty liver disease and gallstones: a cohort retrospective analysis. BMJ Open Gastroenterol. 2017;4:e000178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Wang G, Han T, Wang S, Chen M, Sun Y, Fu Z. Peroxisome Proliferator-Activated Receptor-γ Prevents Cholesterol Gallstone Formation in C57bl Mice by Regulating Bile Acid Synthesis and Enterohepatic Circulation. Biomed Res Int. 2018;2018:7475626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Mao F, Wang MX, Hou X, Zhou Z, Yan YY, Fang LJ, Tan Z, Fang WY, Liu T, He W, Li C, Xie XB, Lu SQ, Sui J, Wang F, Han J, Wang JS, Li W. NTCP Deficiency Causes Gallbladder Abnormalities in Mice and Human Beings. Cell Mol Gastroenterol Hepatol. 2021;11:831-839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Zhang Q, He Z, Liu Z, Gong L. Integrated plasma and liver gas chromatography mass spectrometry and liquid chromatography mass spectrometry metabolomics to reveal physiological functions of sodium taurocholate cotransporting polypeptide (NTCP) with an Ntcp knockout mouse model. J Chromatogr B Analyt Technol Biomed Life Sci. 2021;1165:122531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |